Effect of a Guar Meal Protein Concentrate in Replacement of Conventional Feedstuffs on Productive Performances and Gut Health of Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets and Analysis
2.2. Growth Trial
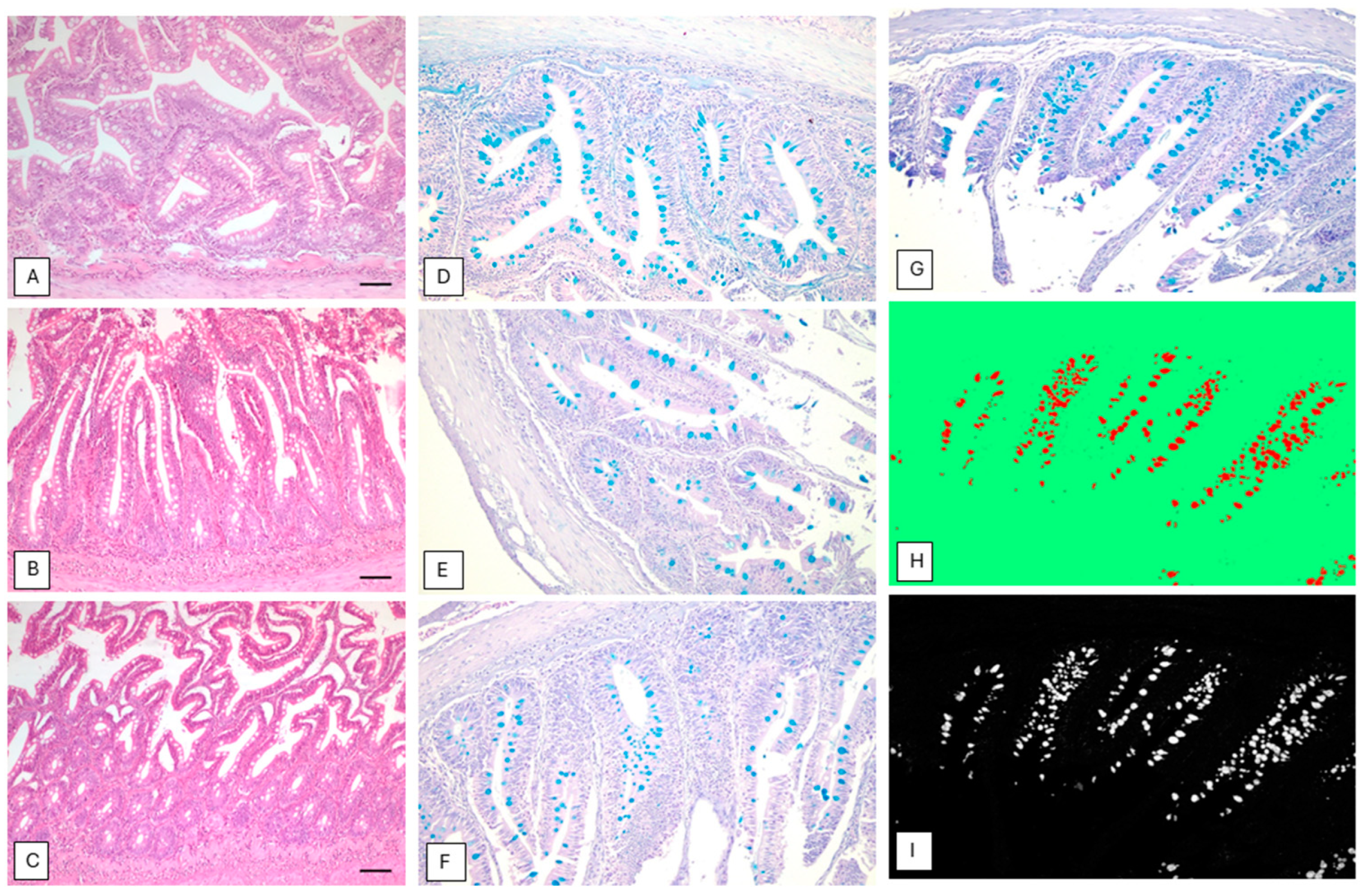
2.3. Histological Analysis
2.4. Statistical Data Analyses
3. Results
3.1. Growth Trial
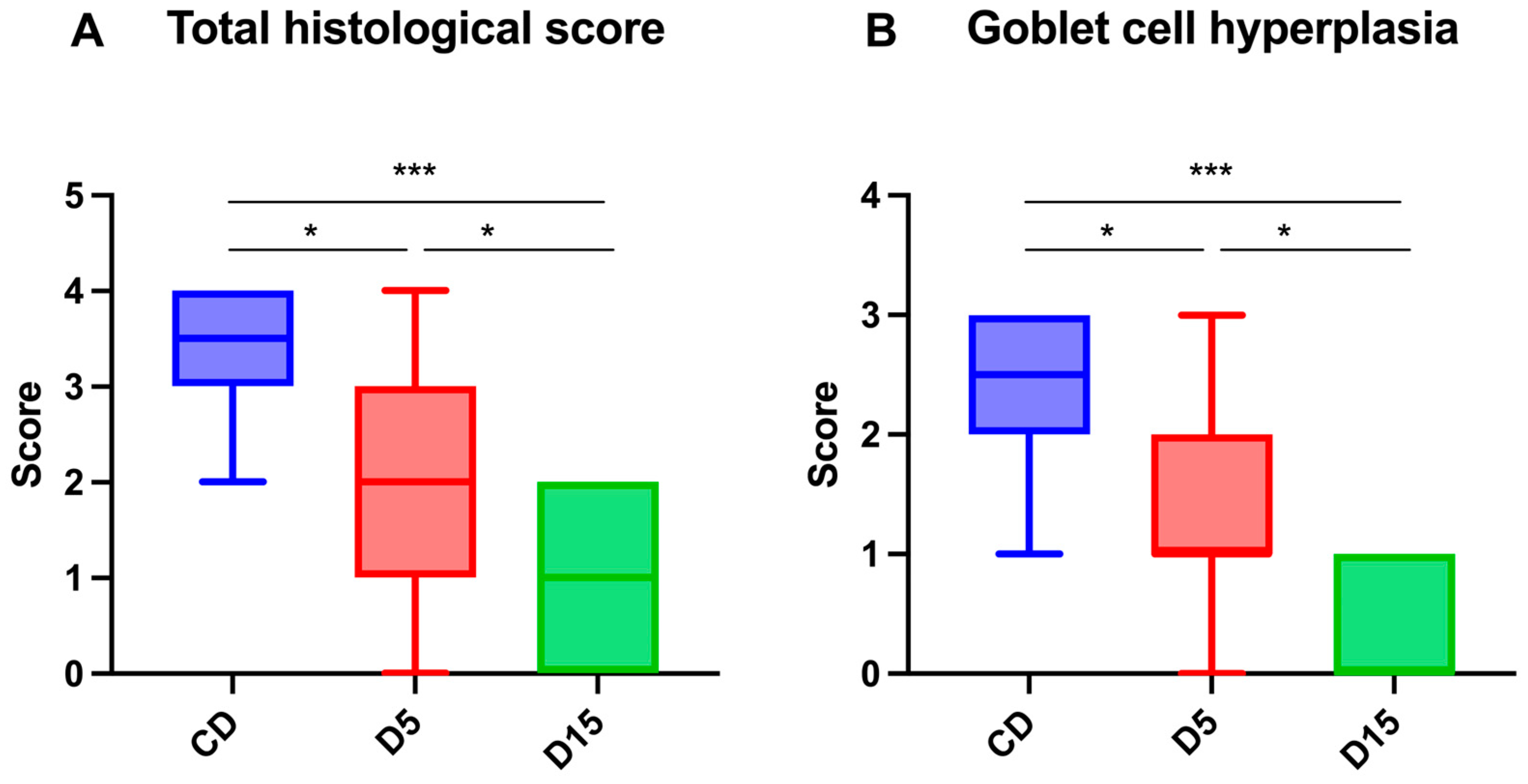
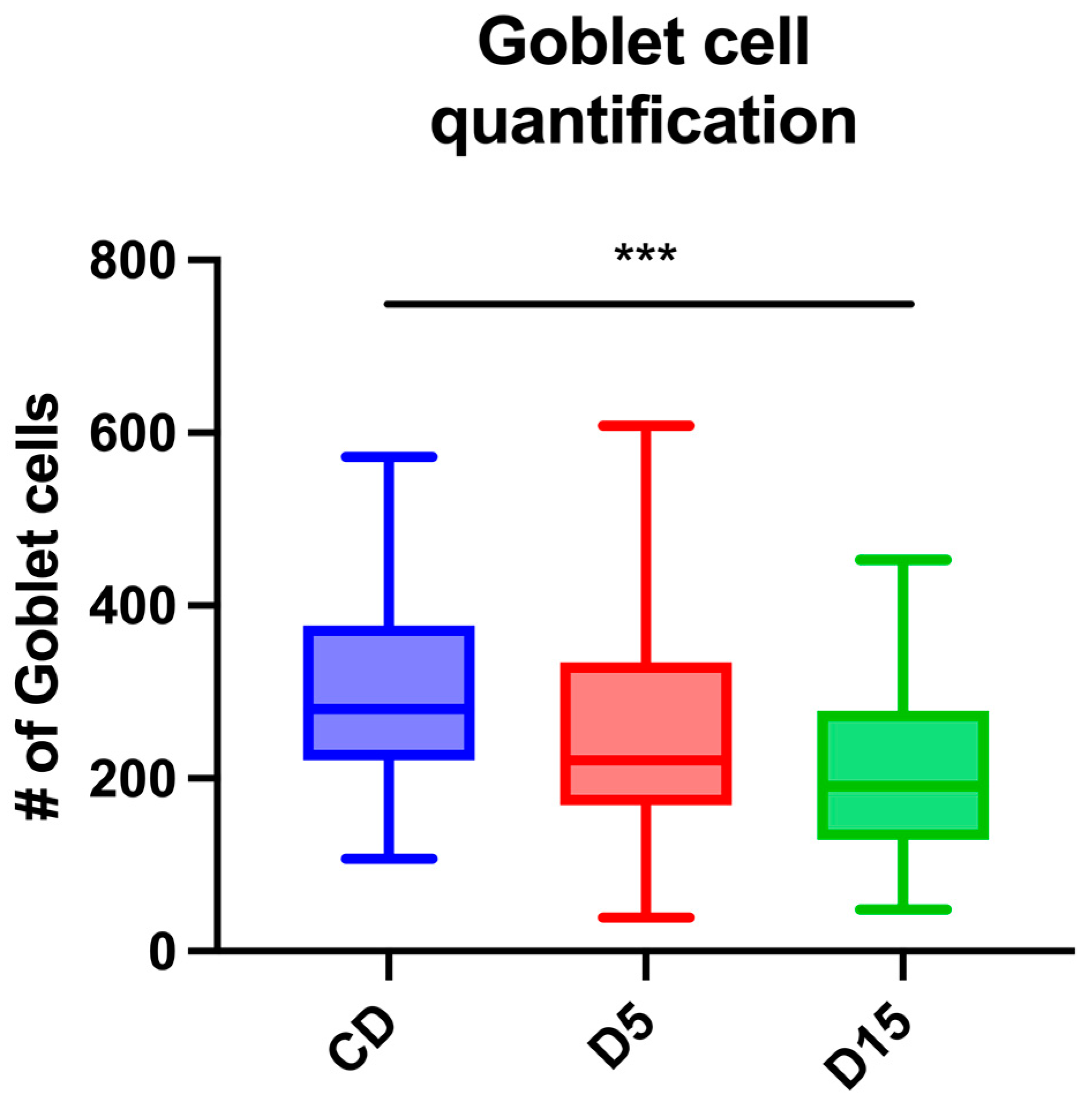
3.2. Gut Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FEAP. European Aquaculture Production Report 2016–2022; FEAP Secretariat: Brussels, Belgium, 2023. [Google Scholar]
- Stankovic, D.; Crivelli, A.J.; Snoj, A. Rainbow trout in Europe: Introduction, Naturalization, and Impacts. Rev. Fish Sci. Aquac. 2015, 23, 39–71. [Google Scholar] [CrossRef]
- Faccenda, F.; Lunelli, F.; Gandolfi, A.; Bozzi, R. Microsatellite-based genetic diversity and admixture history of rainbow trout (Oncorhynchus mykiss—Walbaum, 1792) stocks in Trentino (Italy). Turk. J. Fish. Aquat. Sci. 2018, 18, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Garcìa-Ballesteros, S.; Fernández, J.; Toro, M.A.; Villanueva, B. Benefits of genomic evaluation in aquaculture breeding programs with separate rearing of families. Aquaculture 2021, 543, 737004. [Google Scholar] [CrossRef]
- Albrektsen, S.; Kortel, R.; Skov, P.V.; Ytteborg, E.; Gitlesen, S.; Kleinegris, D.; Mydland, L.T.; Hansen, J.Ø.; Lock, E.-J.; Mørkøre, T.; et al. Future feed resources in sustainable salmonid production: A review. Rev. Aquac. 2022, 14, 1790–1812. [Google Scholar] [CrossRef]
- Parisi, G.; Tulli, F.; Fortina, R.; Marino, R.; Bani, P.; Dalle Zotte, A.; De Angeli, A.; Piccolo, G.; Pinotti, L.; Schiavone, A.; et al. Protein hunger of the feed sector: The alternatives offered by the plant world. Ital. J. Anim. Sci. 2020, 19, 1204–1225. [Google Scholar] [CrossRef]
