Abstract
Cobalt and Nickel (Co, Ni) co-doped magnesium oxide (MgO) nanoparticles (NPs) have been synthesized using the coprecipitation method. The structural, chemical, and optical properties of the as-synthesized NPs are systematically investigated using X-ray Diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR), and UV-visible spectroscopy. It is found that the optical bandgap of co-doped MgO NPs reduces from 2.30 to 1.98 eV (14%) with increasing Ni dopant concentrations up to 7%. The Co0.05Ni0.07Mg0.88O NPs exhibit a high photocatalytic degradation efficiency of 93% for methylene blue dye (MB) under natural sunlight irradiation for 240 min. Our findings indicate that the Co0.05NixMg0.95−xO NPs have strong potential for use as photocatalysts in industrial wastewater treatment.
1. Introduction
The textile industry, considered a backbone of global manufacturing, is currently facing significant environmental challenges, particularly its contribution to water pollution. It is well known that dyeing and finishing processes generate a massive volume of toxic wastewater, which contains various non-biodegradable organic dyes, heavy metals, complex chemicals, and other toxic pollutants that pose serious risks to the ecosystem and human health [1,2]. Different conventional methods such as adsorption, coagulation–flocculation, membrane filtration, ion exchange, and ozonation have been employed to treat wastewater [3,4]. However, these techniques are often expensive, inefficient, or generate secondary unwanted pollutants [5]. Thus, there is a high demand to develop environmentally friendly, sustainable, and cost-effective alternatives for industrial wastewater treatment.
Recently, a promising and cost-effective green technology known as photocatalysis has gained significant attention to treat wastewater by using the sunlight to degrade the pollutants [6]. This technique uses a photocatalyst that actively participates in generating reactive species within the pollutant water. These reactive species oxidize organic contaminants, converting them into non-toxic byproducts such as water and carbon dioxide. However, the selection of photocatalysts is crucial to accelerate the degradation process under sunlight exposure. Generally, semiconducting materials such as zinc oxide (ZnO), titanium dioxide (TiO2), and a wide variety of transition metal oxide (TMO) nanoparticles (NPs) have been extensively reported as effective photocatalysts [7,8,9]. Nevertheless, the practical utilization of these photocatalysts remains limited due to their wide bandgap that restricts their activity to the ultraviolet region of the solar spectrum. Moreover, the potential toxicity of photocatalysts is another concern that should be addressed without compromising their photocatalytic activity.
Magnesium oxide (MgO), an alkaline earth metal oxide, in the form of NPs, are considered as a promising candidate to be used in a wide range of applications, from biomedicines to catalysts, due to their intrinsic characteristics such as their availability in a high natural abundance, environmental friendliness, and, importantly, chemical stability [10,11]. However, MgO nanoparticles exhibit a wide bandgap, typically in the range of 5 to 6 eV, which limits their photocatalytic activity under the exposure of sunlight, particularly within a visible range [12,13,14]. To overcome the limitation, doping of the MgO NPs with transition metals such as copper (Cu), zinc (Zn), nickel (Ni), iron (Fe), and cobalt (Co) has been proposed, as this can introduce low-energy levels within the bandgap and thereby enhance absorption of the visible sunlight [15,16,17].
For this purpose, (Co, Ni) co-doped MgO NPs were successfully synthesized using the coprecipitation method. Dopants Co2+ (0.65 Å) and Ni2+ (0.69 Å) were selected because of their ionic radii are very close to Mg2+ (0.72 Å), enabling substitutional doping within the MgO lattice. To the best of our knowledge, the effect of (Co, Ni) co-doping on the structural and photocatalytic activity of MgO NPs has not been investigated yet comprehensively. Our work shows that the (Co, Ni) co-doped MgO NPs exhibit a remarkable photocatalytic degradation efficiency of up to 93% for methylene blue (MB) dye under sunlight exposure for 240 min. The presented investigations highlight the potential of (Co, Ni) co-doped MgO NPs as efficient and sustainable nanomaterials for industrial wastewater treatment.
2. Results and Discussion
Figure 1 shows the XRD pattern of Co0.05NixMg0.95−xO NPs in the 2θ range of 30° to 80°. The XRD peaks at 2θ of 42.842°, 62.167°, and 78.443° correspond to (200), (220), and (222) planes and confirm the face-centered cubic structure of Co0.05NixMg0.95−xO NPs with the space group Fm3m, which is consistent with the JCPDS card No: No 01-075-0447.
