Comparison of the Intestinal Microbiota Composition and Function of Red Claw Crayfish (Cherax quadricarinatus) Cultured in Ponds and Rice Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animal and Sample Collection
2.3. DNA Library Preparation and Sequencing of Bacterial Genomes
2.4. Analysis of the Intestinal Microbiota by High-Throughput Sequencing and Bioinformatics Analysis (Sequence Analysis)
3. Results
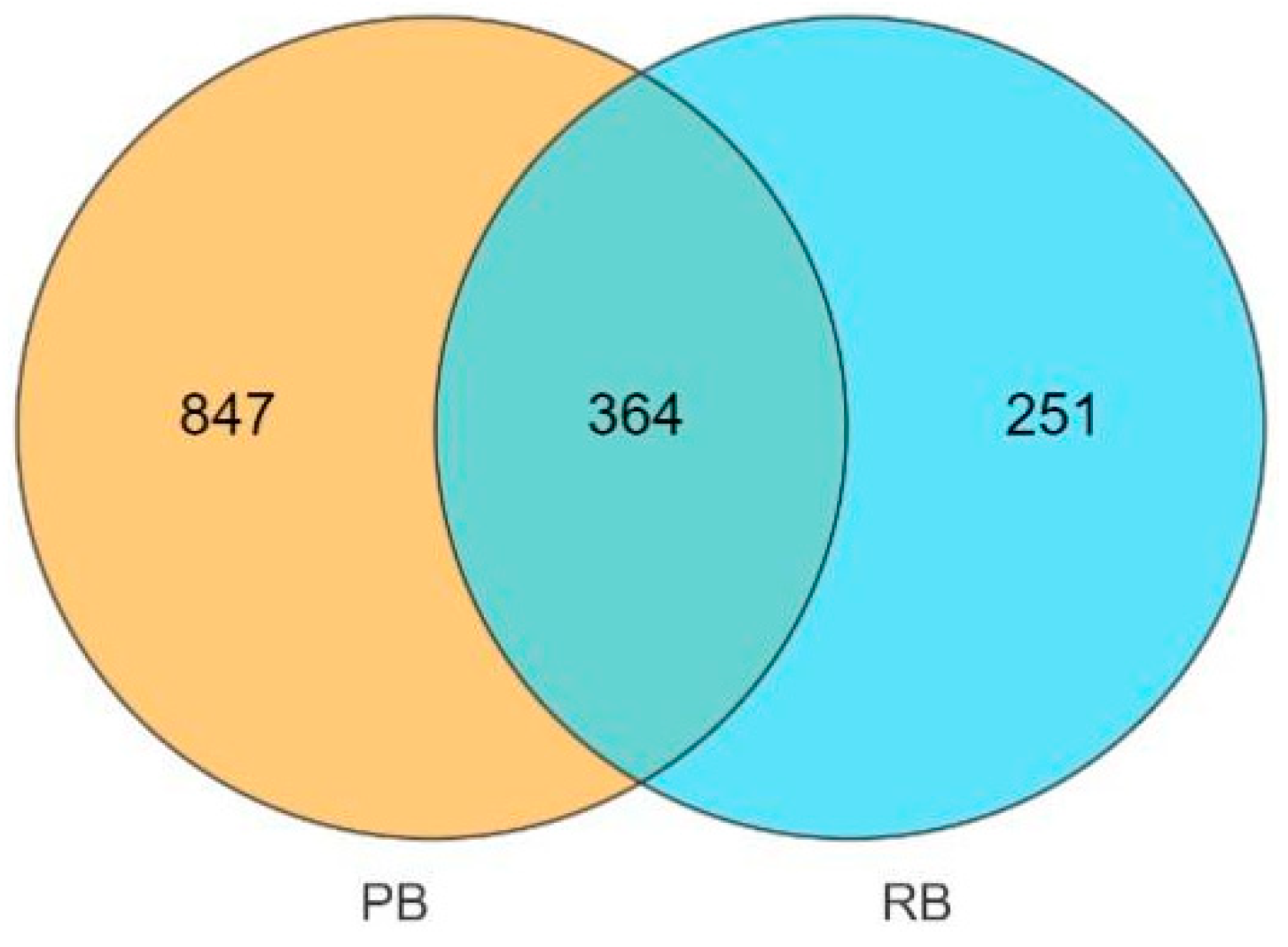
3.1. OTU Analysis
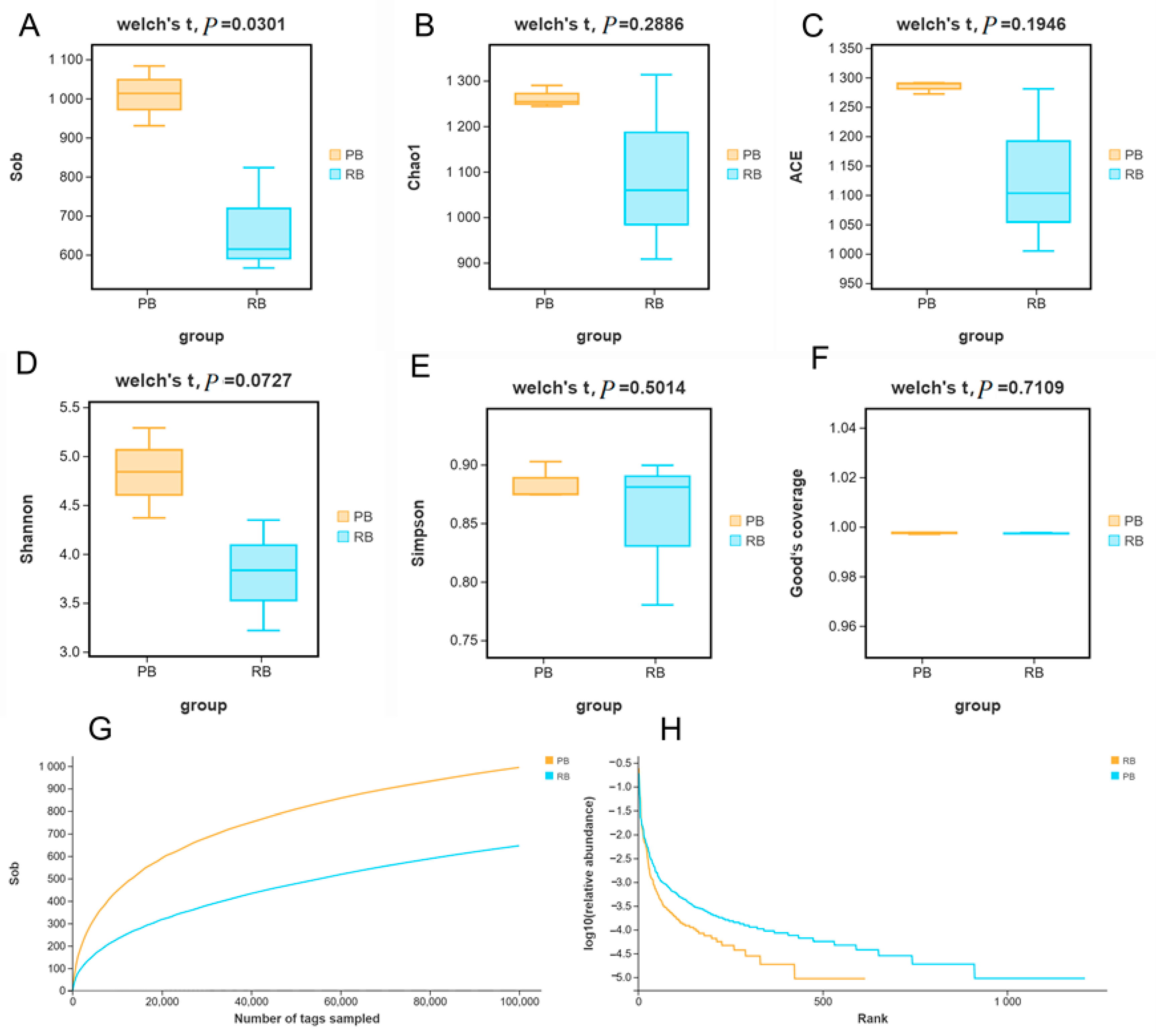
3.2. Alpha Diversities
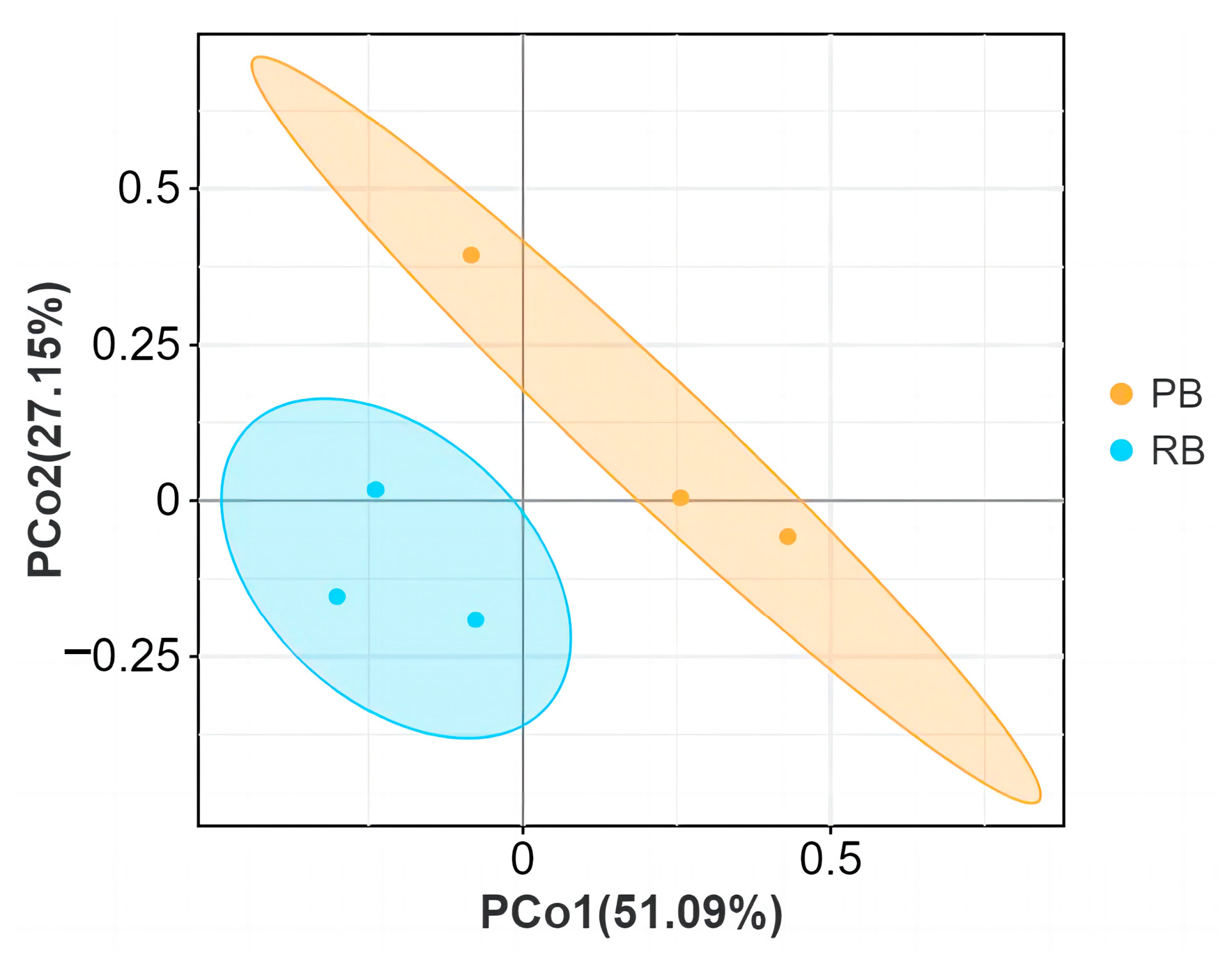
3.3. Beta Diversities
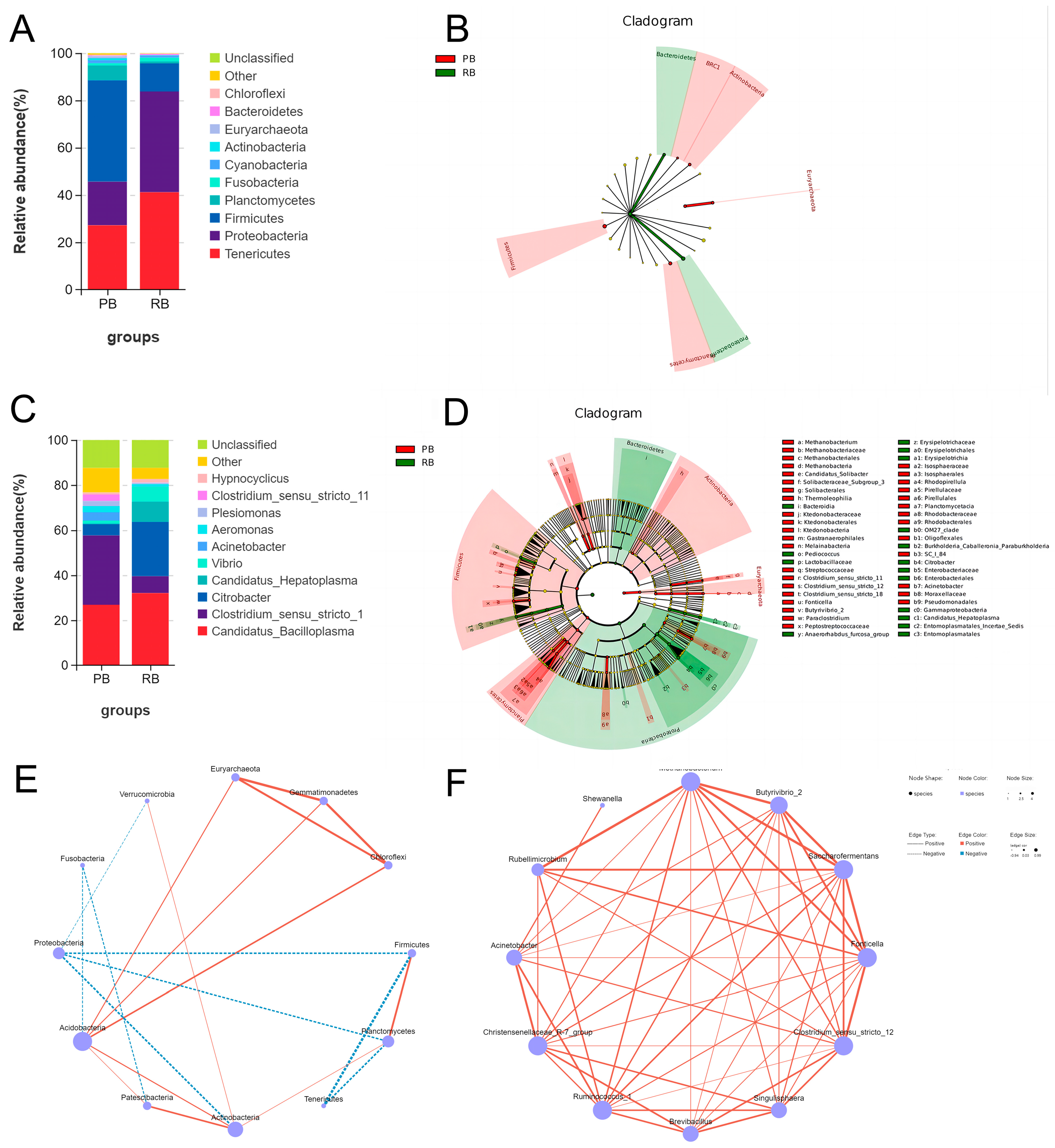
3.4. Microbial Communities Distribution and Correlation Analysis
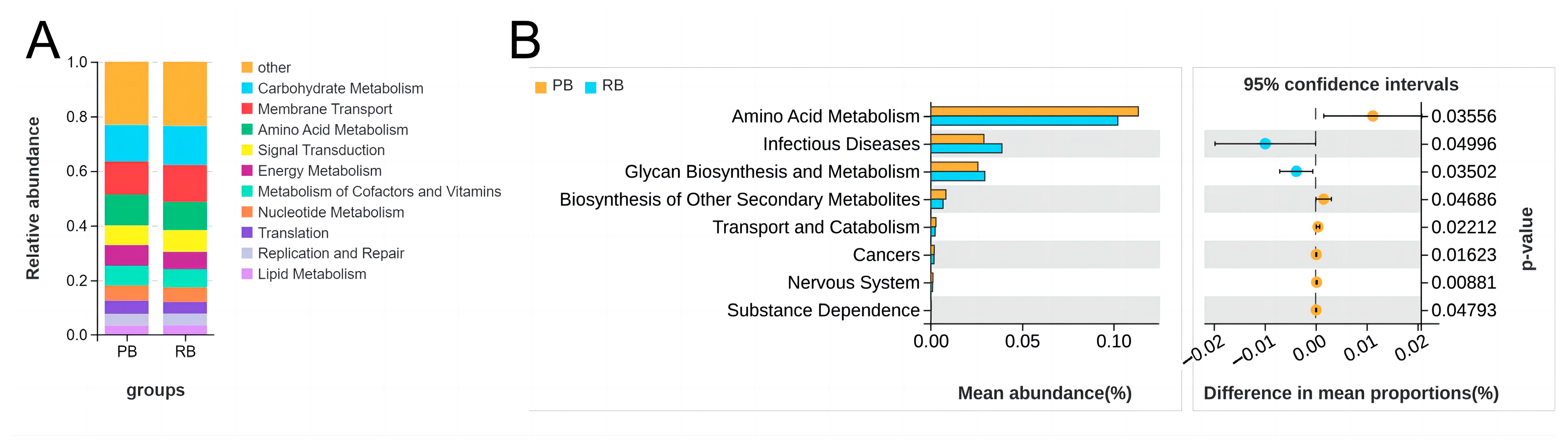
