d° Ferromagnetism of Magnesium Oxide
Abstract
1. Introduction
2. Magnesium Oxide
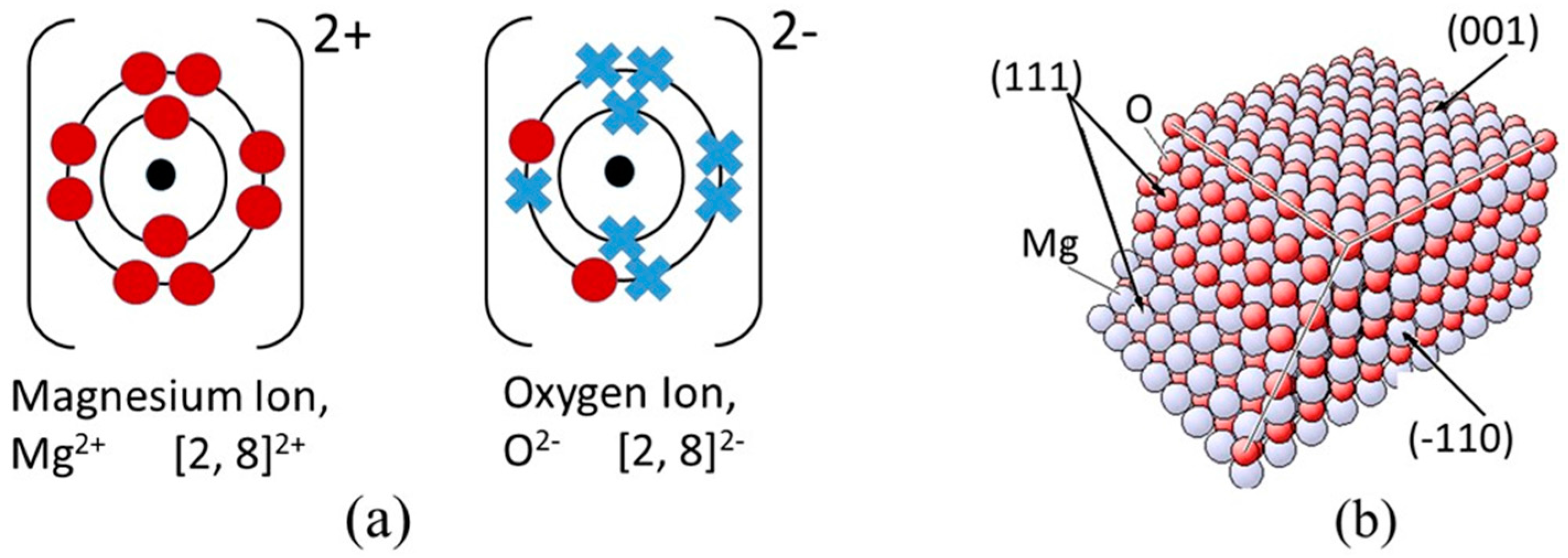
2.1. Electronic Configuration and Structure
2.2. General Properties
2.3. Emerging Phenomena at Low Dimensions
3. Magnetism of MgO: Experimental Approach
3.1. Doping with Magnetic Ions
3.2. Doping with Non-Magnetic Ions
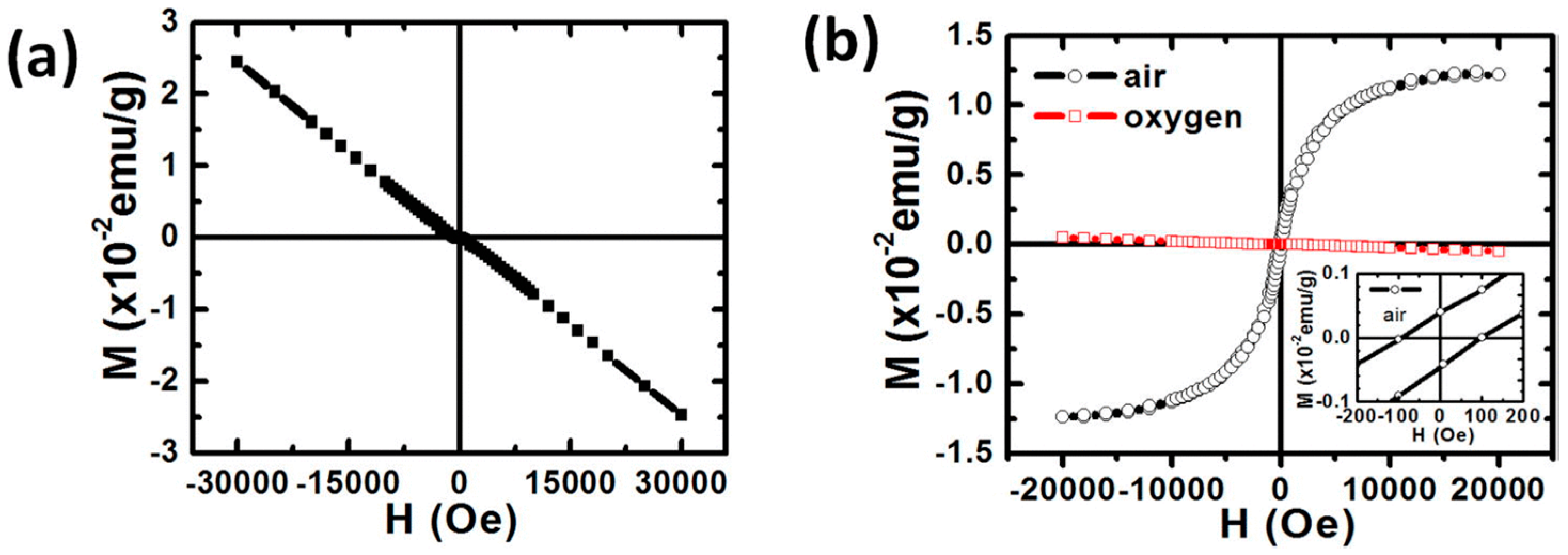
3.3. Non-Doped Systems
4. Magnetism of MgO: Theoretical Approach
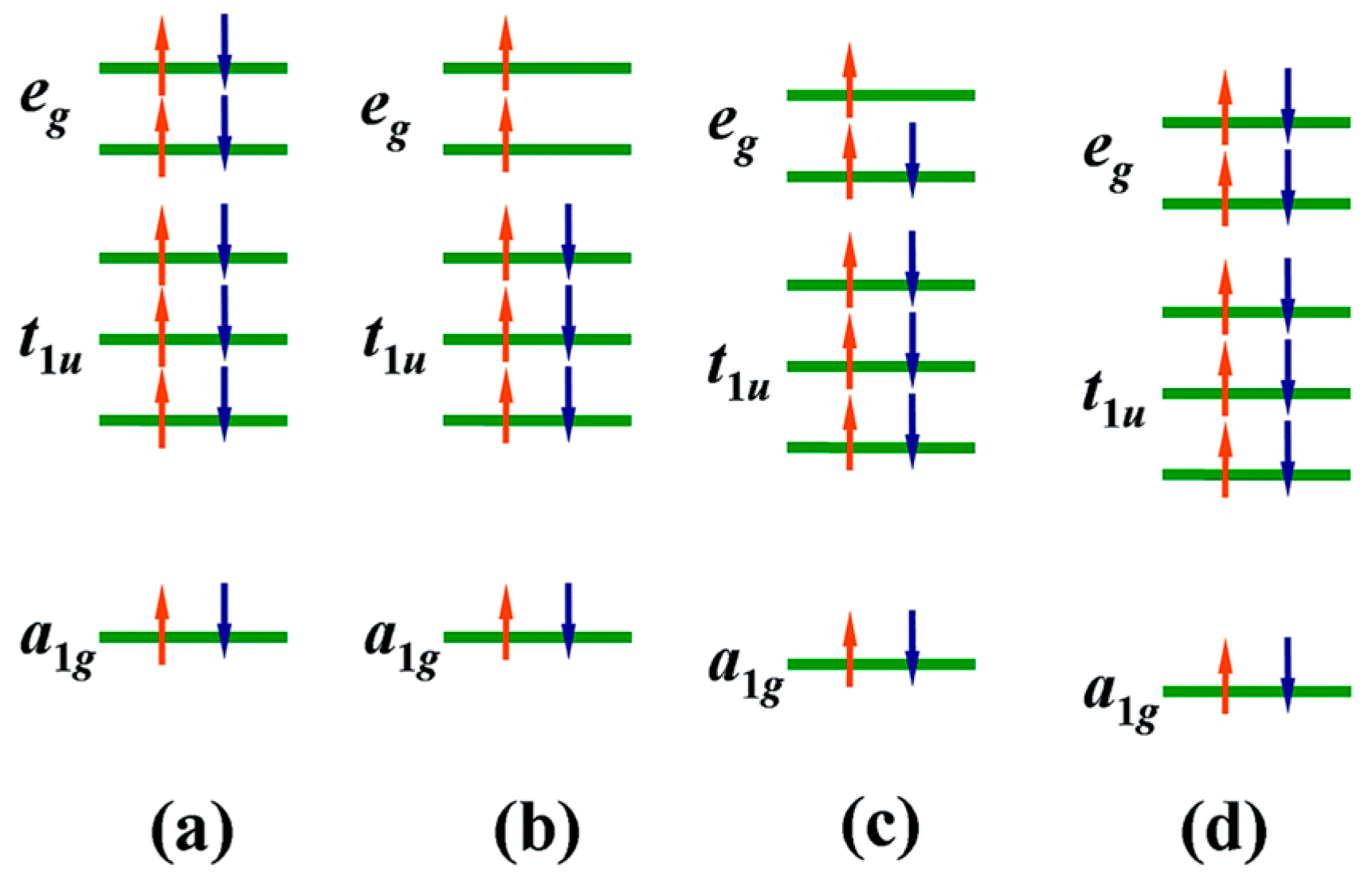
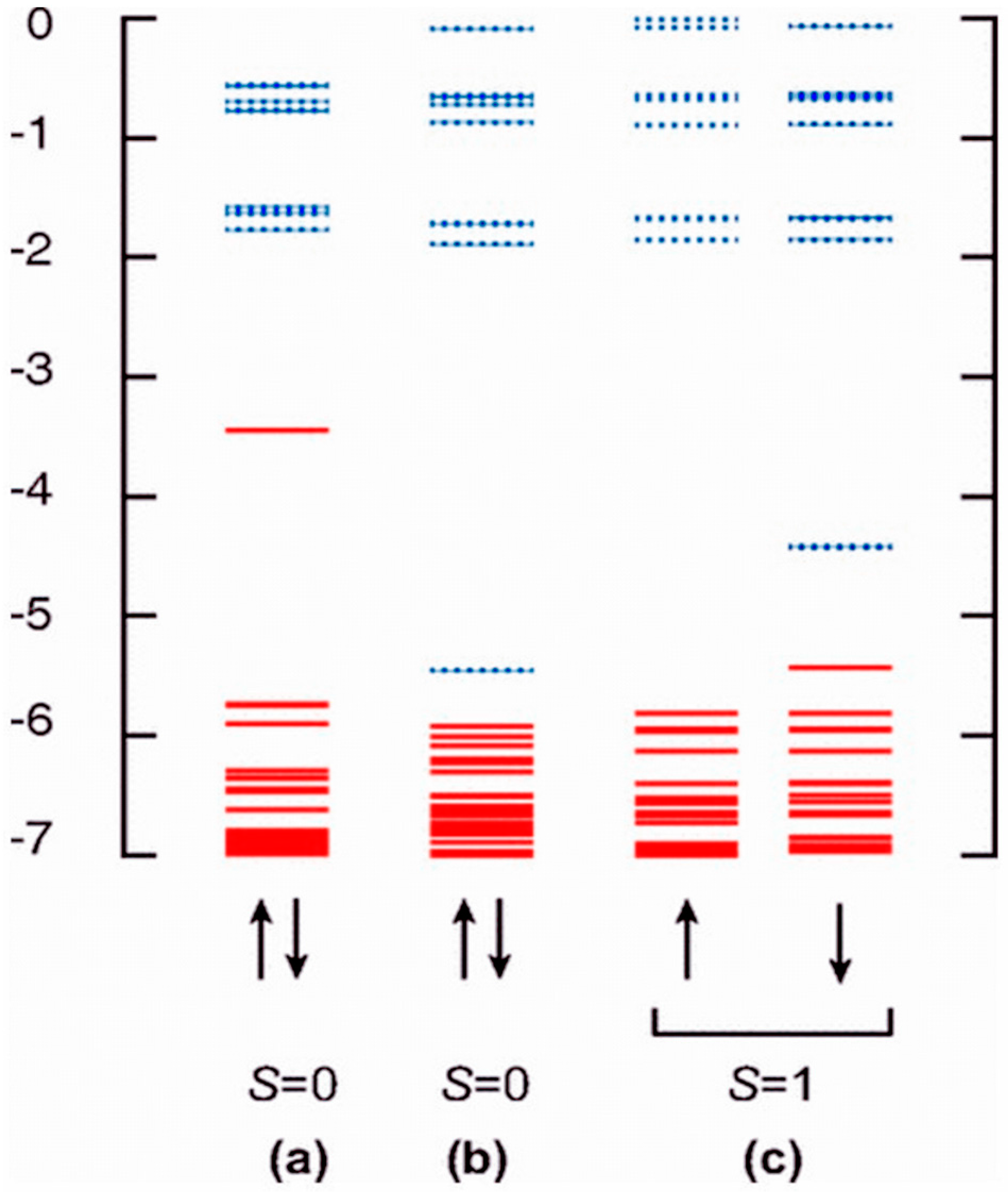
4.1. Mg Vacancy as a Source of Magnetism
4.2. Oxygen Vacancy/Composite Defects as a Source of Magnetism
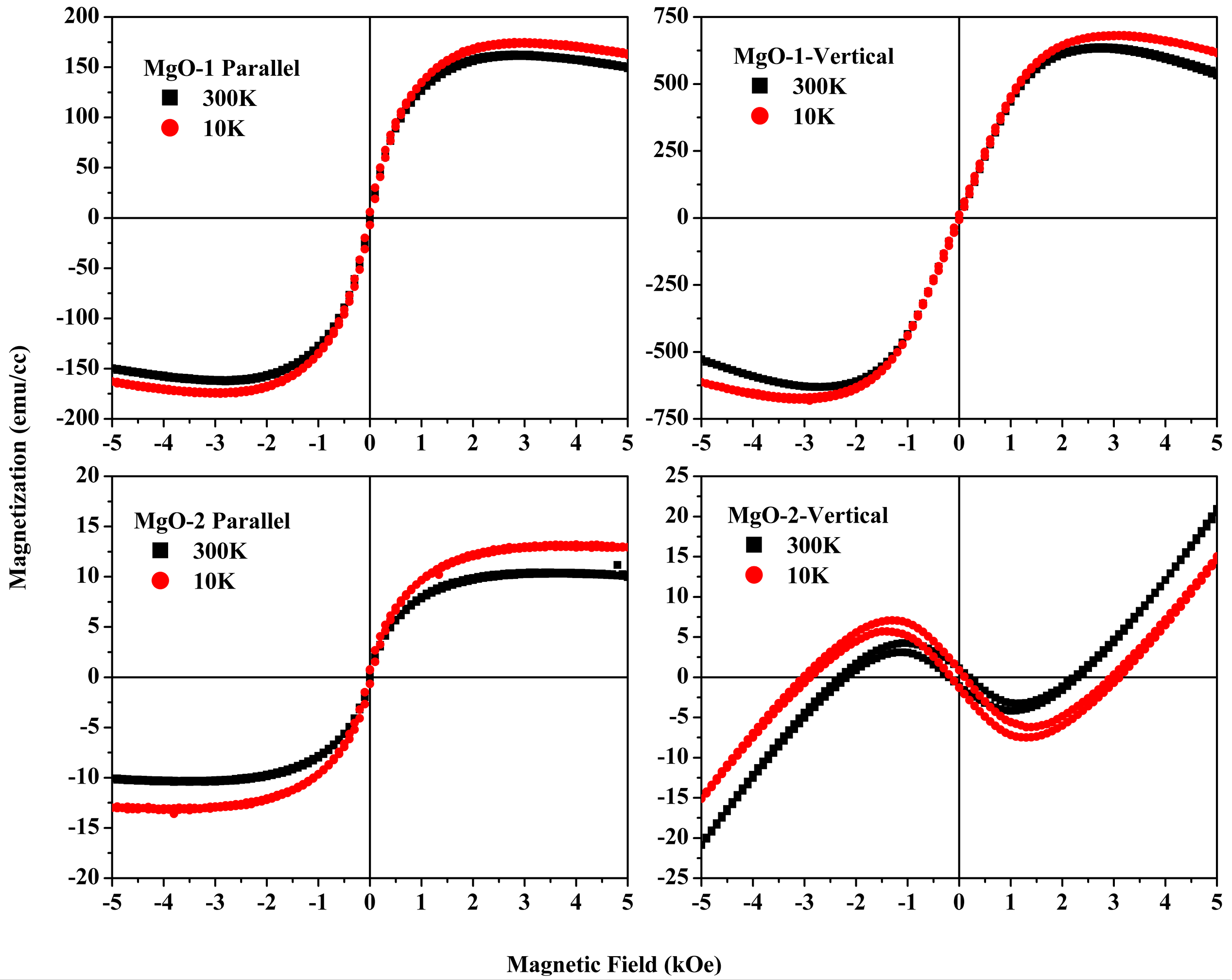
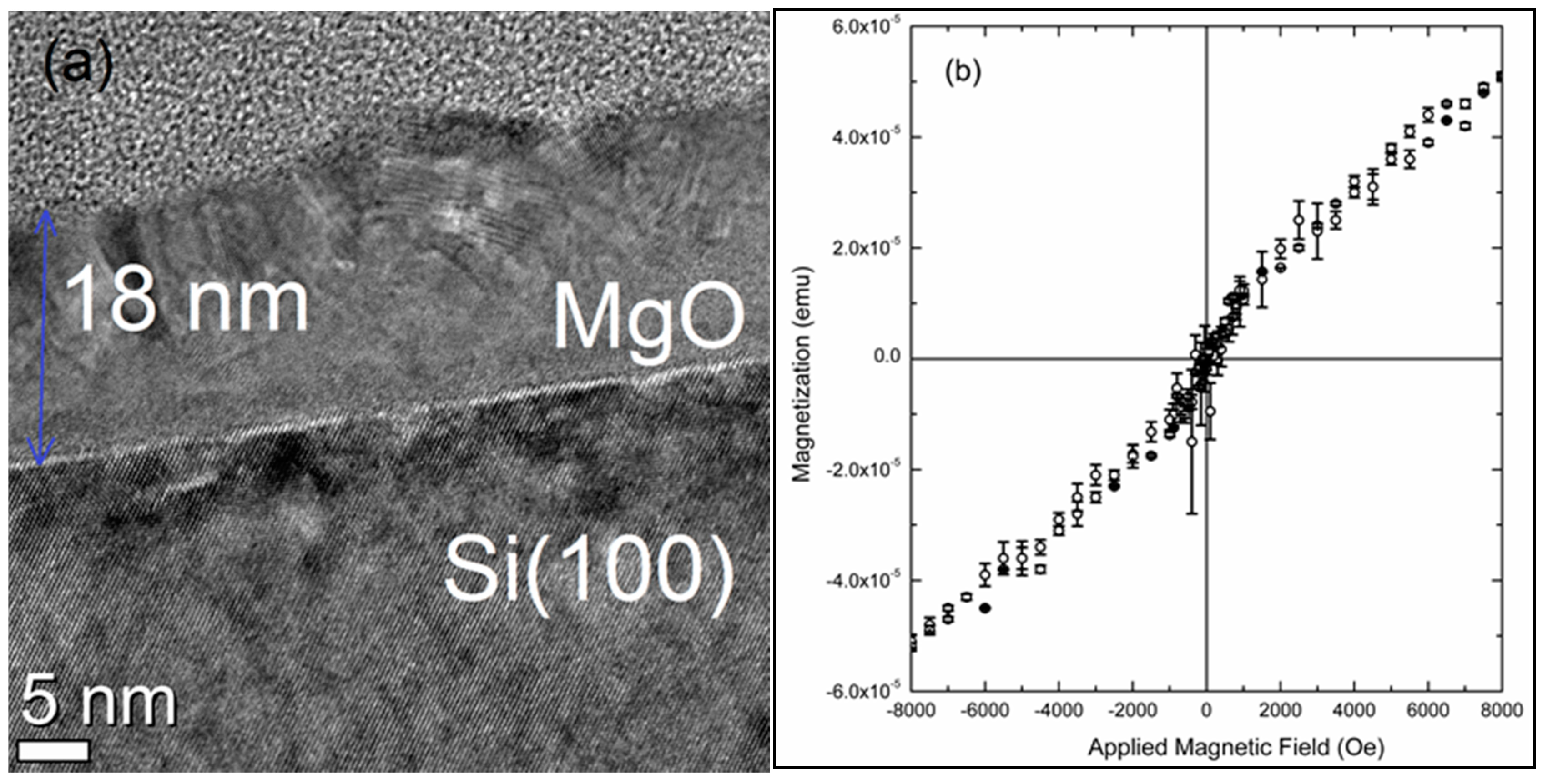
5. Magnetism of Radio-Frequency (RF) Sputtered MgO
6. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cullity, B.D. Introduction to Magnetic Materials; Addision-Wesley: Massachusetts, MA, USA, 1972. [Google Scholar]
- Qin, J.P.J.; Nogués, J.; Mikhaylova, M.; Roig, A.; Muñoz, J.S.; Muhammed, M. Differences in the magnetic properties of Co, Fe, and Ni 250−300 nm wide nanowires electrodeposited in amorphous anodized alumina templates. Chem. Mater. 2005, 17, 1829–1834. [Google Scholar] [CrossRef]
- Singh, J.P.; Srivastava, R.C.; Agrawal, H.M.; Reddy, V.R.; Gupta, A. Observation of bulk like magnetic ordering below the blocking temperature in nanosized zinc ferrite. J. Magn. Magn. Mater. 2012, 324, 2553–2559. [Google Scholar] [CrossRef]
- Chetri, P.; Choudhury, B.; Choudhury, A. Room temperature ferromagnetism in SnO2 nanoparticles: An experimental and density functional study. J. Mater. Chem. C 2014, 2, 9294–9302. [Google Scholar] [CrossRef]
- Díaz-Gallifa, P.; Fabelo, O.; Pasán, J.; Cañadillas-Delgado, L.; Lloret, F.; Julve, M.; Ruiz-Pérez, C. Two-Dimensional 3d–4f Heterometallic Coordination Polymers: Syntheses, Crystal Structures, and Magnetic Properties of Six New Co(II)–Ln(III) Compounds. Inorg. Chem. 2014, 53, 6299–6308. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, M.; Fitzgerald, C.B.; Coey, J.M.D. Thin films: Unexpected magnetism in a dielectric oxide. Nature 2004, 430, 630. [Google Scholar] [CrossRef] [PubMed]
- Si, M.S.; Gao, D.; Yang, D.; Peng, Y.; Zhang, Z.Y.; Xue, D.; Liu, Y.; Deng, X.; Zhang, G.P. Intrinsic ferromagnetism in hexagonal boron nitride nanosheets. J. Chem. Phys. 2014, 140, 204701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Prucnal, S.; Chen, X.; Tong, W.; Yang, Z.; Munnik, F.; Potzger, K.; Skorupa, W.; Gemming, S.; et al. Disentangling defect-induced ferromagnetism in SiC. Phys. Rev. 2014, 89, 014417. [Google Scholar] [CrossRef]
