Abstract
Unlike adult mammalian cardiomyocytes, cardiomyocytes in teleosts display high proliferative capacity throughout adulthood. This study aimed to identify the immunohistochemical profiles of cardiomyocytes and immune cells in the hearts of Molly fish by assessing the immunolabelling expression of key proteins involved in cell proliferation, differentiation, and tissue protection. The cardiac anatomy of Molly fish includes the atrium, ventricle, and bulbus arteriosus. The expression of SOX9, NF-κB, myostatin, and S100 proteins in myocardial cells indicates the proliferative features of the heart in Molly fish. The bulbus arteriosus is characterized by collagenous chambers and smooth muscle cells that express Ach and iba1. The atrium of Molly fish serves as a storage unit for rodlet cells and immune cells. Rodlet cells displayed immunoreactivity to NF-κB, iba1, Olig2, Ach, and S100 proteins, suggesting their roles in the immune response within the heart. Furthermore, telocytes (TCs) have emerged as a significant component of the atrium of Molly fish, expressing Ach, CD68, S100 protein, and iba1. These expressions indicate the involvement of TCs in multiple signaling pathways that contribute to heart architecture. This study delineates the intricate relationship between cardiomyocytes and innate immune cells in Molly fish.
Key Contribution:
The study revealed that Molly fish cardiomyocytes exhibit significant proliferative capabilities with myocardial cells in the atrium and ventricle expressing key markers like Sox9, NF-κB, myostatin, and S100 protein. The role of rodlet cells and telocytes in the immune response and heart structure is emphasized, highlighting their involvement in multiple signaling pathways critical for cardiac regeneration.
1. Introduction
In all vertebrates, the heart is an essential organ that pumps blood throughout the body to provide nutrition and oxygen to tissues while expelling waste. The distinctive heart structure of fish reflects their aquatic lifestyle and metabolic requirements [1]. The fish’s heart is a comparatively simple but highly functional organ consisting of two main chambers: the ventricle and the atrium. It functions as a single circulation loop, which is in contrast to the more intricate four-chambered hearts observed in mammals and birds [2]. Deoxygenated blood returns to the atrium by the body, where it is forced into the ventricle by contraction. With its strong, muscular walls, the ventricle provides the force required to pump blood to the gills where oxygen is received [3,4]. Fish hearts have two additional chambers in addition to the atrium and ventricle: the sinus venosus and bulbus arteriosus. Through the sinus venosus, the collected deoxygenated blood is sent into the atrium.
The hearts of different species can differ greatly in terms of form and function, reflecting the ecological niches and lifestyles of these species [5]. For example, the hearts of swift-moving, energetic fish, such as salmon (Oncorhynchus masou masou) and tuna (Thunnus sp.), are often stronger and have a greater capacity to fulfill their increased energy needs [6]. On the other hand, fish that live in low-oxygen habitats may have evolved adaptations that improve their ability to absorb and use oxygen [7]. Since cardiovascular diseases continue to be the world’s top cause of death, there is an urgent need for novel therapeutic approaches and treatments. The heart’s capacity to repair and regenerate itself after damage is one of the most intriguing topics in regenerative biology. Some fish species have excellent cardiac regeneration abilities, which is in contrast to mammals that have limited cardiac regeneration capacity [8].
In mammals, cardiomyocyte proliferation occurs extensively during early development and for a brief period after birth, and this proliferative capability significantly diminishes within a month after birth, unlike in lower vertebrates, where it persists into adulthood. These differences in the proliferative capacity of adult cardiomyocytes between mammals and lower vertebrates are closely attributed to ontogenetic and phylogenetic factors [9]. Previous studies in adult zebrafish have shown that mature cardiomyocytes can dedifferentiate, re-enter the cell cycle, and proliferate to support the repair and replacement of injured myocardium [10]. Moreover, cardiomyocyte proliferation has been reported as a predominant mechanism responsible for the regeneration of the cardiac apex of the zebrafish heart following resection [11]. Furthermore, previous studies have demonstrated the regeneration process of zebrafish following the experimental induction of ventricular cryoinjury to simulate human myocardial infarction situations [12,13]. When a liquid nitrogen-cooled probe is used to injure tissue, a lesion of necrotic tissue develops. This induces an immediate inflammatory response that recruits different immune cell types to the wound [14]. The subsequent remodeling and regeneration processes in zebrafish are greatly influenced by the actions of these immune cells. In particular, macrophages and regulatory T cells are essential for effective cell regeneration [15,16]. Activated fibroblasts in zebrafish also provide vital signaling molecules that create a regenerative niche by encouraging neovascularization of the wound area and the proliferation of existing cardiomyocytes in the wound border zone [17]. Among the substances that support this signaling are nrg1 (neurogranin 1), which is released from cells originating from the epicardium, and retinoic acid, which is mostly secreted by the endocardial compartment [18,19].