3.5. Tax4Fun Functional Prediction of Microbial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Hao, X.; Dang, Z.; Yang, L.; Wang, X.; Zhang, Y.; Zhang, Y. 2022 Report on the development of crayfish industry in China. China Fish 2022, 6, 47–54. [Google Scholar]
- Austin, C.M. Preliminary Pond Production of the Red Claw Crayfish, Cherax quadricarinatus, in the Central United States. J. Appl. Aquac. 1993, 1, 93–102. [Google Scholar] [CrossRef]
- Shi, R.; Yang, S.; Li, Y. A new insight into the SNP genotyping using high-resolution melting method after the correlation analysis of the SNPs with WSSV-resistant traits. Fish Shellfish Immunol. 2019, 122, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424. [Google Scholar] [CrossRef]
- Dehler, C.E.; Secombes, C.J.; Martin, S.A.M. Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salar L.). Aquaculture 2017, 467, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Xing, S.; Lu, Y.; Zheng, P.; Li, J.; Hao, C.; Xu, J.; Xian, J.; Zhang, L.; et al. A newly isolated strain of Bacillus subtilis W2Z exhibited probiotic effects on juvenile red claw crayfish, Cherax quadricarinatus. Aquaculture 2024, 585, 740700. [Google Scholar] [CrossRef]
- Shui, Y.; Guan, Z.; Liu, G.; Fan, L. Gut microbiota of red swamp crayfish Procambarus clarkii in integrated crayfish-rice cultivation model. AMB Express 2020, 10, 5. [Google Scholar] [CrossRef]
- Li, F.; Gao, J.; Xu, Y.; Nie, Z.; Fang, J.; Zhou, Q.; Xu, G.; Shao, N.; Xu, D.; Xu, P.; et al. Biodiversity and sustainability of the integrated rice-fish system in Hani terraces, Yunnan province, China. Aquac. Rep. 2001, 20, 100763. [Google Scholar] [CrossRef]