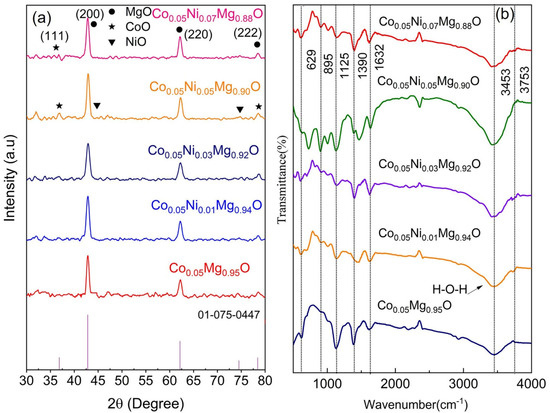
Figure 1.
(a) XRD pattern of Co0.05NixMg0.95−xO nanoparticles with x = 0, 0.01, 0.03, 0.05, and 0.07. The stick pattern of JCPDS card No 01-075-0447 is given at the bottom. (b) The FTIR pattern of Co0.05NixMg0.95−xO nanoparticles with varying concentration of Ni dopant.
The lattice parameter ‘a’ of Co0.05NixMg0.95−xO NPs is determined by using the following relation:
where (hkl) denotes the Miller indices, and d is the interplanar spacing. The lattice parameter (a = b = c) of the face-centered cubic structure of Co0.05Mg0.95O is 4.220 Å. When increasing the concentration of the Ni dopant, the value of the lattice parameter reduces from 4.220 to 4.211 Å. The change in the lattice parameter shows a contraction or expansion in host material due to mismatching of the ionic radius of dopant with the host lattice atom. The decrease in the lattice parameters is due to the smaller ionic sizes of Co2+ (0.65 Å) and Ni2+ (0.69 Å) dopants as compared to the ionic radius of Mg2+ (0.72 Å) [15,18]. This gradual decrease in lattice parameters with increasing concentration of Ni dopant also suggests the possible formation of secondary phases involving Co and Ni oxides. However, no significant change is observed in our XRD data, which provide evidence of the substitutional incorporation of dopant ions into the MgO lattice. Generally, the XRD peak broadening is used to determine the crystallite size of NPs. Several factors such as instrumental broadening, dislocation density, and lattice imperfections can contribute to the broadening of XRD peaks. However, the contribution of instrumental broadening from the measured peak broadening can be reduced by considering the following relation.
The average crystallite size (D) of the Co0.05NixMg0.95−xO NPs is calculated by using the Debye–Scherrer’s expression [4]:
where k is the Scherer constant with a value of 0.9, λ is 0.154 nm, β is the full width at half maximum (FWHM) of the corrected peak broadening, and θ is the Bragg’s diffraction angle in radians. The calculated values of crystallite size are given in Table 1. It can be seen that when increasing the Ni dopant concentration, the average crystallite size decreases from 14.50 to 11.73 nm (19% decrease). This reduction in crystallite size also supports the successful substitution of Ni ions into the MgO crystal lattice.

Table 1.
Determination of average crystallite size, lattice parameter, and dislocation density of Co0.05NixMg0.95−xO NPs using Debye–Scherrer relation.
In addition, the dislocation density (δ = 1/D2) provides a measure of the number of dislocation lines per unit volume of the crystal. It can be seen that dislocation density increases with the increasing concentration of Ni dopant (218% at x = 0.07). In addition, different models such as the modified Scherrer’s method, Williamson Hall method, and size–strain plot method have been employed to evaluate the crystallite size and strain [16,17,18,19,20,21,22,23,24,25,26,27,28] (see Figures S1–S5 in Section S1), and their calculated values are given in Table 2.

Table 2.
Determination of crystallite size and lattice strain of Co0.05NixMg0.95−xO NPs using modified Scherrer model, Williamson Hall model, and size–strain model.
It can be seen that the crystallite size calculated using different XRD models are in good agreement with each other. However, the SSP model is considered as an appropriate model in our study since this model shows the best linear correlation with the experimental data.