Although zebrafish have been considered a potent model for studying adult heart regeneration [11], divergent findings from recent assessments of the capacity for heart regeneration across several teleost fish species show that zebrafish cardiac damage responses are not typical of other teleosts. Certain fish species, such as Mexican cavefish, apanese medaka (Oryzias latipes), and grass carp (Ctenopharyngodon idella), display persistent scarring comparable to that of adult humans [20,21,22], although some fish species, like goldfish (Carassius auratus), exhibit ventricular regeneration [23]. Consequently, it is critical to describe the cellular and molecular aspects of cardiomyocyte proliferative activity in various teleost species, including Molly fish.
Poecilia sphenops is highly adaptable and thrives in various aquatic environments from clear to muddy waters across its native range from the southern United States to the Yucatan Peninsula [24]. Unique for their genetic clonality, female mollies reproduce via parthenogenesis, producing clones without paternal genetic input. This makes them valuable in research, particularly on skin malignancies, thyroid cancer, and infectious diseases [25]. Their robust adaptability and reproductive consistency make them a promising model for various scientific studies [26,27,28].
The present work aims to investigate the immunohistochemical characteristics and proliferative capacity of cardiomyocytes and immune cells in the hearts of Molly fish by assessing the expression of essential proteins involved in cell proliferation, differentiation, and tissue protection. By understanding these cellular interactions and expressions, this study seeks to uncover potential insights and therapeutic avenues for heart conditions in which cardiomyocyte loss is a major concern.
2. Materials and Methods
The current study was completed in compliance with university policies regarding animal care and Egyptian legal requirements. All methods used in this study have been authorized by the National Ethical Committee of the Faculty of Veterinary Medicine, Assiut University, Egypt. The Ethical Number is 04/0015-aun/vet.
2.1. Sample Collection
The study used twenty healthy 6-month-old Molly fish specimens (Poecilia sphenops, Valenciennes, 1846). We bought fish from an ornamental fish shop in Assiut City, Egypt. The standard length of the specimens was 4.50 ± 5.0 cm, and their body weight was 12.00 ± 1.10 g. They were acclimated to their new environment (20 gal flowthrough aquaria with a water temperature of 20 ± 2 °C and a photoperiod of 16 h light and 8 h dark). The fish were kept in glass aquaria (60 L) capacity. About 25% of aquarium water (tap water free from chlorine) was exchanged daily. The aquaria were supplied with continuous aeration by air stones and fish were fed a basal diet. After 2 weeks of acclimation, the fish were randomly selected from the aquariums and euthanized with an overdose of MS-222 (3% tricaine). Then, the hearts were extracted, flushed with PBS to clear the blood, and processed for immunohistochemical analysis.
2.2. Immunohistochemical Analysis
Sections of the hearts were prepared for immunohistochemistry (IHC) using a Pierce Peroxidase Detection Kit (36000, Thermo Fisher Scientific, Waltham, MA, USA). The primary antibodies selected for IHC investigations are well documented for their roles in cell proliferation, differentiation, and tissue protection. The sections underwent a process of xylene deparaffinization, graded ethanol rehydration, and distilled water washing. To enhance epitope exposure, the sections were microwave-heated for 15 min in a sodium citrate buffer (0.01 M, pH 6.0). After 30 min of room temperature cooling, the sections were cleaned using wash buffer (Tris-buffered saline with 0.05% Tween-20 detergent), and after that, to stop the body’s endogenous peroxidase activity, they were incubated for 30 min in a peroxidase suppressor. The tissues were blocked using Universal BlockerTM blocking buffer in TBS for 30 min at room temperature after being rinsed twice for three minutes each time using a wash buffer. The sections were then incubated overnight at 4 °C with diluted (1:100) primary antibodies against a rabbit polyclonal interleukin 1 beta (IL-1β, sc-7884, Santa Cruz Biotechnology, Heidelberg Germany), rabbit polyclonal nuclear factor kappa B (NF-κB, 10745-1-AP, Proteintech, Rosemont, IL, USA), rabbit