Synthesis of Carbon Showing Weak Antiferromagnetic Behavior at a Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
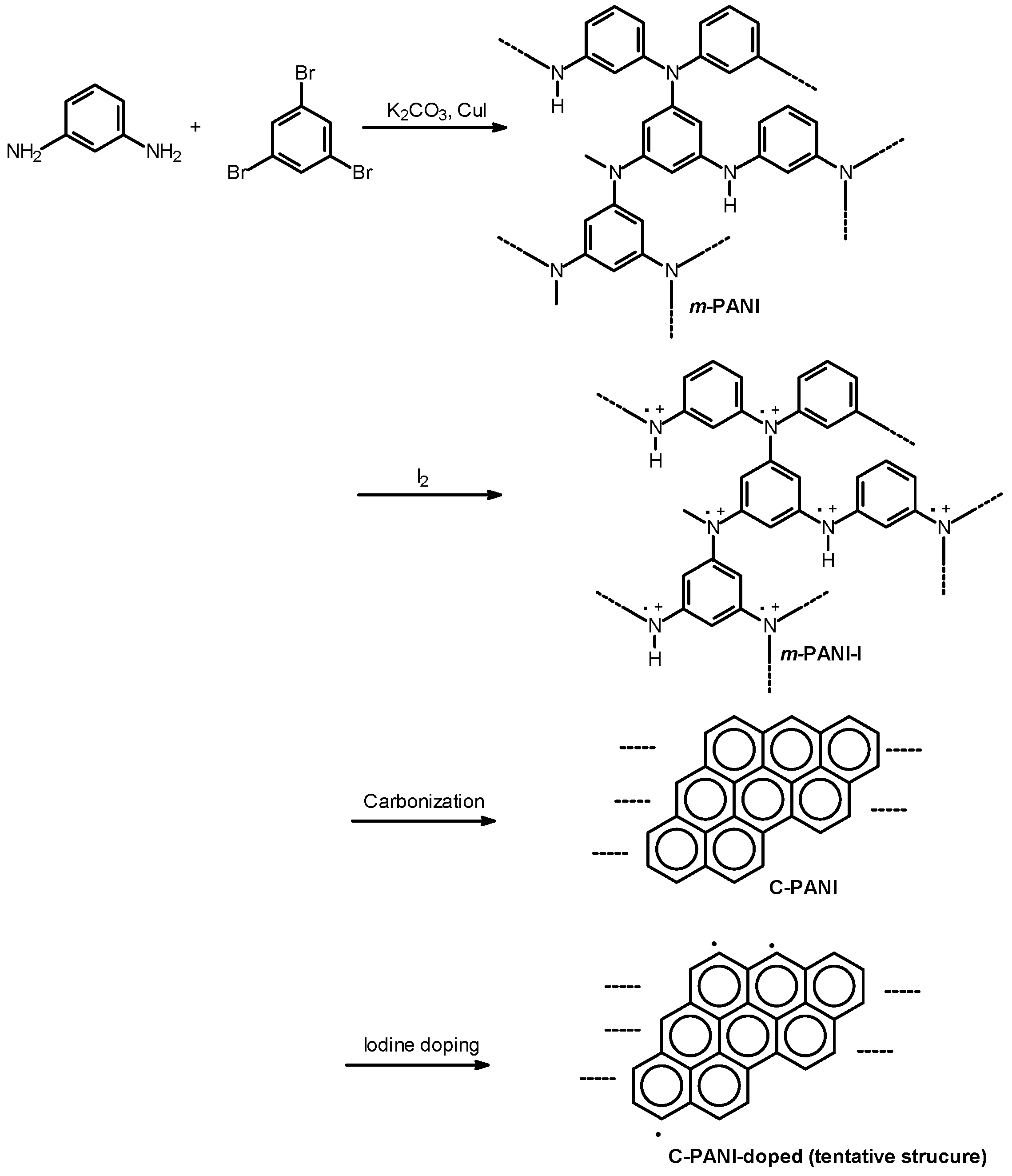
2.1. Synthesis
2.2. Instruments
3. Results
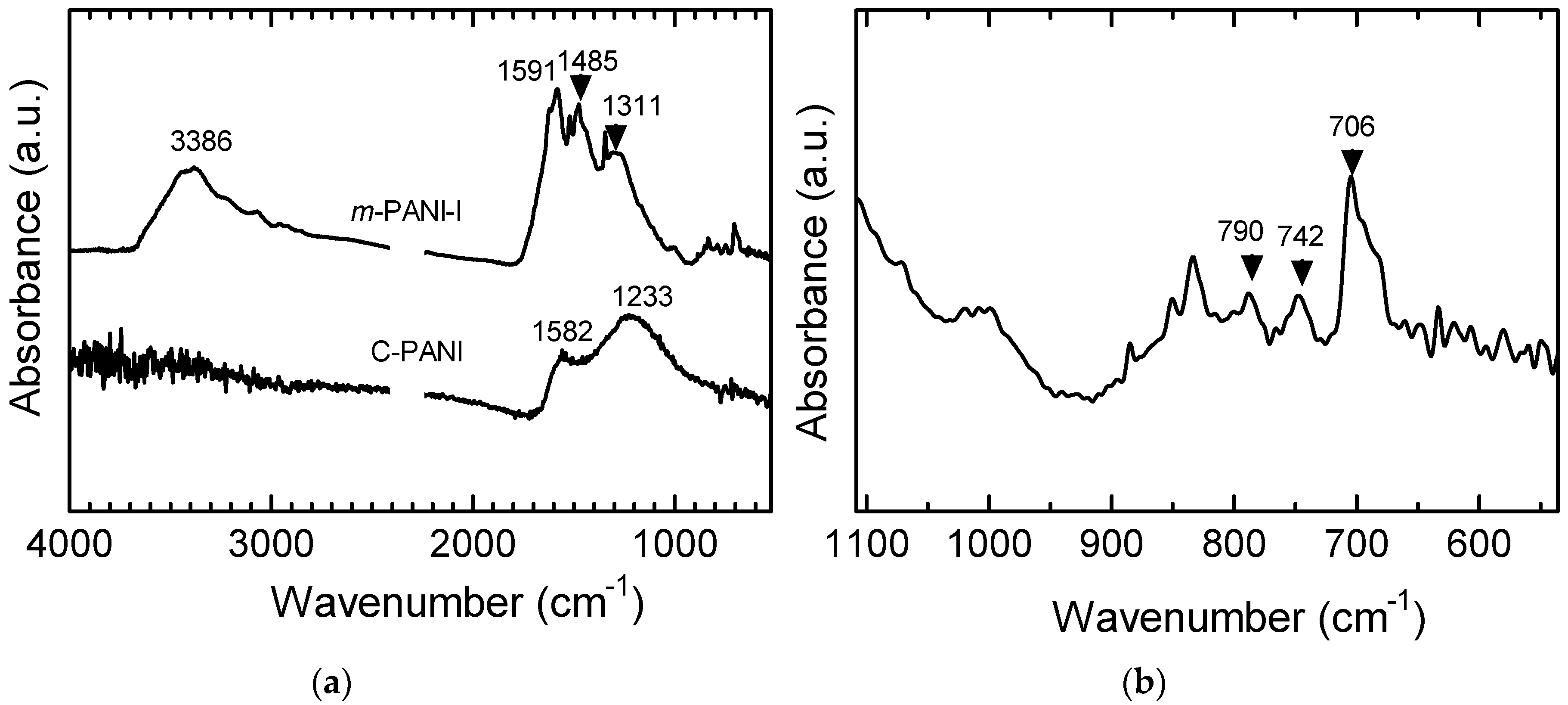
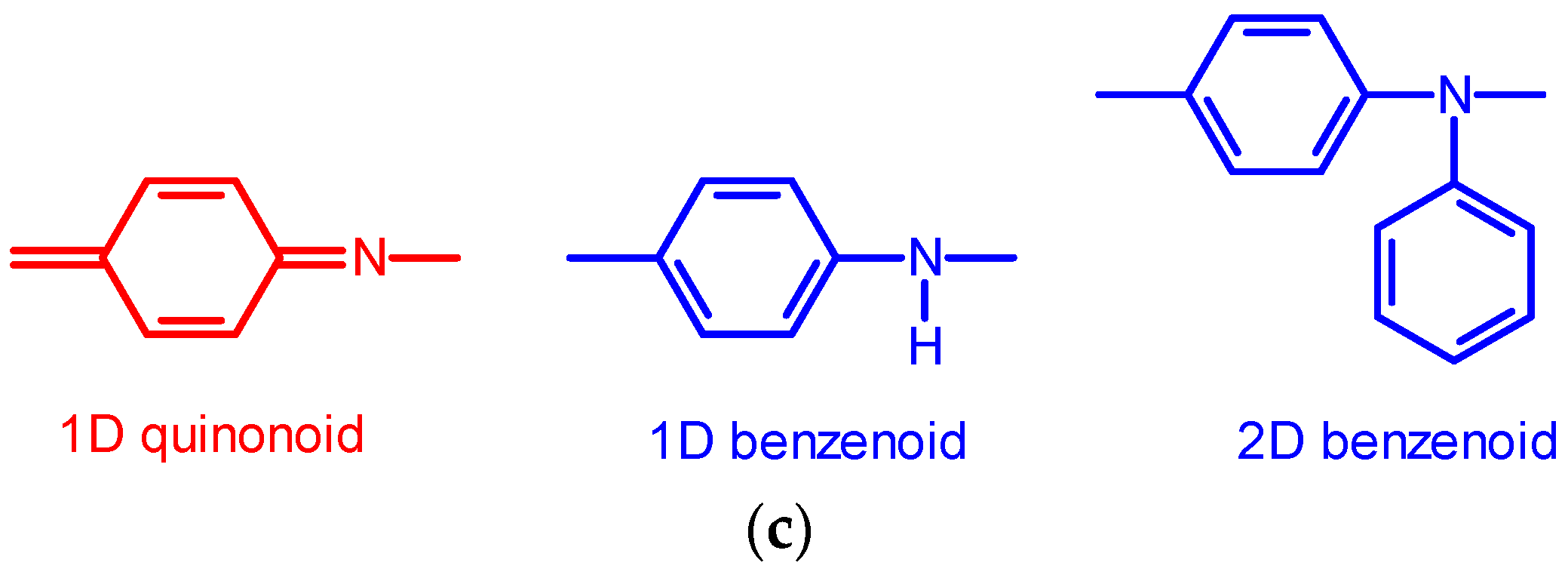
3.1. IR Spectra
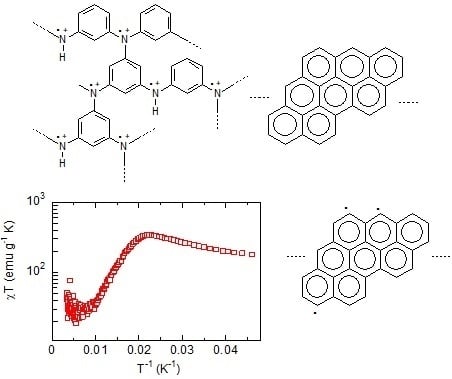
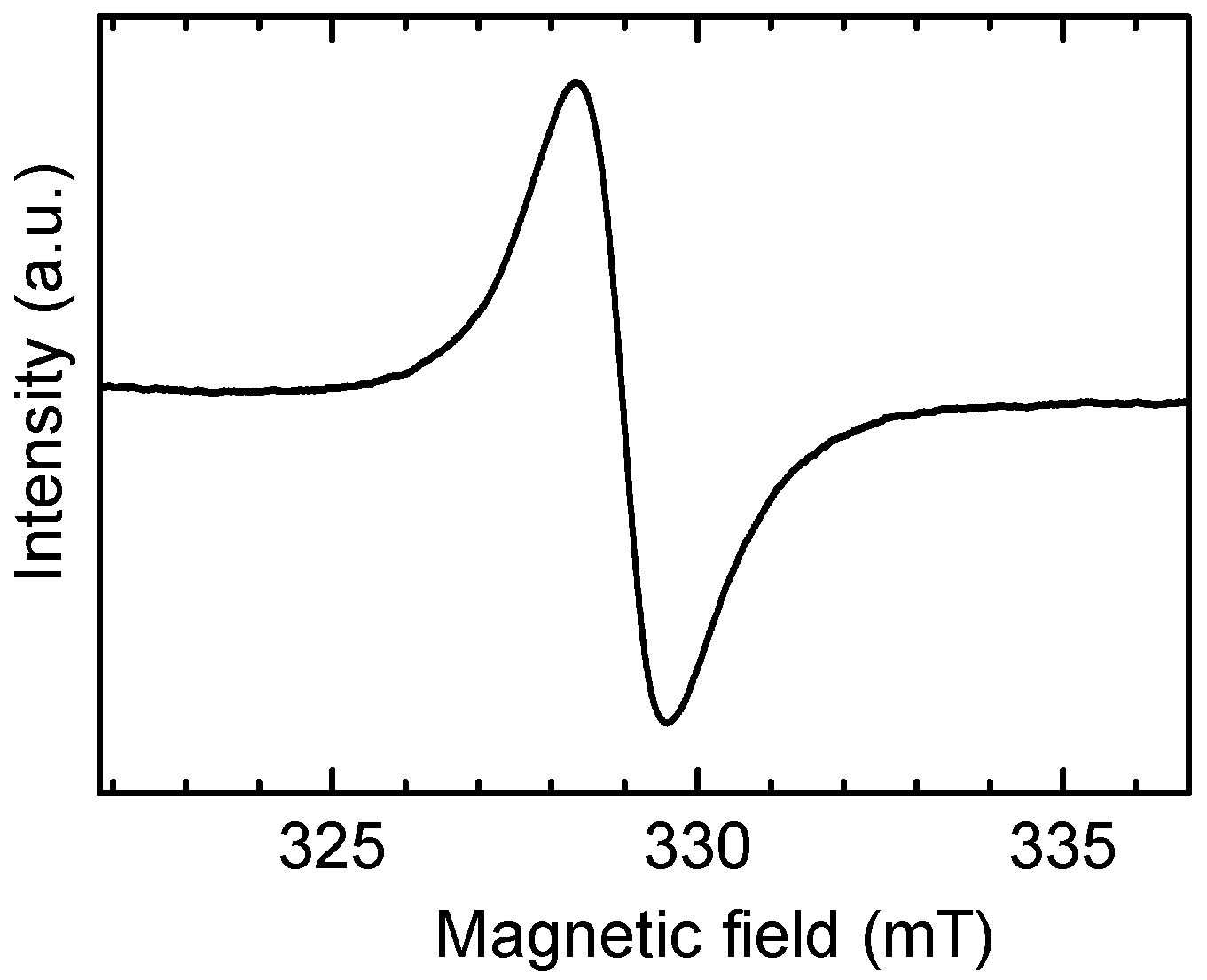
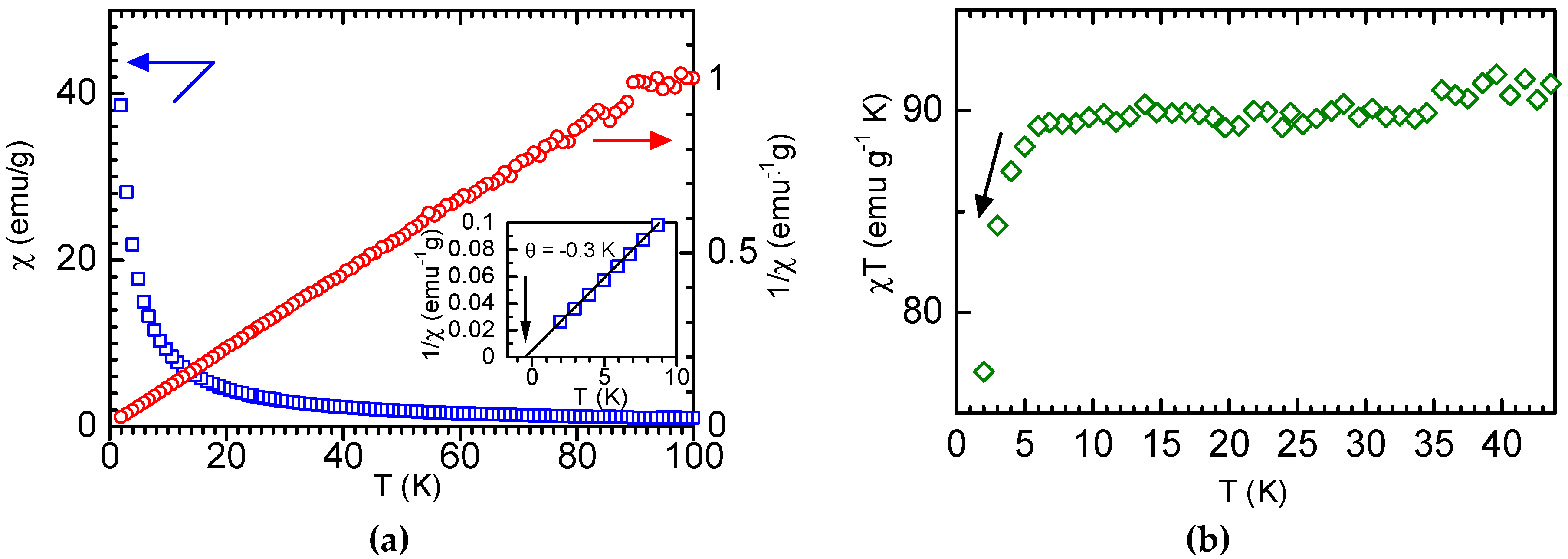
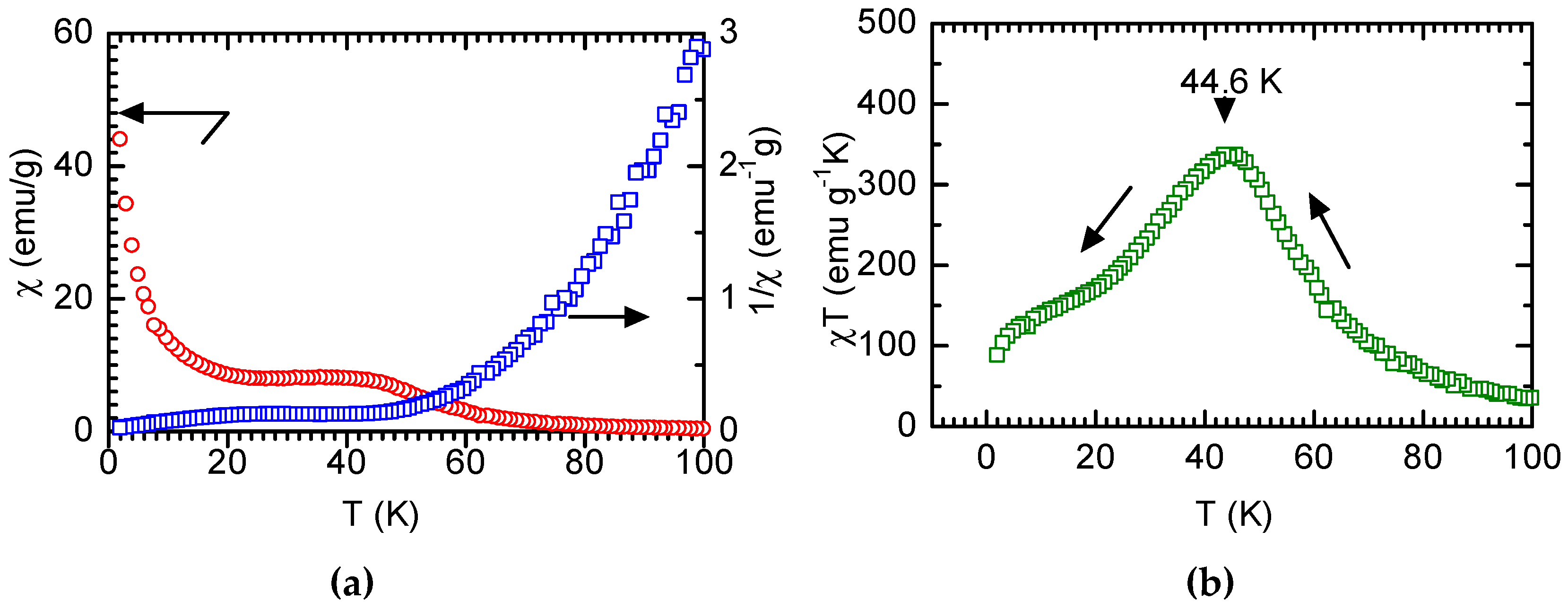
3.2. Magnetic Measurement
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iwamura, H.; Murata, S. Magnetic coupling of two triplet phenylnitrene units joined through an acetylenic or a di-acetylenic linkage. Mol. Cryst. Liq. Cryst. Incorp. Nonlinear Opt. 1989, 176, 33–47. [Google Scholar] [CrossRef]