- Sundaresan, A.; Rao, C.N.R. Ferromagnetism as a universal feature of inorganic nanoparticles. Nano Today 2009, 4, 96–106. [Google Scholar] [CrossRef]
- Rao, C.N.; Nakate, U.T.; Choudhary, R.J.; Kale, S.N. Defect-induced magneto-optic properties of MgO nanoparticles realized as optical-fiber-based low-field magnetic sensor. Appl. Phys. Lett. 2013, 103, 151107. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Si, M.; Zhu, Z.; Yang, G.; Shia, Z.; Xue, D. Origin of the unexpected room temperature ferromagnetism: formation of artificial defects on the surface in NaCl particles. J. Mater. Chem. C 2013, 1, 6216–6222. [Google Scholar] [CrossRef]
- Ma, Y.W.; Lu, Y.H.; Yi, J.B.; Feng, Y.P.; Herng, T.S.; Liu, X.; Gao, D.Q.; Xue, D.S.; Xue, J.M.; Ouyang, J.Y.; et al. Room temperature ferromagnetism in teflon due to carbon dangling bonds. Nat. Commun. 2012, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, X.; Chen, P.; Zhou, D.; Wu, C.; Guo, Y.; Zhang, L.; Zhao, J.; Wu, X.; Xi, Y. Hydrogen dangling bonds induce ferromagnetism in two-dimensional metal-free graphitic-C3N4 nanosheets. Chem. Sci. 2015, 6, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Giesbers, A.J.M.; Uhlířová, K.; Konečný, M.; Peters, E.C.; Burghard, M.; Aarts, J.; Flipse, C.F.J. Interface-induced room-temperature ferromagnetism in hydrogenated epitaxial grapheme. Phys. Rev. Lett. 2013, 111, 166101. [Google Scholar] [CrossRef] [PubMed]
- Dimri, M.C.; Khanduri, H.; Kooskora, H.; Kodu, M.; Jaaniso, R.; Heinmaa, I.; Mere, A.; Krustok, J.; Stern, R. Room-temperature ferromagnetism in Ca and Mg stabilized cubic zirconia bulk samples and thin films prepared by pulsed laser deposition. J. Phys. D Appl. Phys. 2012, 45, 475003. [Google Scholar] [CrossRef]
- Glinchuk, M.D.; Eliseev, E.A.; Khist, V.V.; Morozovska, A.N. Ferromagnetism induced by magnetic vacancies as a size effect in thin films of nonmagnetic oxides. Thin Solid Films 2013, 534, 685–692. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, J.; Jing, J.Z.; Zhaohui, Q.; Shi, W.; Shi, H.; Xue, D. Vacancy-Mediated Magnetism in Pure Copper Oxide Nanoparticles. Nanoscale Res. Lett. 2010, 5, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Thurber, A.P.; Alanko, G.; Beausoleil, G.L., II; Dodge, K.N.; Hanna, C.B.; Punnoose, A. Unusual crystallite growth and modification of ferromagnetism due to aging in pure and doped ZnO nanoparticles. J. Appl. Phys. 2012, 111, 07C319. [Google Scholar] [CrossRef]