To confirm the structural modifications in Co0.05NixMg0.95−xO NPs, the FTIR pattern of MgO NPs is shown in Supplementary Figure S6 (Section S2). The IR peaks at 846, and 1422 cm−1 are related to Mg-O-Mg and CH2 bending vibrations [12]. The IR peak at 2355 cm−1 is related to the asymmetric stretching of carbon dioxide from ambient air within the spectrometer [29]. This is due to the incomplete background subtraction of atmospheric carbon dioxide, which can cause the corresponding peak to appear either as positive or negative in the spectra, depending on baseline conditions. Similarly, the IR peaks in the wavenumber range of 1600–1630 cm−1 and 3300–3600 cm−1 are related to O-H stretching vibrations [30]. In contrast, the spectra of Co0.05NixMg0.95−xO NPs show characteristic IR peaks at 629, 895, 1125, 1390, 1632, 3453, and 3753 cm−1 (see Figure 1b). The FTIR peaks in the wave number range of 550–670 cm−1 and 850–900 cm−1 confirm the presence of Mg–O–Mg vibrations [12]. The FTIR peaks between 1026 and 1278 cm−1 are related to C-O stretching vibrations [31]. A minor peak at 1390 cm−1 is attributed to the adsorption of carbonate species from the environment [32]. At 1632 cm−1, the IR peak appeared due to the presence of adsorbed water molecules (H-O-H bending). In addition, a wide band in the wave number range of 3300–3600 cm−1 is related to the O–H stretching vibration of water molecules [33]. Similarly, the minor peak at 3753 cm−1 is attributed to an adsorbed species and is most likely due to the incomplete background subtraction of the stretching vibrations of gas-phase water molecules [34]. The presence of IR peaks due to carbonate, carbonyl, and hydroxyl bonding is related to the adsorption of atmospheric CO2 and H2O molecules.
The optical bandgap energies of Co0.05NixMg0.95−xO NPs are determined from the UV-visible absorption data by using the following Tauc relation for direct band transition,
where α is the absorption coefficient, A is the constant of proportionality, and hν is the photon energy. The optical bandgap energies are found by plotting (αhν)2 versus hν and extrapolating the linear part of the curve to the intercept (hν = 0) (see Supplementary Figure S7 in Section S3). The estimated bandgap energy values are mentioned in Figure 2a. The optical bandgap of Co0.05Mg0.95O NPs reduces from 5.34 eV (MgO) [12] to 2.3 eV, which is consistent with the reported bandgap of co-doped MgO [35]. With the increasing concentration of the Ni dopant (at x = 0.07), the optical bandgap reduces to 1.98 eV (14%). The observed reduction in the optical bandgap is attributed to the successful incorporation of both Co2+ and Ni2+ ions into the MgO host lattice. In addition, the existence of structural defects along with the formation of additional electronic states due to the incorporation of dopant ions facilitate the transition from the valence band to the conduction band [36]. Moreover, the similar ionic radii of Co2+ (0.65 Å), Ni2+ (0.69 Å), and Mg2+ (0.72 Å) support the structural compatibility of these dopants within the MgO lattice [37]. Such doping not only enhances the surface area but also reduces the bandgap energy, making the synthesized NPs promising candidates for photocatalytic degradation [12,38,39].
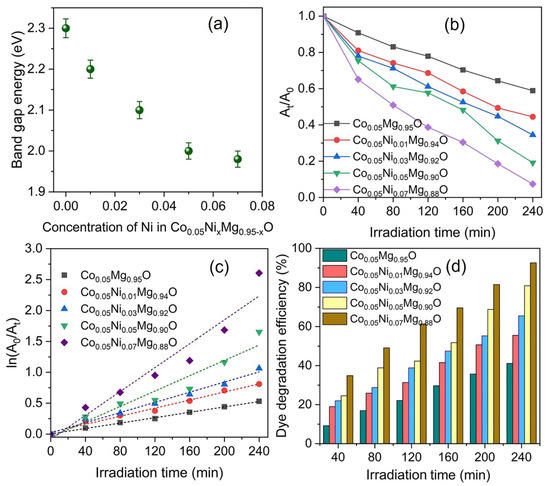
Figure 2.
(a) Optical bandgap energies of Co0.05NixMg0.95−xO NPs with varying concentrations of Ni dopant; (b) the plot of At/A0 versus irradiation time for the degradation of MB; and (c) kinetic fit for the degradation of MB. The dotted line shows the linear fit to experimental data, (d) the degradation efficiencies of Co0.05NixMg0.95−xO NPs against MB with exposure time.