- Tahmouzi, S.; Meftahizadeh, H.; Eyshi, S.; Mahmoudzadeh, A.; Alizadeh, B.; Mollakhalili-Meybodi, N.; Hatami, M. Application of guar (Cyamopsis tetragonoloba L.) gum in food technologies: A review of properties and mechanisms of action. Food Sci. Nutr. 2023, 11, 4869–4897. [Google Scholar] [CrossRef] [PubMed]
- Pach, F.; Nagel, F. Replacing the substitute-Guar meal as an alternative for non-genetically modified soybean meal in the nutrition of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792). Aquac. Nutr. 2018, 24, 666–672. [Google Scholar] [CrossRef]
- Janphirom, T.; Chaiprasert, P.; Thongthieng, T.; Suwannathep, S.; Songkasiri, W. Increasing fish feed stability using guar gum: Case study with Channa striata. Asian J. Food Agro-Ind. 2010, 3, 363–370. [Google Scholar]
- Chiofalo, B.; Lo Presti, V.; D’Agata, A.; Rao, R.; Ceravolo, G.; Gresta, F. Qualitative profile of degummed guar (Cyamopsis tetragonoloba L.) seeds grown in a Mediterranean area for use as animal feed. J. Anim. Physiol. Anim. Nutr. 2018, 102, 260–267. [Google Scholar] [CrossRef]
- Vohra, P.; Keatzer, F.H. The use of guar meal in chicken rations. Poult. Sci. 1964, 43, 502–503. [Google Scholar] [CrossRef]
- Hasan, M.S.; Humphrey, R.M.; Yang, Z.; Crenshaw, M.A.; Brett, J.; Liao, S.F. Effects of dietary inclusion of GuarPro F-71 on the growth performance and nutrient metabolism in young growing pig. J. Appl. Anim. Nutr. 2020, 8, 143–149. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Abo-State, H.A.; Tahoun, A.A. Evaluation of guar meal as a dietary protein source for Nile tilapia (Oreochromis niloticus) reared in hapa-in-pond system. J. Fish Aquat. Sci. 2016, 11, 317–322. [Google Scholar] [CrossRef]
- Barlaya, G.; Kumar, B.S.A.; Huchchappa, R.R.C.; Basumatary, P.; Kannur, H. Effect of fish meal replacement with toasted guar meal on growth, food conversion, digestive enzyme activity and final carcass composition of rohu, Labeo rohita. Aquac. Res. 2021, 52, 5551–5557. [Google Scholar] [CrossRef]