polyclonal nuclear factor erythroid 2-related factor 2 (Nrf2, sc-722, Santa Cruz Biotechnology, Heidelberg, Germany), rabbit polyclonal myostatin (AB3239, Sigma-Aldrich, Madrid, Spain), rabbit polyclonal SRY-Box transcription factor 9 (Sox9, AB5535, Sigma-Aldrich, Madrid, Spain), mouse monoclonal anti-CD68 (sc-17832, Santa Cruz Biotechnology, Heidelberg, Germany), mouse monoclonal anti-Olig2 (sc-515947, Santa Cruz Biotechnology, Heidelberg, Germany), mouse monoclonal Ionized calcium binding adaptor molecule (Iba-1, sc-32725, Santa Cruz Biotechnology, Heidelberg, Germany, diluted 1:200), rabbit polyclonal Nicotinic Acetylcholine R alpha 7 NACHRA7 (Ach, A7844, ABclonal, Woburn, MA, USA), and rabbit polyclonal S100 protein (Z0311, Dako, Glostrup, Denmark). In parallel, tissue specimens, in which the S100 protein, Nrf2, and SOX9 primary antibodies were omitted and replaced with buffer, served as negative controls (Figure S1). The slides were washed two times for 3 min with wash buffer and were incubated with diluted (1:1000) goat anti-rabbit IgG (65-6140, Invitrogen, Waltham, MA, USA) and diluted (1:100) goat anti-mouse IgG (31800, Invitrogen, Waltham, MA, USA) secondary antibodies for 30 min at room temperature. Following that, the slides were washed three times for 3 min each with a wash buffer, and the tissues were incubated with the diluted (1:500) Avidin-HRP (43-4423, Invitrogen, Waltham, MA, USA) in a universal blocker blocking buffer for 30 min. The slides were then washed three times for 3 min each with a wash buffer. The tissues were treated for 5 to 15 minutes, or until the required staining was obtained, with a 1× metal-enhanced 3,3′ diaminobenzidine (DAB) substrate working solution, which was made by adding stable peroxide buffer to the 10× DAB/Metal Concentrate. The sections were then counterstained with hematoxylin, which was changed twice by Harris, for three minutes each, and mounted using mounting medium.
In IHC, target antigens are detected directly through chromogenic means, in which antibodies are conjugated to horseradish peroxidase enzyme (HRP). Following incubation with DAB substrate, the enzyme activity led to the precipitation of insoluble, which were colored precipitates at the antigen localization site. Using light microscopy, the IHC expression can be detected by visualizing these colored precipitates. The absence of these precipitates indicates the negative IHC expression.
3. Results
3.1. General structure of Molly Fish’s Heart
Like many teleost species, the heart of Molly fish consists of atria, ventricles, and bulbus arteriosus (Figure 1A). The bulbus arteriosus was pear in shape and characterized by a thick wall structure extending between the single ventricle and ventral aorta. A valve was observed between the ventricle and the bulbus. The inner surface was composed of longitudinal unbranched ridges. It consisted of the endocardium, middle layer, and external layer. The endocardium was a thin layer of endothelium. The middle layer was formed of layers of smooth muscle fibers, which was separated by bundles of collagenous fibers. The external layer was composed of wavy collagenous bundles and fibroblast-like cells (Figure 1A). The middle layer of the bulbus expressed iba1 (Figure 1B), whereas the external layer expressed Ach (Figure 1C).
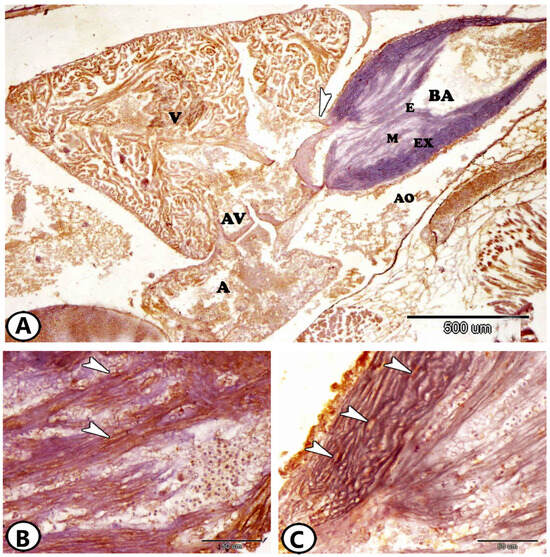
Figure 1.
General view of the heart of Molly fish stained by Ach and iba1. (A) The heart of atrium (A), ventricle (V), and bulbus arteriosus (BA). The bulbous consisted of the endocardium (E), middle (M), and external layers (EX). Note the presence of the atrioventricular valve (AV) and aorta (AO) and the valve (arrowhead) between the ventricle and the bulbous. (B) The middle layer bulbous arteriosus expressed iba1 (arrowheads). (C) The external layer of the bulbus expressed Ach (arrowheads).
3.2. Architecture of the Atrium and Atrioventricular Regions
The single atrium consisted of a thin myocardium and a network of thin trabeculae (Figure 2A,B). The atrioventricular (AV) region is formed by a ring of myocardium, which supports the AV valves (Figure 2A). Typically, two leaflets with a dense core composed of many cells and a substantial quantity of connective tissue create the AV valves. This connective tissue core contained macrophages and lymphocytes that expressed Ach (Figure 2C) and dendritic cells that expressed iba1 (Figure 2D).