In addition, the photocatalytic activity of Co0.05NixMg0.95−xO NPs is investigated by monitoring the photodegradation of MB in an aqueous solution under natural sunlight exposure for 240 min. The MB solution before sunlight exposure is regarded as a reference sample (0 min) to evaluate the photocatalytic degradation efficiency (see Supplementary Figure S8 in Section S4). As the sunlight exposure time increases from 0 to 240 min, the optical absorbance gradually reduces. However, in the absence of Co0.05NixMg0.95−xO nanoparticles, the MB showed only 15% degradation efficiency after sunlight exposure for 240 min. The maximum reduction in optical absorbance is observed when using Co0.05Ni0.07Mg0.88O NPs as a catalyst. From the optical absorbance data, the ratio is determined and plotted in Figure 2b versus the sunlight exposure time, where At is the optical absorbance at a specific irradiation time and A0 relates to the optical absorbance at t = 0. It can be seen that the ratio decreases stepwise with increasing concentrations of the Ni dopant and sunlight exposure time.
The photodegradation of Co0.05NixMg0.95−xO NPs is determined using a pseudo-first-order kinetic model by using the following equation [40],
where k is the rate constant. The rate constant values are determined from the slope of the linear fit to the experimental data in Figure 2c, and their numerical values are mentioned in Table 3.

Table 3.
The calculated values of pseudo-first-order kinetic parameters of Co0.05NixMg0.95−xO NPs.
Moreover, the degradation efficiency of Co0.05NixMg0.95−xO NPs against the MB is determined by using the following relation [40]:
The calculated degradation efficiencies of Co0.05NixMg0.95−xO NPs with respect to exposure time are shown in Figure 2d. It can be seen that Co0.05Ni0.07Mg0.88O NPs degrade the MB up to 93% with a sunlight exposure of 240 min at max. In addition, with increasing concentrations of the Ni dopant, both the rate constant and degradation efficiency increase (see Supplementary Figure S9 in Section S4). This significantly higher degradation efficiency of Co0.05Ni0.07Mg0.88O NPs against MB may be attributed to the narrower optical bandgap.
The photocatalytic mechanism of Co0.05NixMg0.95−xO NPs is shown in Figure 3. With sunlight exposure, electrons (e−) in the valence band (VB) are excited to the conduction band (CB). The generated electron and hole pairs are separated due to the presence of Ni and Co dopants that act as trapping centers in the bandgap and result in suppressing the electron hole recombination [12]. The excited electrons react with adsorbed oxygen molecules and reduce them to superoxide radicals. In addition, holes react with water molecules and generate hydroxyl radicals. These superoxide and hydroxyl radicals actively participate in the degradation of MB. The following chemical reactions are involved in the photocatalytic activity of Co0.05NixMg0.95−xO NPs [41,42,43].
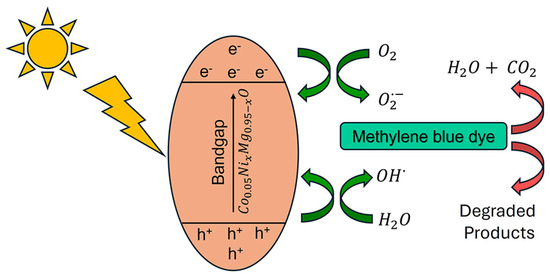
Figure 3.
The photocatalytic mechanism involved in the degradation of methylene blue dye.
3. Experimentation
3.1. Materials
For the presented investigation, magnesium nitrate hexahydrate (Mg(NO3)2.6H2O), cobalt nitrate hexahydrate (Co(NO3)2.6H2O), and nickel nitrate hexahydrate (Ni(NO3)2.6H2O) were used for the synthesis of Co0.05NixMg0.95−xO NPs without further purification. Sodium hydroxide (NaOH) was used as a precipitant.
3.2. Synthesis of Co0.05NixMg0.95−xO Nanoparticles
A stoichiometric amount of Mg(NO3)2.6H2O and (Co(NO3)2.6H2O) was dissolved separately in 100 mL double distilled water to prepare a homogenous solution. Both solutions were then mixed and magnetically stirred for 60 min at 70 °C. During constant stirring, a 2 M solution of NaOH was added dropwise into the homogeneous solution of salts until the desired pH value was reached at 11. This solution was vigorously stirred for 2 h and then aged for 24 h at room temperature to complete the following chemical reaction.