- Yadollahi, V.; Shamsaie, M.; Javaheri Baboli, M.; Yadollahi, F.; Hajivar, E.N.; Moëzzi, F. Replacement of soybean meal with guar meal in the diet of rainbow trout, Oncorhynchus mykiss (Walbaum 1792): Biological parameters and flesh quality. Asian Fish Sci. 2018, 31, 181–190. [Google Scholar] [CrossRef]
- Welker, T.-L.; Liu, K.; Overtuf, K.; Abernathy, J.; Barrows, F.T. Effect of soy protein products and gum inclusion in feed on fecal particle size profile of rainbow trout. Aquac. J. 2021, 1, 14–25. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 60, 497–509. [Google Scholar] [CrossRef]
- Christopherson, S.W.; Glass, R.L. Preparation of milk methyl esters by alcoholysis in an essentially non-alcoholic solution. J. Dairy Sci. 1969, 52, 1289–1290. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Meat and meat products. In Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Volume 2, pp. 931–948. [Google Scholar]
- Kasumyan, A.O. Gustatory reception and feeding behaviour in fish. J. Ichthyol. 1997, 37, 72–86. [Google Scholar]
- Kasumyan, A.O.; Døving, K.B. Taste preferences in fish. Fish Fish. 2003, 4, 289–347. [Google Scholar] [CrossRef]
- Steffens, W. Principles of Fish Nutrition; Ellis Horwood: Chichester, UK, 1989. [Google Scholar]
- Hooft, J.M.; Montero, R.; Morales-Lange, B.; Blihovde, V.F.; Purushothaman, K.; Press, C.M.; Mensah, D.D.; Agboola, J.O.; Javed, S.; Mydland, L.T.; et al. Paecilomyces variotii (PEKILO®) in novel feeds for Atlantic salmon: Effects on pellet quality, growth performance, gut health, and nutrient digestibility and utilization. Aquaculture 2024, 589, 740905. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, A.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Kaushik, S.; Panserat, S.; Schrama, J.W. Carbohydrates. In Fish Nutrition, 4th ed.; Hardy, R.W., Kaushik, S.J., Eds.; Elsevier: London, UK, 2022; pp. 555–591. [Google Scholar]