- Peng, H.; Xiang, H.J.; Wei, S.H.; Li, S.S.; Xia, J.B.; Li, J. Origin and enhancement of hole-induced ferromagnetism in first-row d0 semiconductors. Phys. Rev. Lett. 2009, 102, 017201. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhang, J.; Yang, G.; Qi, J.; Si, M.; Xue, D. Ferromagnetism Induced by Oxygen Vacancies in Zinc Peroxide Nanoparticles. J. Phys. Chem. C 2011, 115, 16405–16410. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lu, Y.H.; Huang, T.W.; Yan, D.C.; Dong, C.L. Oxygen Vacancy Dependent Magnetism of CeO2 Nanoparticles Prepared by Thermal Decomposition Method. J. Phys. Chem. C 2010, 114, 19576–19581. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, D.; Tao, K.; Zhang, J.; Shi, Z.; Xu, Q.; Shia, S.; Xue, D. A series of unexpected ferromagnetic behaviors based on the surface-vacancy state: An insight into NiO nanoparticles with a core–shell structure. RSC Adv. 2014, 4, 46133–46140. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, P.; Zhou, W. Tunable bandgap and ferromagnetism in sputtered epitaxial Sn1−xMgxO2 thin films. Appl. Phys. Lett. 2012, 101, 182406. [Google Scholar] [CrossRef]
- Hua, P.; Sha, Z.; Fa-Shen, L. Electronic structures and magnetic couplings of B-, C-, and N-doped BeO. Chin. Phys. B 2013, 22, 047504. [Google Scholar] [CrossRef]
- Farvid, S.S.; Sabergharesou, T.; Hutfluss, L.N.; Hegde, M.; Prouzet, E.; Radovanovic, P.V. Evidence of charge-transfer ferromagnetism in transparent diluted magnetic oxide nanocrystals: Switching the mechanism of magnetic interactions. J. Am. Chem. Soc. 2014, 136, 7669–7679. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, C.D.; Sanvito, S. Ferromagnetism driven by intrinsic point defects in HfO2. Phys. Rev. Lett. 2005, 94, 217205. [Google Scholar] [CrossRef] [PubMed]
- Mellor, J.W. A Comprehensive Treatise on Inorganic and Theoretical Chemistry; Longmans: London, UK, 1924; pp. 280–284. [Google Scholar]
- Verma, N.K.; Verma, N. Academic Chemistry IX; Laxmi Publications: New Delhi, India, 2012. [Google Scholar]
- Chang, K.J.; Cohen, M.I. High-pressure behavior of MgO: Structural and electronic properties. Phys. Rev. B 2008, 30, 4774–4781. [Google Scholar] [CrossRef]
- Magnesium oxide (MgO) crystal structure, lattice parameters, thermal expansion. In II-VI and I-VII Compounds; Semimagnetic Compounds; Madelung, O., Rössler, U., Schulz, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 41B, pp. 1–6. [Google Scholar]
- Lempicki, A. The Electrical Conductivity of MgO Single. Crystals at High Temperatures. Proc. Phys. Soc. B 1953, 66, 281–283. [Google Scholar] [CrossRef]
- Wilson, I.O. Magnesium oxide as a high-temperature insulant. IEE Proc. 1981, 128, 159–164. [Google Scholar] [CrossRef]
- Yamaka, K.; Sawamoto, K. Photoinduced Hall effect in MgO. Phys. Rev. 1956, 101, 565–566. [Google Scholar] [CrossRef]