The precipitate of Co0.05Mg0.95(OH)2 was settled at the bottom of the reaction beaker. These precipitates were filtered and washed three to four times with double distilled water to remove the unwanted impurities. The filtrate was dried at 120 °C for 24 h. After drying, the calcination of the resultant sample was performed at 500 °C for 2 h. Note that for these present investigations, the Co concentration was kept constant at 5% as a control parameter. At this concentration, co-doped MgO NPs exhibit optimized properties with a decrease in bandgap to 2.3 eV.
Similarly, for the synthesis of Co0.05NixMg0.95−xO (x = 0, 0.01, 0.03, 0.05, and 0.07) the stoichiometric amounts of Ni(NO3)2.6H2O were added to the homogeneous solution of Mg and Co nitrates to perform the following chemical reaction.
All other synthesis steps were repeated in a similar manner. The precipitate of Co0.05NixMg0.95−x (OH)2 was washed three to four times with double distilled water and then dried at 120 °C for 24 h. Afterwards, calcination was conducted at 500 °C to obtain Co0.05NixMg0.95−xO NPs. However, the designations, such as Co0.05NixMg0.95−xO, used throughout the study only indicate the nominal molar ratios of metal precursors used during synthesis. The actual composition of the nanoparticles has not been directly measured due to limitations in available instrumentation. The compositional analysis will be conducted in a future study to confirm the stoichiometry and elemental distribution of the synthesized materials.
3.3. Characterization
The structural properties of NPs were investigated by using they BRUKER D8-X-ray advanced diffractometer. The XRD patterns were obtained in the 2θ range of 30°–90° with Cu-Kα radiation. The presence of different functional groups was examined using the IRTracer-100 Fourier transform infrared spectrometer. In addition, the optical absorbance spectra were recorded with the double beam UV–Visible (UV-2800) spectrophotometer in the range of 200–900 nm. MgO NPs were used as a catalyst, and their photocatalytic activity was analyzed using the (UV-2800) spectrophotometer. The photocatalytic activity was conducted under natural sunlight exposure between 11:00 AM to 3:00 PM in May. During this period, the average solar irradiance was 734 W/m2. For this purpose, a 10-ppm solution was prepared by dissolving 1 mg of MB in 100 mL distilled water and adding 30 mg of the photocatalyst. The solution was exposed to sunlight for 240 min. After each 40 min of exposure, a sample of 5 mL of the solution was collected, and the optical absorbance of the MB solution was evaluated in the wavelength range of 400–800 nm. Note that the solution was continuously stirred during the exposure period. After exposure, stirring was stopped, and the samples were kept in the dark to allow the particles to settle down at the bottom. A small quantity of the sample was collected for UV-visible spectroscopic analysis. Prior to the measurement, this sample was subjected to ultracentrifugation to remove the remaining NPs from the liquid sample.
4. Conclusions
Co and Ni co-doped Co0.05NixMg0.95−xO NPs are successfully synthesized via the coprecipitation method. XRD analysis revealed the face-centered cubic structure of the NPs along with a systematic reduction in crystallite size that supports ionic substitution. The crystallite size and lattice strain are investigated using a modified Scherrer’s plot, Williamson Hall method, and size–strain method; the obtained results are found to be in good agreement with each other. The decrease in the optical bandgap with an increase in Ni concentration resulted in an increase in the photocatalytic degradation of methylene blue dye. Interestingly, Co0.05Ni0.07Mg0.88O NPs showed up to 93% dye degradation efficiency with open sunlight exposure for 240 min. These findings suggest that Co0.05NixMg0.95−xO NPs can be considered potential candidates for further utilization in industrial wastewater treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/condmat10030041/s1, Figure S1: Determination of crystallite size and strain of Co0.05NixMg0.95−xO nanoparticles using Modified Scherer method; Figure S2: Determination of crystallite size and strain of Co0.05NixMg0.95−xO nanoparticles using uniform deformation model (UDM); Figure S3: Determination of crystallite size and strain of Co0.05NixMg0.95−xO nanoparticles using uniform stress deformation model (USDM); Figure S4: Determination of crystallite size and strain of Co0.05NixMg0.95−xO nanoparticles using uniform deformation energy density model (UDEDM); Figure S5: Determination of crystallite size and strain of Co0.05NixMg0.95−xO nanoparticles using Size Strain plot (SSP); Figure S6: FTIR pattern of MgO nanoparticles; Figure S7: Determination of optical bandgap energy using Tauc relation for direct bandgap; Figure S8: Optical absorbance of Methylene blue (MB) dye without Co0.05NixMg0.95−xO nanoparticles under the sunlight exposure of 0, 120 and 240 min. The optical absorbance spectrum at 0 min is regarded as reference sample. The MB dye show up to 15% degradation efficiency in the absence of Co0.05NixMg0.95−xO nanoparticles with sunlight exposure of 240 min; Figure S9: The variation in (a) rate constant and (b) dye degradation efficiency (%) of Co0.05NixMg0.95−xO nanoparticles under the sunlight exposure.