- Fanizza, C.; Trocino, A.; Stejskal, V.; Prokesova, M.D.; Zare, M.; Tran, H.Q.; Brambilla, F.; Xiccato, G.; Bordignon, F. Practical low-fishmeal diets for rainbow trout (Oncorhynchus mykiss) reared in RAS: Effects of protein meals on fish growth, nutrient digestibility, feed physical quality, and faecal particle size. Aquac. Rep. 2023, 28, 101435. [Google Scholar] [CrossRef]
- Seong, P.N.; Cho, S.H.; Park, K.M.; Kang, G.H.; Park, B.Y.; Moon, S.S.; Ba, H.V. Characterization of chicken by-products by mean of proximate and nutritional compositions. Korean J. Food Sci. Anim. Resour. 2015, 35, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Brinker, A.; Friedrich, C. Fish meal replacement by plant protein substitution and guar gum addition in trout feed. Part II: Effects on faeces stability and rheology. Biorheology 2012, 49, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Becke, C.; Schumann, M.; Steinhagen, D.; Rojas-Tirado, P.; Geist, J.; Brinker, A. Effects of unionized ammonia and suspended solids on rainbow trout (Oncorhynchus mykiss) in recirculating aquaculture systems. Aquaculture 2019, 499, 348–357. [Google Scholar] [CrossRef]
- Reid, G.K.; Liutkus, M.; Robinson, S.M.C.; Chopin, T.R.; Blair, T.; Lander, T.; Mullen, J.; Page, F.; Moccia, R.D. A review of the biophysical properties of salmonid faeces: Implications for aquaculture waste dispersal models and integrated multi-trophic aquaculture. Aquac. Res. 2009, 40, 257–273. [Google Scholar] [CrossRef]
- Brinker, A. Improving the mechanical characteristic of faecal waste in rainbow trout: The influence of fish size and treatment with a non-starch polysaccharide (guar gum). Aquac. Nutr. 2009, 15, 229–240. [Google Scholar] [CrossRef]
- Welker, T.L.; Overturf, K.; Snyder, S.; Liu, K.; Abernathy, J.; Frost, J.; Barros, F.T. Effects of feed processing method (extrusion and expansion-compression pelleting) on water quality and growth of rainbow trout in a commercial setting. J. Appl. Aquac. 2018, 30, 97–124. [Google Scholar] [CrossRef]