- Reiling, G.H.; Henslzy, E. Fundamental optical absorption in magnesium oxide. Phys. Rev. 1958, 112, 1106–1111. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Shannon, R.D.; Chai, B.H.T.; Abraham, M.M.; Wintersgil, M.C. Dielectric Constants of BeO, MgO, and CaO Using the Two-Terminal Method. Phys. Chem. Miner. 1989, 16, 741–746. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, B.; Gautam, S.; Lim, W.C.; Asokan, K.; Chae, K.H. Chemical effects at interfaces of Fe/MgO/Fe magnetic tunnel junction. Superlattices Microstruct. 2016, 100, 560–586. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Guo, Z.; Zeng, R.; Dou, S.; Chen, X. Peashell-like nanostructure—A new kind of one-dimensional nanostructure: The case of magnesium oxide. Chem. Commun. 2010, 46, 3887–3889. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Won, S.O.; Lim, W.C.; Shim, C.H.; Chae, K.H. Optical behavior of MgO nanoparticles investigated using diffuse reflectance and near edge X-ray absorption spectroscopy. Mater. Lett. 2017, 198, 34–37. [Google Scholar] [CrossRef]
- Uchino, T.; Okutsu, D. Broadband laser emission from color centers inside MgO microcrystals. Phys. Rev. Lett. 2008, 101, 117401. [Google Scholar] [CrossRef] [PubMed]
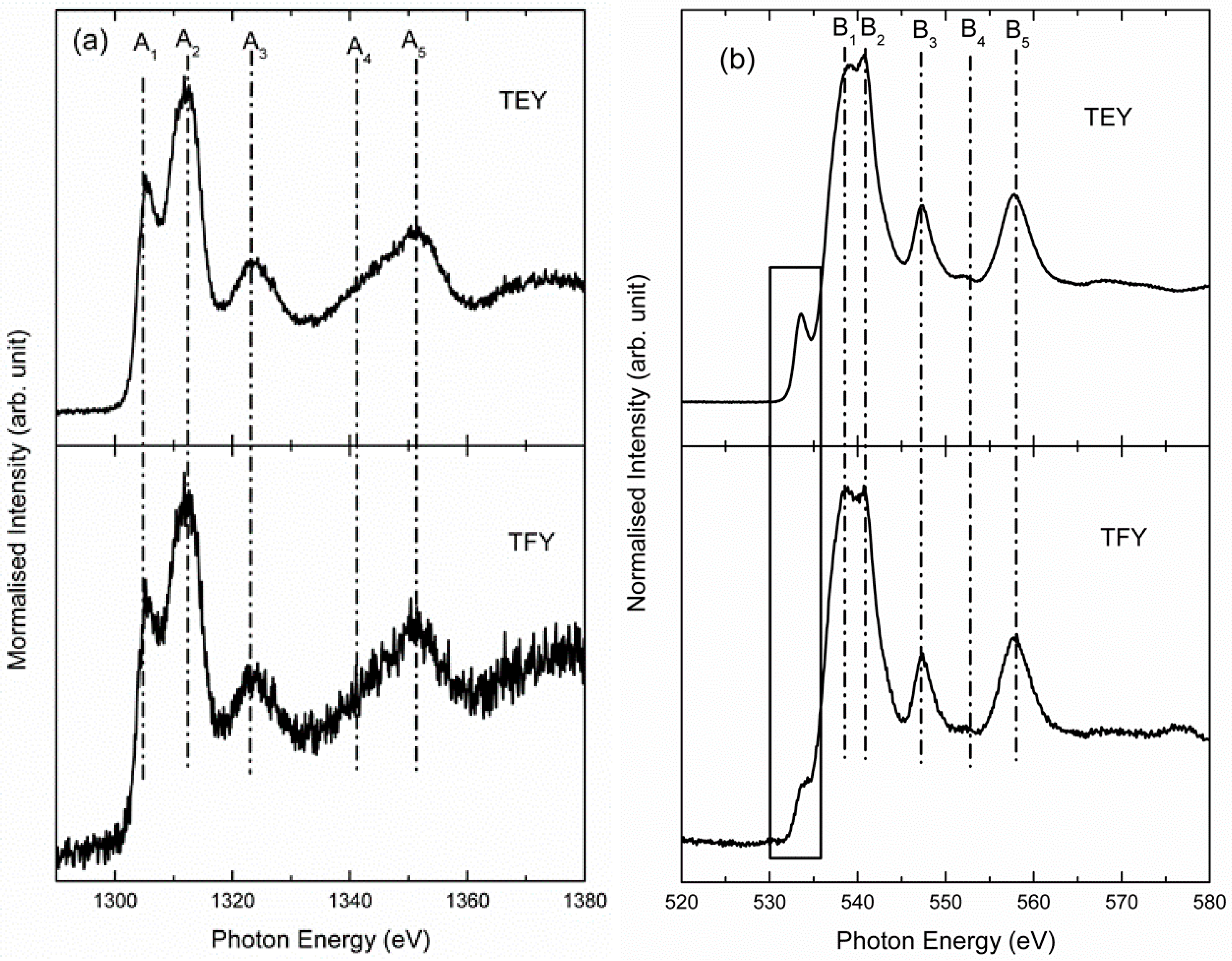
- Singh, J.P.; Chen, C.L.; Dong, C.L.; Prakash, J.; Kabiraj, D.; Kanjilal, D.; Pong, W.F.; Asokan, K. Role of surface and subsurface defects in MgO thin film: XANES and magnetic investigations. Superlattice Microstruct. 2015, 77, 313–324. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Schmid, A.K.; Qiu, Z.Q. Spin-Dependent Quantum Interference from Epitaxial MgO Thin Films on Fe(001). Phys. Rev. Lett. 2006, 97, 217205. [Google Scholar] [CrossRef] [PubMed]
- Jambois, O.; Carreras, P.; Antony, A.; Bertomeu, J.; Martínez-Boubeta, C. Resistance switching in transparent magnetic MgO films. Solid State Commun. 2011, 151, 1856–1859. [Google Scholar] [CrossRef]
- Nomura, K.; Taya, S.; Okazawa, A.; Kojima, N. Sol-gel synthesis and dilute magnetism of nano MgO powder doped with Fe. Hyperfine Interact. 2014, 226, 161–169. [Google Scholar] [CrossRef]
- Ramachandrana, S.; Narayan, J.; Prater, J.T. Magnetic properties of Ni-doped MgO diluted magnetic insulators. Appl. Phys. Lett. 2007, 90, 132511. [Google Scholar] [CrossRef]
- Narayan, J.; Nori, S.; Pandya, D.K.; Avasthi, D.K.; Smirnov, A.I. Defect dependent ferromagnetism in MgO doped with Ni and Co. Appl. Phys. Lett. 2008, 93, 082507. [Google Scholar] [CrossRef]
- Chun-Ming, L.; Hai-Quan, G.; Xia, X.; Yan, Z.; Yong, J.; Meng, C.; Xiao-Tao, Z. Optical and magnetic properties of nitrogen ion implanted MgO single crystal. Chin. Phys. B 2011, 20, 047505. [Google Scholar] [CrossRef]
- Li, Y.; Deng, R.; Yao, B.; Xing, G.; Wang, D.; Wu, T. Tuning ferromagnetism in MgxZn1−xO thin films by band gap and defect engineering. Appl. Phys. Lett. 2010, 97, 102506. [Google Scholar] [CrossRef]