Author Contributions
Conceptualization, S.A. and M.S.A.; methodology, A.S.; formal analysis, S.A. and A.S.; investigation, A.S.; data curation, A.S.; writing—original draft preparation, S.A., A.S. and M.S.A.; writing—review and editing, S.A. and M.S.A.; visualization, S.A. and M.S.A.; supervision, S.A.; project administration, S.A. and M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abid, Z.; Sarwar, Z.; Munir, N.; Safi, S.Z.; Arshad, M. Effluent Treatment in Textile Industry to Achieve SDGs. In Enzymes in Textile Processing: A Climate Changes Mitigation Approach: Textile Industry, Enzymes, and SDGs; Springer: Singapore, 2025; pp. 363–390. [Google Scholar]
- Ejaz, M.; Sharif, M.; Safi, S.Z.; Nawaz, S.; Jamil, S.; Syed, M.A.; Ahmed, W. Microbial Enzymes in Bioremediation of Water Polluted by Textile Industry Effluents. In Enzymes in Textile Processing: A Climate Changes Mitigation Approach: Textile Industry, Enzymes, and SDGs; Springer: Singapore, 2025; pp. 391–417. [Google Scholar]
- Ruan, H.; Guo, L.; Ding, N.; Cui, H.; Lu, Y.; Qiu, Y.; Yao, Y.; Liao, J.; Shen, J. Enhanced recovery of p-Aminophenol from high-salt wastewater via optimized bipolar membrane electrodialysis in a Water-Ethanol system. Sep. Purif. Technol. 2025, 360, 131038. [Google Scholar] [CrossRef]
- Shamshad, J.; Rehman, R.U. Innovative approaches to sustainable wastewater treatment: A comprehensive exploration of conventional and emerging technologies. Environ. Sci. Adv. 2025, 4, 189–222. [Google Scholar] [CrossRef]
- Kanwal, A.; Rehman, R.; Imran, M.; Samin, G.; Jahangir, M.M.; Ali, S. Phytoremediative adsorption methodologies to decontaminate water from dyes and organic pollutants. RSC Adv. 2023, 13, 26455–26474. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Lecture Notes in Chemistry. In Photocatalysis; Springer: Singapore, 2018; pp. 1–17. [Google Scholar]
- AlMohamadi, H.; Awad, S.A.; Sharma, A.K.; Fayzullaev, N.; Távara-Aponte, A.; Chiguala-Contreras, L.; Amari, A.; Rodriguez-Benites, C.; Tahoon, M.A.; Esmaeili, H. Photocatalytic activity of metal-and non-metal-anchored ZnO and TiO2 nanocatalysts for advanced photocatalysis: Comparative study. Catalysts 2024, 14, 420. [Google Scholar] [CrossRef]
- Arif, S.; Javaid, I.; Israr, Z.; Gillani, S.; Anwar, M. Sunlight-driven degradation of water pollutants using pomegranate-synthesized CuO nanoparticles. Mater. Sci. Eng. B 2024, 310, 117749. [Google Scholar] [CrossRef]
- Cheng, C.; Amini, A.; Zhu, C.; Xu, Z.; Song, H.; Wang, N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014, 4, 4181. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Velavan, R.; Batoo, K.M.; Raslan, E.H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Hornak, J. Synthesis, properties, and selected technical applications of magnesium oxide nanoparticles: A review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Arif, S.; Nawaz, M.; Siddique, S.; Ayub, R.; Saleem, S. Synthesis, characterization and photocatalytic activity of Mg1− xCuxO nanoparticles for wastewater treatment. Mater. Today Commun. 2022, 33, 104361. [Google Scholar] [CrossRef]
- Rotti, R.B.; Sunitha, D.; Manjunath, R.; Roy, A.; Mayegowda, S.B.; Gnanaprakash, A.; Alghamdi, S.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M. Green synthesis of MgO nanoparticles and its antibacterial properties. Front. Chem. 2023, 11, 1143614. [Google Scholar] [CrossRef]