- Daudaa, A.B.; Ajadib, A.; Tola-Fabunmic, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Hilton, J.W.; Cho, C.Y.; Slinger, S.J. Effect of extrusion processing and steam pelleting diets on pellet durability, pellet water absorption, and the physiological response of rainbow trout (Salmo gairdneri R.). Aquaculture 1981, 25, 185–194. [Google Scholar] [CrossRef]
- Brinker, A.B.; Koppe, W.; Rosch, R. Optimised effluent treatment by stabilized trout faeces. Aquaculture 2004, 249, 125–144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Fan, J.; Zhou, H.; Huang, H.; Cao, Y.; Jiang, W.; Zhang, W.; Deng, J.; Tan, B. Effects of different viscous guar gums on growth, apparent nutrient digestibility, intestinal development and morphology in juvenile Largemouth Bass, Micropterus salmoides. Front. Physiol. 2022, 13, 927819. [Google Scholar] [CrossRef] [PubMed]
- Verdile, N.; Pasquariello, R.; Scolari, M.; Scirè, G.; Brevini, T.A.; Gandolfi, F. A detailed study of rainbow trout (Onchorhynchus mykiss) intestine revealed that digestive and absorptive functions are not linearly distributed along its length. Animals 2020, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Verdile, N.; Cardinaletti, G.; Faccenda, F.; Brevini, T.A.; Gandolfi, F.; Tibaldi, E. Ectopic stem cell niches sustain rainbow trout (Oncorhynchus mykiss) intestine absorptive capacity when challenged with a plant protein-rich diet. Aquaculture 2023, 564, 739031. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Bosi, G.; De Pasquale, J.A.; Manera, M.; Giari, L. Fish innate immunity against intestinal helminths. Fish Shellfish. Immunol. 2016, 50, 274–287. [Google Scholar] [CrossRef]
- Seibel, H.; Chikwati, E.; Schulz, C.; Rebl, A. A multidisciplinary approach evaluating soybean meal-induced enteritis in Rainbow Trout Oncorhynchus mykiss. Fishes 2022, 7, 22. [Google Scholar] [CrossRef]
| Proximate Composition (% as Fed) | |
|---|---|
| Moisture | 6.0 |