- Yao, L.; Zi, Y.; Xiao, Y. Research on the Artificial Culture of Chinese Cipangopaludina cathayensis in Rice Fields. Fish. Sci. 2002, 21, 23–24. [Google Scholar]
- Cai, C.; Li, G.; Zhu, J.; Peng, L.; Wu, Q. Effects of rice-crawfish rotation on soil physicochemical properties in Jianghan plain. Acta Pedol. Sin. 2019, 56, 217–226. [Google Scholar]
- Si, G.; Peng, C.; Xu, X.; Xu, D.; Yuan, J.; Li, J. Effect of integrated rice-crayfish farming system on soil physico-chemical properties in waterlogged paddy soils. Chin. J. Eco-Agric. 2017, 25, 61–68. [Google Scholar]
- Si, G.; Peng, C.; Yuan, J.; Xu, X.; Zhao, S.; Xu, D.; Wu, J. Changes in soil microbial community composition and organic carbon fractions in an integrated rice–crayfish farming system in subtropical China. Sci. Rep. 2017, 7, 2856. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Webster, D.C.; Thompson, R.K.; Cummins, C.V., Jr.; Gannam, L.A.; Twibell, G.R.; Albers Koch, J.F. Effects on growth, survival, body composition, processing traits and water quality when feeding a diet without vitamin and mineral supplements to Australian red claw crayfish (Cherax quadricarinatus) grown in ponds. Aquac. Res. 2015, 11, 2716–2727. [Google Scholar] [CrossRef]
- Cheng, S.; Jia, Y.; Chi, M.; Zheng, J.; Liu, S.; Gu, Z. Culture model of Cherax quadricarinatus: Temporary shelter in shed and pond culture. Aquaculture 2020, 526, 735359. [Google Scholar]
- National Standard of the People’s Republic of China–Fisheries Water Quality Standards (GB11607-89). Environ. Prot. 1989, 12, 25–27.