- Mishra, D.; Mandal, B.P.; Mukherjee, R.; Naik, R.; Lawes, G.; Nadgorny, B. Oxygen vacancy enhanced room temperature magnetism in Al-doped MgO nanoparticles. Appl. Phys. Lett. 2013, 102, 182404. [Google Scholar] [CrossRef]
- Li, Q.; Ye, B.; Hao, Y.; Liu, J.; Zhang, J.; Zhang, L.; Kong, W.; Weng, H.; Ye, B. Room-temperature ferromagnetism observed in C-/N-/O-implanted MgO single crystals. Chem. Phys. Lett. 2013, 556, 237–241. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, B.; Singh, J.P.; Kumar, A.; Chae, K.H.; Asokan, K.; Kanjilal, D. Room temperature ferromagnetism in MgO single crystals via N-implantation. In Proceedings of the International Conference on Nano Structuring by Ion Beam, Indore, India, 11–13 October 2017; p. 40. [Google Scholar]
- Araujo, C.M.; Kapilashrami, M.; Jun, X.; Jayakumar, O.D.; Nagar, S.; Wu, Y.; Århammar, C.; Johansson, B.; Belova, L.; Ahuja, R.; et al. Room temperature ferromagnetism in pristine MgO thin films. Appl. Phys. Lett. 2010, 96, 232505. [Google Scholar] [CrossRef]
- Kapilashrami, M.; Xu, J.; Rao, K.V.; Belova, L.; Carlegrim, E.; Fahlman, M. Experimental evidence for ferromagnetism at room temperature in MgO thin films. J. Phys. Condens. Matter 2010, 22, 345004. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhan, Y.; Fahlman, M.; Fang, M.; Rao, K.V.; Belova, L. In-situ solution processed room temperature ferromagnetic MgO thin films printed by inkjet technique. MRS Proc. 2011, 1292, 12–26. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Li, Y.; Yang, D.; Xu, Y.; Yan, M. Origin of room temperature ferromagnetism in MgO films. Appl. Phys. Lett. 2013, 102, 072406. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Microstructural, optical and magnetic properties study of nanocrystalline MgO. Mater. Res. Exp. 2014, 1, 025026. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Zhao, M.; Qin, H.; Jiang, M. Room-temperature ferromagnetism in MgO nanocrystalline powders. Appl. Phys. Lett. 2008, 93, 192503. [Google Scholar] [CrossRef]
- Kumar, N.; Jagadeesan, D.; Pillai, P.B.; Chacko, M.; Eswaramoorthy, M.; Sundaresan, A. Ferromagnetism in thin-walled hollow spheres of non-magnetic inorganic materials. Chem. Phys. Lett. 2011, 504, 189–192. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Fan, J.; Biswas, A.; Sreelath, K.S.; Belova, L.; Rao, K.V. Magnetism of amorphous and nano-crystallized DC-sputter deposited MgO thin films. Nanomater 2013, 3, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, J.; Priya, S. Defect and adsorbate induced ferromagnetic spin-order in magnesium oxide nanocrystallites. Appl. Phys. Lett. 2012, 100, 192404. [Google Scholar] [CrossRef]