| Crude protein | 66.0 |
| Crude lipid | 10.0 |
| Fibre | 6.0 |
| Ash | 4.0 |
| Fatty acid content (% total FA) | |
| Linolenic acid | 39.34 |
| Oleic acid | 29.98 |
| Palmitic acid | 15.34 |
| Stearic acid | 6.93 |
| Linolenic acid | 2.95 |
| Amino acid profile (g/100 g) | |
| Aspartic acid | 6.51 |
| Glutamic acid | 13.1 |
| Alanine | 2.30 |
| Arginine | 9.23 |
| Phenylalanine | 2.61 |
| Glycine | 3.34 |
| Isoleucine | 1.92 |
| Histidine | 1.71 |
| Leucine | 3.56 |
| Lysine | 3.30 |
| Proline | 2.35 |
| Tyrosine | 2.00 |
| Threonine | 1.94 |
| Cysteine | 0.75 |
| Valine | 2.22 |
| Tryptophan | 1.00 |
| Methionine | 0.70 |
| CD | D5 | D15 | |
|---|---|---|---|
| Fish meal | 16 | 15.5 | 14.5 |
| Chicken meal | 16.31 | 15.9 | 11.1 |
| Soybean meal | 13.99 | 11.9 | 7.6 |
| Guar protein concentrate | 0 | 5 | 15 |
| Porcine haemoglobin meal | 12.6 | 12.6 | 12.6 |
| Wheat meal | 12.53 | 12.53 | 12.53 |
| Wheat distillers | 3.2 | 3.2 | 3.2 |
| Wheat gluten | 2.5 | 2.5 | 2.5 |
| Soybean lecithin | 0.5 | 0.5 | 0.5 |
| Fish oil | 10 | 10 | 10 |
| Soybean oil | 8.5 | 8.5 | 8.6 |
| L-lysine | 0.3 | 0.3 | 0.3 |
| DL-methionine | 0.22 | 0.22 | 0.22 |
| Mineral–vitamin premix | 1 | 1 | 1 |
| Choline liquid 75% | 0.35 | 0.35 | 0.35 |
| Proximate composition (%): | |||
| Moisture | 7.75 | 7.5 | 7.12 |
| Crude protein | 43.04 | 43.5 | 43.01 |
| Crude fat | 25.31 | 25.01 | 25.01 |
| Fibre | 1.4 | 1.5 | 2.01 |
| Ash Gross energy (MJ/kg) | 8.66 22.83 | 8.7 22.83 | 7.74 22.71 |
| CD | D5 | D15 | |
|---|---|---|---|
| Temperature °C | 13.5 ± 0.4 | 13.5 ± 0.6 | 13.5 ± 0.7 |
| Dissolved oxygen | 8.7 ± 0.5 | 8.7 ± 0.6 | 8.7 ± 0.4 |
| pH | 7.86 ± 0.4 | 7.82 ± 0.6 | 7.84 ± 0.5 |
| TAN | 0.24 ± 0.09 b | 0.22 ± 0.06 b | 0.44 ± 0.01 a |
| NO2-N | 0.02 ± 0.002 | 0.02 ± 0.01 | 0.01 ± 0.001 |
| NO3-N | 1.1 ± 0.4 | 1 ± 0.2 | 0.9 ± 0.1 |
| Parameters | CD | D5 | D15 |
|---|---|---|---|
| Initial mean weight (g) | 50.0 ± 1.4 | 50.0 ± 1.4 | 50.0 ± 1.4 |