- Guo, K.; Ruan, G.; Fan, W.; Fang, L.; Wang, Q.; Luo, M.; Yi, T. The effect of nitrite and sulfide on the antioxidant capacity and microbial composition of the intestines of red swamp crayfish, Procambarus clarkii. Fish Shellfish Immunol. 2020, 96, 290–296. [Google Scholar] [CrossRef]
- Li, J.; Hou, J.; Zhang, P.; Liu, Y.; Xia, R.; Ma, X. Comparative study of intestinal microbial community structure in different species of carp in aquaponics system. South China Fish. Sci. 2016, 12, 42–50. [Google Scholar]
- Zhang, B.-Y.; Yao, Q.; Zhang, D.-M.; Wang, N.; Liu, H.-J.; Wan, J.-W.; Chen, Y.-K.; Wang, Q.-J.; Guo, Z.-X. Comparative study on growth, digestive function and intestinal microbial composition of female Chinese mitten crab Eriocheir sinensis selected at different growth stages in rice-crab culture systems. Aquaculture 2022, 554, 738120. [Google Scholar] [CrossRef]
- Ye, C.; Geng, S.; Zhang, Y.; Qiu, H.; Zhou, J.; Zeng, Q.; Zhao, Y.; Wu, D.; Yu, G.; Gong, H.; et al. The impact of culture systems on the gut microbiota and gut metabolome of bighead carp (Hypophthalmichthys nobilis). Anim. Microbiome 2023, 5, 20. [Google Scholar] [CrossRef]
- Foysal, J.; Fotedar, R.; Siddik, M.; Tay, C. Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii). Sci. Rep. 2020, 10, 5196. [Google Scholar] [CrossRef]
- Foysal, M.; Fotedar, R.; Tay, C.; Gupta, S. Biological filters regulate water quality, modulate health status, immune indices and gut microbiota of freshwater crayfish, marron (Cherax cainii, Austin, 2002). Chemosphere 2020, 247, 125821. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ye, X.; Wei, L.; Wen, Y.; Yang, Z.; Ten, Z.; Liu, K.; Lv, Y. Analysis of Difference in Intestinal Bacterial Flora betwee en Common Carp Cyprinus carpio var. Jinbian and C. carpio var. Jian Cultured in Paddy Fields and in Ponds. Fish. Sci. 2020, 39, 509–516. [Google Scholar]
- Liu, Z.; Yan, L.; Jie, L.; Qiao, L.; Jian, Z.; Jun, D.; Song, Y. Analysis and comparison of intestinal microbiota, immune enzyme activities, and muscle flavor of Jian carp in two culture modes. J. Fish. Sci. China 2021, 28, 48–56. [Google Scholar]
- Nayak, K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Christian, D.; Anderson, S.; Luiz, F.; Juliano, Z. Environmental contamination alters the intestinal microbial community of the livebearer killifish Phalloceros caudimaculatus. Heliyon 2020, 6, e04190. [Google Scholar]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, W.; Yu, Y.; Lv, Q. Effects of different cultural patterns on microbial communities in the intestine of Macrobrachium rosenbergii and interactions with environment factors. J. Shanghai Ocean Univ. 2019, 28, 501–510. [Google Scholar]
- Zeng, S.; Huang, Z.; Hou, D.; Liu, J.; Weng, S.; He, J. Composition, diversity and function of intestinal microbiota in pacific white shrimp (Litopenaeus vannamei) at different culture stages. PeerJ 2017, 5, e3986. [Google Scholar] [CrossRef]
- Amoah, K.; Huang, Q.-C.; Tan, B.-P.; Zhang, S.; Chi, S.-Y.; Yang, Q.-H.; Liu, H.-Y.; Dong, X.-H. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 796–808. [Google Scholar] [CrossRef]
- Turnbaugh, P.; Ley, R.; Mahowald, M.; Magrini, V.; Mardis, E.; Gordon, J. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Iskhakova, Z.; Zhuravleva, D.; Heim, C.; Hartmann, M.; Laykov, A.; Forchhammer, K.; Kayumov, A. PotN represents a Novel Energy-State Sensing PII Subfamily, Occurring in Firmicutes. FEBS J. 2022, 289, 5305–5321. [Google Scholar] [CrossRef]
- Vitorino, I.; Lage, O. The Planctomycetia: An overview of the currently largest class within the phylum Planctomycetes. Antonie Van Leeuwenhoek 2022, 115, 169–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.; Zhou, Y.; Almeida, A.; Finn, R.D.; Danchin, A.; He, L. Phylogenomics of expanding uncultured environmental Tenericutes provides insights into their pathogenicity and evolutionary relationship with Bacilli. BMC Genom. 2020, 21, 408. [Google Scholar] [CrossRef]