- Maoz, B.M.; Tirosh, E.; Sadan, M.B.; Markovich, G. Defect-induced magnetism in chemically synthesized nanoscale sheets of MgO. Phy. Rev. B 2011, 83, 161201(R). [Google Scholar] [CrossRef]
- Balcells, L.; Beltrán, J.I.; Martínez-Boubeta, C.; Konstantinović, Z.; Arbiol, J.; Martínez, B. Aging of magnetic properties in MgO films. Appl. Phys. Lett. 2011, 97, 252503. [Google Scholar] [CrossRef]
- Liu, G.; Ji, S.; Yin, L.; Fei, G.; Ye, C. An investigation of the electronic properties of MgO doped with group III, IV, and V elements: Trends with varying dopant atomic number. J. Phys. Condens. Matter 2010, 22, 046002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Mi, W.; Song, Q.; Yan, H. Ferromagnetism in Cu-doped MgO: Density-functional calculations. Solid State Commun. 2014, 194, 1–5. [Google Scholar] [CrossRef]
- Beltrána, J.I.; Montyb, C.; Balcellsc, L.; Martínez-Boubetad, C. Possible d° ferromagnetism in MgO. Solid State Commun. 2009, 149, 1654–1657. [Google Scholar] [CrossRef]
- Volnianska, O.; Boguslawski, P. Magnetism of solids resulting from spin polarization of p orbitals. J. Phys. Condens. Matter 2010, 22, 073202. [Google Scholar] [CrossRef] [PubMed]
- Pardo, V.; Pickett, W.E. Magnetism from 2p states in alkaline earth monoxides: Trends with varying N impurity concentration. Phys. Rev. B 2008, 78, 134427. [Google Scholar] [CrossRef]
- Seike, M.; Dinh, V.A.; Fukushima, T.; Sato, K.; Katayama-Yoshida, H. Self-organized nanostructures and high blocking temperatures in MgO-based d° ferromagnets. Jpn. J. Appl. Phys. 2012, 51, 050201. [Google Scholar] [CrossRef]
- Seike, M.; Sato, K.; Yoshida, H.K. The magnetic properties of hole-doped MgO. Jpn. J. Appl. Phys. 2011, 50, 090204. [Google Scholar] [CrossRef]
- Kim, D.; Yang, J.; Hong, J. Mg Vacancy Defect Induced Half Metallic MgO(001) Film. J. Korean Phys. Soc. 2010, 56, L1729–L1732. [Google Scholar] [CrossRef]
- Droghetti, A.; Pemmaraju, C.D.; Sanvito, S. Polaronic distortion and vacancy-induced magnetism in MgO. Phys. Rev. B 2010, 81, 092403. [Google Scholar] [CrossRef]
- Kuang, F.-G.; Kang, S.-Y.; Kuang, X.-Y.; Cheng, Q.-F. An ab initio study on the electronic and magnetic properties of MgO with intrinsic defects. RSC Adv. 2014, 4, 51366. [Google Scholar] [CrossRef]
- Osorio-Guill’en, J.; Lany, S.; Barabash, S.V.; Zunger, A. Magnetism without magnetic ions: percolation, exchange, and formation energies of magnetism-promoting intrinsic defects in CaO. Phys. Rev. Lett. 2006, 96, 107203. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.P.; Shluger, A.L. First-principles calculations of defects near a grain boundary in MgO. Phys. Rev. B 2009, 79, 224116. [Google Scholar] [CrossRef]