| Final mean weight (g) | 198.8 ± 3.8 a | 201.0 ± 3.7 a | 171.2 ± 5.1 b |
| Final mean length (cm) KI | 23.2 ± 3.5 1.59 ± 0.11 a | 25.1 ± 0.9 1.27 ± 0.08 b | 23.7 ± 1.1 1.29 ± 0.1 b |
| WG (g) | 148.8 ± 26 | 151.0 ± 24 | 121.2 ± 18 |
| SGR (%/day) | 1.65 ± 0.18 | 1.68 ± 0.11 | 1.35 ± 0.30 |
| FCR | 1.03 ± 0.02 b | 1.04 ± 0.01 b | 1.09 ± 0.02 a |
| SR (%) | 97.33 ± 2.89 | 98.07 ± 1.29 | 98.00 ± 1.70 |
| Palatability | 100 ± 0.0 a | 100 ± 0.0 a | 98 ± 0.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roncarati, A.; Galosi, L.; Di Cerbo, A.; Quagliardi, M.; Marchetti, F.; Fiordelmondo, E.; Mariotti, F.; Magi, G.E. Effect of a Guar Meal Protein Concentrate in Replacement of Conventional Feedstuffs on Productive Performances and Gut Health of Rainbow Trout (Oncorhynchus mykiss). Fishes 2024, 9, 295. https://doi.org/10.3390/fishes9080295
Roncarati A, Galosi L, Di Cerbo A, Quagliardi M, Marchetti F, Fiordelmondo E, Mariotti F, Magi GE. Effect of a Guar Meal Protein Concentrate in Replacement of Conventional Feedstuffs on Productive Performances and Gut Health of Rainbow Trout (Oncorhynchus mykiss). Fishes. 2024; 9(8):295. https://doi.org/10.3390/fishes9080295
Chicago/Turabian StyleRoncarati, Alessandra, Livio Galosi, Alessandro Di Cerbo, Martina Quagliardi, Francesco Marchetti, Elisa Fiordelmondo, Francesca Mariotti, and Gian Enrico Magi. 2024. "Effect of a Guar Meal Protein Concentrate in Replacement of Conventional Feedstuffs on Productive Performances and Gut Health of Rainbow Trout (Oncorhynchus mykiss)" Fishes 9, no. 8: 295. https://doi.org/10.3390/fishes9080295
APA StyleRoncarati, A., Galosi, L., Di Cerbo, A., Quagliardi, M., Marchetti, F., Fiordelmondo, E., Mariotti, F., & Magi, G. E. (2024). Effect of a Guar Meal Protein Concentrate in Replacement of Conventional Feedstuffs on Productive Performances and Gut Health of Rainbow Trout (Oncorhynchus mykiss). Fishes, 9(8), 295. https://doi.org/10.3390/fishes9080295