- Wang, Y.; Stingl, U.; Anton-Erxleben, F.; Geisler, S.; Brune, A.; Zimmer, M. “Candidatus Hepatoplasma crinochetorum”, a New, Stalk-Forming Lineage of Mollicutes Colonizing the Midgut Glands of a Terrestrial Isopod. Appl. Environ. Microbiol. 2004, 70, 6166–6172. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Lv, D.; Ge, Y.; Li, H.; You, Y. Change in the intestinal bacterial community structure associated with environmental microorganisms during the growth of Eriocheir sinensis. MicrobiologyOpen 2019, 8, e00727. [Google Scholar] [CrossRef]
- Kostanjsek, R.; Strus, J.; Avgustin, G. “Candidatus bacilloplasma”, a Novel Lineage of Mollicutes Associated with the Hindgut Wall of the Terrestrial Isopod Porcellio scaber (Crustacea: Isopoda). Appl. Environ. Microbiol. 2007, 73, 5566–5573. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Yang, J.; Mutanda, I.; Li, S.; Zhang, Q.; Zhang, Y.; Zhang, Y.; Wang, Y. Pathway-specific enzymes from bamboo and crop leaves biosynthesize anti-nociceptive C-glycosylated flavones. Commun. Biol. 2020, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, X.; Zhang, R.; Zhao, Y.; Wu, G.; Liu, J.; Zhu, X.; Li, L. Comparative analysis of the intestinal bacterial community and expression of gut immunity genes in the Chinese Mitten Crab (Eriocheir sinensis). AMB Express 2018, 8, 192. [Google Scholar] [CrossRef]
- Meng, X.; Wu, S.; Hu, W.; Zhu, Z.; Yang, G.; Zhang, Y.; Qin, C.; Yang, L.; Nie, G. Clostridium butyricum improves immune responses and remodels the intestinal microbiota of common carp (Cyprinus carpio L.). Aquaculture 2021, 530, 735753. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Chen, C.; Feng, Y.; Ren, N.; Sun, K. Effects of cadmium on intestinal histology and microbiota in freshwater crayfish (Procambarus clarkii). Chemosphere 2019, 242, 125101–125109. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luo, X.; Lin, H.; Han, F.; Qin, J.G.; Chen, L.; Li, E. Growth, Health, and Gut Microbiota of Female Pacific White Shrimp, Litopenaeus vannamei Broodstock Fed Different Phospholipid Sources. Aquaculture 2022, 11, 1143. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Q.; Qian, C.; Shen, K.; Zhu, X.; Zhou, D.; Lu, W.; Sun, Z.; Liu, H.; Li, K.; et al. In Vitro Susceptibility and Florfenicol Resistance in Citrobacter Isolates and Whole-Genome Analysis of Multidrug-Resistant Citrobacter freundii. Int. J. Genom. 2019, 2019, 7191935. [Google Scholar]
- Huang, Y.; Li, C.; Qin, S.; Lu, W.; Zhang, B.; Sun, J.; Zhang, W.; Tong, T.; Zhang, Q. Isolation, Identification and Drug Resistance Analysis of Citrobacter braakii from Cherax quadricarinatus. Chin. J. Vet. Med. 2024, 60, 59–65. [Google Scholar]
- Xiao, P.; Jin, S.; Zheng, C. Research progress on major diseases of Cherax quadricarinatus. Fish. Sci. 2009, 28, 485–488. [Google Scholar]
- Chen, H.; Wang, Y.; Zhang, J.; Bao, J. Intestinal microbiota in white spot syndrome virus infected red swamp crayfish (Procambarus clarkii) at different health statuses. Aquaculture 2021, 542, 736826. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Xue, M.; Yang, T.; Luo, X.; Fan, Y.; Meng, Y.; Liu, W.; Lin, G.; Li, B.; et al. Effects of Dietary Saccharomyces cerevisiae YFI-SC2 on the Growth Performance, Intestinal Morphology, Immune Parameters, Intestinal Microbiota, and Disease Resistance of Crayfish (Procambarus clarkia). Animals 2021, 11, 1963. [Google Scholar] [CrossRef]
- Chen, C.; Xu, C.; Yang, X.; Jia, Y.; Gu, Z.; Li, E. The Optimum Lipid Level for the Juvenile Redclaw Crayfish Cherax quadricarinatus: Practical Diets with Soybean Oil as the Lipid Source. Aquac. Nutr. 2022, 2022, 2640479. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xing, D.; Bai, G.; Li, X. Studies on disease of mitten ceab caused by Citrobacter freudii. Acta Hydrobiol. Sin. 2001, 25, 217–223. [Google Scholar] [CrossRef]