- Wang, F.; Pang, Z.; Lin, L.; Fang, S.; Dai, Y.; Han, S. Magnetism in undoped MgO studied by density functional theory. Phys. Rev. B 2009, 80, 144424. [Google Scholar] [CrossRef]
- Stoneham, A.M.; Pathak, A.P.; Bartram, R.H. The ground state of two-hole centres in oxide. J. Phys. C: Solid State Phys. 1976, 9, 73–80. [Google Scholar] [CrossRef]
- Uchino, T.; Yoko, T. Spin-polarized ground states and ferromagnetic order induced by low-coordinated surface atoms and defects in nanoscale magnesium oxide. Phys. Rev. B 2013, 87, 144414. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Kuga, T.; Sato, T.; Hatanaka, M.; Tanaka, K.; Yamabe, T. Through-Bond and Through-Space Interactions of Organic Radicals Coupled by m-Phenylene. Bull. Chem. Soc. Jpn. 1996, 69, 3443–3450. [Google Scholar] [CrossRef]
- Jin, H.; Dai, Y.; Huang, B.B.; Whangbo, M.-H. Ferromagnetism of undoped GaN mediated by through-bond spin polarization between nitrogen dangling bonds. Appl. Phys. Lett. 2009, 94, 162505. [Google Scholar] [CrossRef]
- Bannikov, V.; Shein, I.R.; Ivanovskii, A.L. Novel magnetic half-metallic materials based on ionic insulators doped with nonmagnetic impurities: MgO + B, C, N Systems. Tech. Phys. Lett. 2007, 33, 541–544. [Google Scholar] [CrossRef]
- Sharma, V.; Lowther, J.E. Ferromagnetism in nitrogen doped oxide: A first principle study. J. Nano-Electron. Phys. 2011, 3, 453–459. [Google Scholar]
- Zhang, Y.F.; Feng, M.; Shao, B.; Lu, Y.; Liu, H.; Zuo, X. Ab initio calculations on magnetism induced by composite defects in magnesium oxide. J. Appl. Phys. 2014, 115, 17A926. [Google Scholar] [CrossRef]
- Mir, A.; Bekkouche, B.; Boukortt, A.; Kacimi, S.; Djermouni, M.; Zaoui, A. Enhancement of ferromagnetic ordering curie temperature in N-doped MgO under hydrostatic pressure. Model. Numer. Simul. Mater. Sci. 2012, 2, 37–42. [Google Scholar] [CrossRef]
- Pesci, M.; Gallino, F.; Valentin, C.D.; Pacchioni, G. Nature of defect states in nitrogen-doped MgO. J. Phys. Chem. C 2010, 114, 1350–1356. [Google Scholar] [CrossRef]
- Kuang, F.-G.; Kang, S.-Y.; Xu, Y.-Q.; Xiong, Z.-Z.; Liao, J.-F.; Yu, H.-J.; Zhang, X.-K.; Sun, T.-Z.; Cao, J. Effect of oxygen and magnesium vacancies on the d° magnetism of C-monodoped MgO using ab initio method. J. Alloys Compd. 2017, 712, 526–534. [Google Scholar] [CrossRef]
- Singh, J.P.; Kumar, M.; Lee, I.J.; Chae, K.H. X-ray reflectivity and near edge X-ray absorption fine structure investigations of MgO thin films. Appl. Sci. Lett. 2017, in press. [Google Scholar]
- Singh, J.P.; Lin, W.C.; Lee, J.; Song, J.; Lee, I.J.; Chae, K.H. Surface and local electronic structure modification of MgO film using Zn and Fe ion implantation. Appl. Surface Sci. 2017, in press. [Google Scholar] [CrossRef]