- Sim, H.T.; Gençaslan, M.; Merdan, M. Synthesis of MgO nanoparticles via the sol-gel method for antibacterial applications, investigation of optical properties and comparison with commercial MgO. Discov. Appl. Sci. 2024, 6, 577. [Google Scholar] [CrossRef]
- Arif, S.; Tahir, J.; Fatima, H.; Anwar, M. Investigation of structural defects and magnetic ordering in Co-doped magnesium oxide nanoparticles. Inorg. Chem. Commun. 2025, 178, 114655. [Google Scholar] [CrossRef]
- Chauhan, D.; Kumar, R.; Thakur, N.; Kumar, K. Exploring transition metal (Co, Cu, and Zn) doped magnesium oxide nanoparticles for their environmental remediation potential. Mater. Sci. Eng. B 2024, 302, 117256. [Google Scholar] [CrossRef]
- El-Shobaky, G.; El-Molla, S.; Ali, A. Catalytic promotion of NiO/MgO system by doping with some transition metal cations. Appl. Catal. A Gen. 2003, 253, 417–425. [Google Scholar] [CrossRef]
- He, T.; Chen, L.; Su, Y.; Lu, Y.; Bao, L.; Chen, G.; Zhang, Q.; Chen, S.; Wu, F. The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni-Rich cathode materials. J. Power Sources 2019, 441, 227195. [Google Scholar] [CrossRef]
- Lim, J.S.; Yam, F.K. Structural parameters of CVD synthesized Ga2O3 nanostructures from X-ray diffraction analysis derived by Scherrer, Williamson-Hall, Size-Strain Plot and Halder-Wagner methods–A comparative study. Phys. B Condens. Matter 2025, 699, 416798. [Google Scholar] [CrossRef]
- Arif, S.; Shahzadi, K.; Sabah, A.; Anwar, M. Antibacterial and solar-driven photocatalytic activities of Co x Sn1− x O2− δ nanoparticles for wastewater treatment. Appl. Phys. A 2024, 130, 208. [Google Scholar] [CrossRef]
- Balzar, D.; Ledbetter, H. Voigt-function modeling in Fourier analysis of size-and strain-broadened X-ray diffraction peaks. Appl. Crystallogr. 1993, 26, 97–103. [Google Scholar] [CrossRef]
- Bodke, M.; Gawai, U.; Patil, A.; Dole, B. Estimation of accurate size, lattice strain using Williamson-Hall models, SSP and TEM of Al doped ZnO nanocrystals. Matériaux Tech. 2018, 106, 602. [Google Scholar] [CrossRef]
- Dave, M.S.; Giri, R.K.; Vaidya, R.D.; Patel, K.R.; Bharucha, S.R.; Solanki, M.B. Unravelling NbSe2 single crystal: First principle insights, optical properties, synthesis and X-ray diffraction profile investigation. Next Mater. 2025, 7, 100361. [Google Scholar] [CrossRef]
- Ditto, A.; Joseph, D.P.; Baby, B.L.; Mohan, D.B. Structural Analysis of Ag Doped SnS Nanorods. In Proceedings of the International Conference on Emerging Multifunctional Materials and Devices for Sustainable Technologies: IEMDST-2024, Hanamkonda, India, 4–5 July 2025; p. 109. [Google Scholar]
- Gueddim, A.; Bouarissa, N.; Villesuzanne, A. Pressure dependence of elastic constants and related parameters for rocksalt MgO. Comput. Mater. Sci. 2010, 48, 490–494. [Google Scholar] [CrossRef]
- Jamal, M.; Asadabadi, S.J.; Ahmad, I.; Aliabad, H.R. Elastic constants of cubic crystals. Comput. Mater. Sci. 2014, 95, 592–599. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles-a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Obeid, M.M.; Edrees, S.J.; Shukur, M.M. Synthesis and characterization of pure and cobalt doped magnesium oxide nanoparticles: Insight from experimental and theoretical investigation. Superlattices Microstruct. 2018, 122, 124–139. [Google Scholar] [CrossRef]