- Liu, H.; Chen, G.; Li, L.; Lin, Z.; Tan, B.; Dong, X.; Zhou, X. Supplementing artemisinin positively influences growth, antioxidant capacity, immune response, gut health and disease resistance against Vibrio parahaemolyticus in Litopenaeus vannamei fed cottonseed protein concentrate meal diets. Fish Shellfish Immunol. 2022, 131, 105–118. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Wang, C.; Zhang, L.; Tu, K.; Zheng, Z. Insights into the intestinal microbiota of several aquatic organisms and association with the surrounding environment. Aquaculture 2019, 507, 196–202. [Google Scholar] [CrossRef]
- Mendoza-Barberá, E.; Merino, S.; Tomás, J. Surface glucan structures in Aeromonas spp. Mar. Drugs 2021, 19, 649. [Google Scholar] [CrossRef] [PubMed]
- Azzam-Sayuti, M.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Annas, S.; Yusof, M.T.; Monir, M.S.; Mohamad, A.; Muhamad-Sofie, M.H.N.; Lee, J.Y.; Chin, Y.K.; et al. Comparative Pathogenicity of Aeromonas spp. in Cultured Red Hybrid Tilapia (Oreochromis niloticus × O. mossambicus). Biology 2021, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (g/kg Dry Weight) | Nutritional Composition (%) | ||
|---|---|---|---|
| Fish meal | 240 | Crude protein | 430 |
| Antarctic krill powder | 80 | Crude fiber | 120 |
| Fish oil | 20 | Crude ash content | 160 |
| Soybean meal | 160 | Crude fat | 50 |
| Peanut bran | 120 | Total phosphorus | 30 |
| Squid paste | 30 | Lysine | 24 |
| Shrimp paste | 30 | Calcium | 30 |
| Flour | 270 | ||
| Plant extract | 30 | ||
| Choline chloride | 5 | ||
| Salt | 5 | ||
| Vitamin premix 1 | 5 | ||
| Mineral premix 2 | 5 | ||
| Water Quality Index | Radius |
|---|---|
| pH | 8.0 ± 0.8 |
| Dissolved oxygen | ≥6.0 ppm |
| Ammonia nitrogen | ≤0.02 mg/L |
| Phosphates | ≤0.5 mg/L |
| Temperature | 23.5~29.5 °C |
| Nitrite | ≤0.2 mg/L |
| Groups | Replicates | Initial Body Weight/g | Initial Body Length/cm | Weight/g | Body Length/cm |
|---|---|---|---|---|---|
| PB | 1 | 12.94 ± 1.55 | 4.23 ± 0.69 | 79.58 ± 10.04 | 10.14 ± 0.52 |
| 2 | 13.12 ± 1.43 | 4.50 ± 0.43 | 81.18 ± 11.28 | 10.38 ± 0.25 | |
| 3 | 11.99 ± 1.62 | 4.14 ± 0.52 | 70.38 ± 6.52 | 9.78 ± 0.31 | |
| RB | 1 | 12.23 ± 2.54 | 4.53 ± 0.66 | 77.50 ± 8.18 | 10.5 ± 0.35 |
| 2 | 13.65 ± 3.12 | 4.22 ± 0.53 | 73.76 ± 14.15 | 10.08 ± 0.66 | |
| 3 | 12.65 ± 2.11 | 4.19 ± 0.42 | 72.22 ± 6.55 | 9.96 ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Lu, T.; Lu, X.; Shi, J.; Huang, Y.; Du, X.; Wang, D.; Liang, Y.; Lei, Y.; Wang, L.; et al. Comparison of the Intestinal Microbiota Composition and Function of Red Claw Crayfish (Cherax quadricarinatus) Cultured in Ponds and Rice Fields. Fishes 2024, 9, 345. https://doi.org/10.3390/fishes9090345
Huang L, Lu T, Lu X, Shi J, Huang Y, Du X, Wang D, Liang Y, Lei Y, Wang L, et al. Comparison of the Intestinal Microbiota Composition and Function of Red Claw Crayfish (Cherax quadricarinatus) Cultured in Ponds and Rice Fields. Fishes. 2024; 9(9):345. https://doi.org/10.3390/fishes9090345
Chicago/Turabian StyleHuang, Libin, Tianhe Lu, Xiaohua Lu, Jingu Shi, Yin Huang, Xuesong Du, Dapeng Wang, Yi Liang, Yanju Lei, Lianggang Wang, and et al. 2024. "Comparison of the Intestinal Microbiota Composition and Function of Red Claw Crayfish (Cherax quadricarinatus) Cultured in Ponds and Rice Fields" Fishes 9, no. 9: 345. https://doi.org/10.3390/fishes9090345
APA StyleHuang, L., Lu, T., Lu, X., Shi, J., Huang, Y., Du, X., Wang, D., Liang, Y., Lei, Y., Wang, L., Wang, R., & Yang, H. (2024). Comparison of the Intestinal Microbiota Composition and Function of Red Claw Crayfish (Cherax quadricarinatus) Cultured in Ponds and Rice Fields. Fishes, 9(9), 345. https://doi.org/10.3390/fishes9090345