- Hwang, H.-N.; Kim, H.-S.; Kim, B.; Hwang, C.C.; Moon, S.W.; Chung, S.M.; Jeon, C.; Park, C.-Y.; Chae, K.H.; Choi, W.K. Construction of a soft X-ray beamline at the PLS. Nucl. Instrum. Method. Phys. Res. A 2007, 581, 850–855. [Google Scholar] [CrossRef]
- Yoshida, T.; Tanaka, T.; Yoshida, H.; Funabiki, T.; Yoshida, S. Study of Dehydration of Magnesium Hydroxide. J. Phys. Chem. 1995, 99, 10890–10896. [Google Scholar] [CrossRef]
- Singh, J.P.; Gautam, S.; Singh, B.B.; Chaudhary, S.; Kabiraj, D.; Kanjilal, D.; Chae, K.H.; Kotnala, R.; Lee, J.-M.; Chen, J.-M.; et al. Magnetic, electronic structure and interface study of Fe/MgO/Fe multilayer. Adv. Mater. Lett. 2014, 5, 372–377. [Google Scholar] [CrossRef]
- McLeod, J.A.; Wilks, R.G.; Skorikov, N.A.; Finkelstein, L.D. Bandgaps and Electronic Structure of Alkaline Earth and Post-Transition Metal Oxides. Phys. Rev. B 2010, 81, 245123. [Google Scholar] [CrossRef]
- Singh, J.P.; Sulania, I.; Prakash, J.; Gautam, S.; Chae, K.H.; Kanjilal, D.; Asokan, K. Study of surface morphology and grain size of irradiated MgO thin films. Adv. Mater. Lett. 2012, 3, 112–117. [Google Scholar] [CrossRef]
- Linder, T.; Sauer, H.; Engel, W.; Kmabe, K. Near-edge structure in electron-energy-loss spectra of MgO. Phys. Rev. B 2006, 33, 22–24. [Google Scholar] [CrossRef]
- Bianconi, A. Surface X-Ray absorption spectroscopy: Surface EXAFS and surface XANES. Appl. Surf. Sci. 1980, 63, 392–418. [Google Scholar] [CrossRef]
- Garcia, J.; Bianconi, A.; Benfatto, M.; Natoli, C.R. Coordination geometry of transition metal ions in dilute solutions by XANES. J. Phys. Colloq. 1986, 47, C8-49-C8-54. [Google Scholar] [CrossRef]
- Bianconi, A.; Marcelli, A. Synchrotron Radiation Research: Advances in Surface and Interface Science Techniques; Springer: Boston, MA, USA, 1992. [Google Scholar]
- Bianconi, A. Core excitons and inner well resonances in surface soft x-ray absorption (SSXA) spectra. Surf. Sci. 1979, 89, 41–50. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.P.; Chae, K.H. d° Ferromagnetism of Magnesium Oxide. Condens. Matter 2017, 2, 36. https://doi.org/10.3390/condmat2040036
Singh JP, Chae KH. d° Ferromagnetism of Magnesium Oxide. Condensed Matter. 2017; 2(4):36. https://doi.org/10.3390/condmat2040036
Chicago/Turabian StyleSingh, Jitendra Pal, and Keun Hwa Chae. 2017. "d° Ferromagnetism of Magnesium Oxide" Condensed Matter 2, no. 4: 36. https://doi.org/10.3390/condmat2040036
APA StyleSingh, J. P., & Chae, K. H. (2017). d° Ferromagnetism of Magnesium Oxide. Condensed Matter, 2(4), 36. https://doi.org/10.3390/condmat2040036