- Araki, H.; Matsuoka, R.; Yoshino, K. Ferromagnetic behavior of pyrolyzed o,m,p-phenylenediamine and triazine derivatives. Solid State Commun. 1991, 79, 443–446. [Google Scholar] [CrossRef]
- Michinobu, T.; Takahashi, M.; Tsuchida, E.; Nishide, H. Robust Triplet Molecule: Cationic Diradical of 3,4‘-Bis(diphenylamino)stilbene. Chem. Mater. 1999, 11, 1969–1971. [Google Scholar] [CrossRef]
- Dobrzyńska, E.; Jouni, M.; Gawryś, P.; Gambarelli, S.; Mouesca, J.-M.; Djurado, D.; Dubois, L.; Wielgus, I.; Maurel, V.; Kulszewicz-Bajer, I. Tuning of ferromagnetic spin interactions in polymeric aromatic amines via modification of their π-conjugated system. J. Phys. Chem. B. 2012, 116, 14968–14978. [Google Scholar] [CrossRef] [PubMed]
- Skorka, L.; Kurzep, P.; Chauviré, T.; Dubois, L.; Mouesca, J.-M.; Maurel, V.; Kulszewicz-Bajer, I. High-spin polymers: ferromagnetic coupling of s = 1 hexaazacyclophane units up to a pure s = 2 polycyclophane. J. Phys. Chem. B. 2017, 121, 4293–4298. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xia, H.; Qin, X.; Li, W.; Dai, Y.; Liu, X.; Zhao, M.; Xia, Y.; Yan, S.; Wang, B. Correlation between the vacancy defects and ferromagnetism in graphite. Carbon 2009, 47, 1399–1406. [Google Scholar] [CrossRef]
- Ali, M.; Pi, X.; Liu, Y.; Yang, D. Electronic and magnetic properties of graphene, silicone and germanene with varying vacancy concentration. AIP Adv. 2017, 7, 045308. [Google Scholar] [CrossRef]