- Schott, J.A.; Do-Thanh, C.-L.; Shan, W.; Puskar, N.G.; Dai, S.; Mahurin, S.M. FTIR investigation of the interfacial properties and mechanisms of CO2 sorption in porous ionic liquids. Green Chem. Eng. 2021, 2, 392–401. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, N.K.; Sachan, K.; Ali, M.A.; Gupta, S.S.; Singh, R. Trimetallic oxide nanocomposites of transition metals titanium and vanadium by sol-gel technique: Synthesis, characterization and electronic properties. Mater. Res. Express 2018, 5, 045037. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared spectrum characteristics and quantification of OH groups in coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Hanif, A.; Dasgupta, S.; Nanoti, A. Facile synthesis of high-surface-area mesoporous MgO with excellent high-temperature CO2 adsorption potential. Ind. Eng. Chem. Res. 2016, 55, 8070–8078. [Google Scholar] [CrossRef]
- Zviagina, B.B.; McCarty, D.K.; Środoń, J.; Drits, V.A. Interpretation of infrared spectra of dioctahedral smectites in the region of OH-stretching vibrations. Clays Clay Miner. 2004, 52, 399–410. [Google Scholar] [CrossRef]
- Kang, M.; Kumaravel, V. Photocatalytic Hydrogen Evolution; MDPI AG: Basel, Switzerland, 2020. [Google Scholar]
- Shaji, R.P.; Kunjumon, J.; Aleena, P.; Jose, A.K.; Shaiju, S.; Nair, S.S.; Vinitha, G.; Alex, J.; George, M.; Sajan, D. Influence of cobalt doping on the structural and third order nonlinear optical properties of MgO nanostructures. Mater. Sci. Eng. B 2025, 321, 118507. [Google Scholar] [CrossRef]
- Zhang, A.; Liang, Y.; Zhang, H.; Geng, Z.; Zeng, J. Doping regulation in transition metal compounds for electrocatalysis. Chem. Soc. Rev. 2021, 50, 9817–9844. [Google Scholar] [CrossRef]
- Almontasser, A.; Parveen, A. Probing the effect of Ni, Co and Fe doping concentrations on the antibacterial behaviors of MgO nanoparticles. Sci. Rep. 2022, 12, 7922. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Subash, M.; Perumal, V.; Panimalar, S.; Aravindan, S.; Uthrakumar, R.; Inmozhi, C.; Isaev, A.B.; Muniyasamy, S.; Raja, A. Specific charge separation of Sn doped MgO nanoparticles for photocatalytic activity under UV light irradiation. Sep. Purif. Technol. 2022, 294, 121189. [Google Scholar] [CrossRef]
- Gebreaneniya, M.F.; Berhe, G.G.; Teklu, T. Synthesis, Characterization, and Photocatalytic Activity of Cu-Doped MgO Nanoparticles on Degradation of Methyl Orange (MO). Adv. Mater. Sci. Eng. 2024, 2024, 9969064. [Google Scholar] [CrossRef]
- Arif, S.; Fatima, H.; Tahir, J.; Anwar, M. Doped hydroxyapatite photocatalyst for efficient degradation of Methylene blue dye. Inorg. Chem. Commun. 2025, 172, 113684. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Bovali, N.; Lagopati, N.; Pavlatou, E.A. MgO nanoparticles as a promising photocatalyst towards rhodamine B and rhodamine 6G degradation. Molecules 2024, 29, 4299. [Google Scholar] [CrossRef] [PubMed]
- Muhaymin, A.; Mohamed, H.E.A.; Hkiri, K.; Safdar, A.; Azizi, S.; Maaza, M. Green synthesis of magnesium oxide nanoparticles using Hyphaene thebaica extract and their photocatalytic activities. Sci. Rep. 2024, 14, 20135. [Google Scholar] [CrossRef]
- Tahir, H.; Anwer, M.; Khan, S.; Saad, M. Enhancement of adsorption and photocatalytic activity of MgO nanoparticles for the treatment of textile dye using ultrasound assisted process by Response Surface Methodology. Desalination Water Treat. 2024, 319, 100429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).