- Shimizu, D.; Osuka, A. A benzene-1,3,5-triaminyl radical fused with ZnII-porphyrins: remarkable stability and a high-spin quartet ground state. Angew. Chem. Int. Ed. 2018, 57, 3733–3736. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, F.; Bielecki, J. Synthesis in the biphenyl series. Ber. Dtsch. Chem. 1901, 34, 2174. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Tanaka, K.; Yamabe, T.; Yamauchi, J. Ferromagnetic interaction in poly(m-aniline): Electron spin resonance and magnetic susceptibility. J. Chem. Phy. 1992, 96, 5516–5522. [Google Scholar] [CrossRef]
- Mougel, V.; Chatelain, L.; Hermle, J.; Caciuffo, R.; Colineau, E.; Tuna, F.; Magnani, N.; de Geyer, A.; Pécaut, J.; Mazzanti, M. A Uranium-based UO2+–Mn2+ single-chain magnet assembled trough cation–cation Interactions. Angew. Chem. Int. Ed. 2014, 53, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Coulon, C.; Clerac, R.; Lecren, L.; Wernsdorfer, W.; Miyasaka, H. Glauber dynamics in a single-chain magnet: From theory to real systems. Phys. Rev. B. 2004, 69, 132408. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Ishikawa, R.; Breedlove, B.; Yamashita, M. Single-chain magnets: Beyond the Glauber model. RSC Adv. 2013, 3, 3772–3798. [Google Scholar] [CrossRef]
- Gałecka, M.; Wielgus, I.; Zagórska, M.; Pawłowski, M.; Kulszewicz-Bajer, I. High-spin radical cations of poly(m−p-anilines) and poly(m−p−p-anilines): Synthesis and spectroscopic properties. Macromolecules 2007, 40, 4924–4932. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Teng, P.-Y.; Yeh, C.-H.; Koshino, M.; Chiu, P.-W.; Suenaga, K. Structural and chemical dynamics of pyridinic-nitrogen defects in graphene. Nano Lett. 2015, 15, 7408–7413. [Google Scholar] [CrossRef] [PubMed]


| Compound | Role | Chemical Structure | Quantity |
|---|---|---|---|
| m-Phenylenediamine | Monomer |  | 0.12 (g) |
| Tribromobenzene | Monomer | 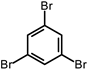 | 0.37 (g) |
| Copper iodide | Catalyst | CuI | 0.019 (g) |
| Potassium carbonate | Co-catalyst | K2CO3 | 0.47 (g) |
| Nitrobenzene | Solvent |  | 2.35 mL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaki, M.; Hirokawa, S.; Goto, H. Synthesis of Carbon Showing Weak Antiferromagnetic Behavior at a Low Temperature. Condens. Matter 2019, 4, 33. https://doi.org/10.3390/condmat4010033
Otaki M, Hirokawa S, Goto H. Synthesis of Carbon Showing Weak Antiferromagnetic Behavior at a Low Temperature. Condensed Matter. 2019; 4(1):33. https://doi.org/10.3390/condmat4010033
Chicago/Turabian StyleOtaki, Masashi, Shota Hirokawa, and Hiromasa Goto. 2019. "Synthesis of Carbon Showing Weak Antiferromagnetic Behavior at a Low Temperature" Condensed Matter 4, no. 1: 33. https://doi.org/10.3390/condmat4010033
APA StyleOtaki, M., Hirokawa, S., & Goto, H. (2019). Synthesis of Carbon Showing Weak Antiferromagnetic Behavior at a Low Temperature. Condensed Matter, 4(1), 33. https://doi.org/10.3390/condmat4010033