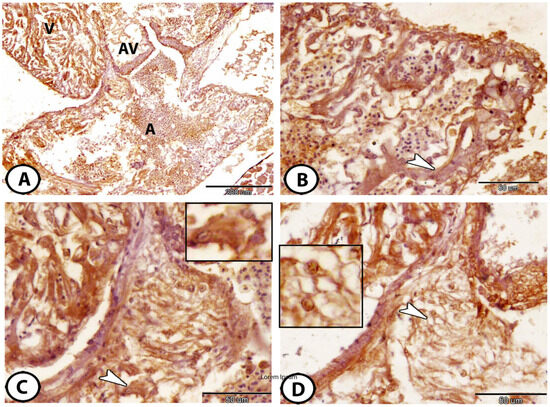
Figure 2.
Morphology of atrium and AV valves. (A) The atrioventricular valve (AV) is located between the atrium (A) and ventricle (V), immunostained against Ach. (B) Higher magnification of the single that consisted of a thin myocardium and a network of thin trabeculae (arrowhead), immunostained against Ach. (C) The connective tissue core of AV valves immunostained against Ach showed an expression of macrophages and lymphocytes (arrowhead and boxed area). (D) The AV connective tissue core of the valves immunostained against iba1 showed dendritic cells (arrowhead and boxed area).
3.3. Immunohistochemical Properties of the Atrial Immune Cells
The atrium showed a large proportion of immune cells, including dendritic-like cells that expressed Ach (Figure 3A), macrophages that expressed Ach (Figure 3B), CD68 (Figure 3C), and iba1 (Figure 3D). Moreover, granulocytes could be identified by S100 protein (Figure 3E). We can identify various immune cells according to their morphology. Dendritic cells were characterized by a high nuclear-to-cytoplasmic ratio and the presence of dendrite-like processes. Macrophages were characterized by eccentric nuclei and pseudopodia. On the other hand, the granulocytes were observed near RBCs with pleomorphic nuclei and some pseudopodia.
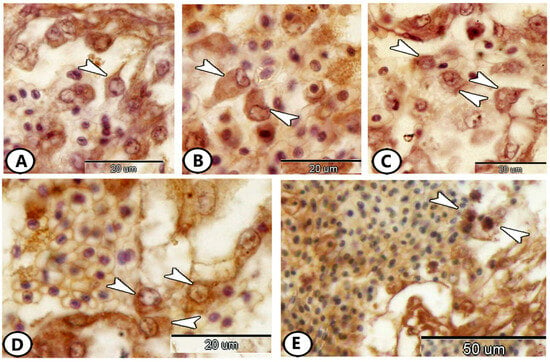
Figure 3.
Immunohistochemistry of immune cells in the atrium. (A) Dendritic cells (arrowhead) expressed Ach. (B) Macrophages (arrowheads) expressed Ach. (C,D) Macrophages (arrowheads) expressed CD68 and iba1, respectively. (E) Granulocytes (arrowheads) expressed S100 Protein.
Rodlet cells are immune cells scattered throughout the atrium. They were rounded to polyhedral cells surrounded by a capsule and contained an eccentric nucleus. These cells exhibited a wide range of immunoreactivity to various antibodies. The cytoplasm of these cells expressed Ach (Figure 4A), NF-κB (Figure 4C), and iba1 (Figure 4E,F), while their capsule expressed S100 protein (Figure 4B) and Olig-2 (Figure 4D).
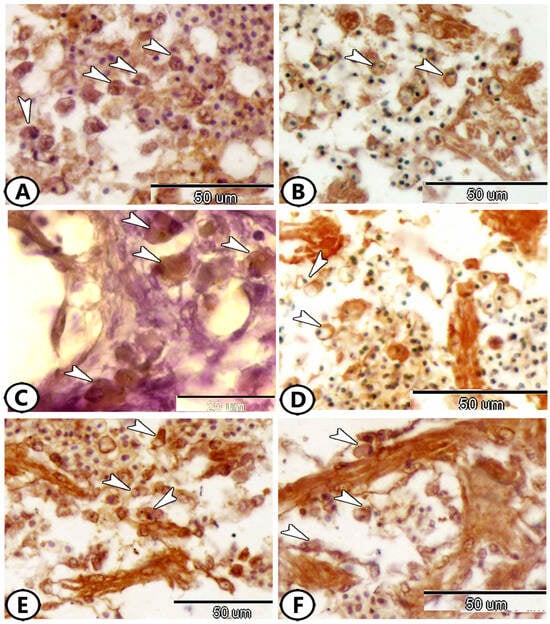
Figure 4.
Immunohistochemistry of rodlet cells in the atrium. (A) Rodlet cells (arrowheads) expressed Ach. (B) Rodlet cells (arrowheads) expressed S100 Protein. (C) Rodlet cells (arrowheads) expressed NF-κB. (D) Rodlet cells (arrowheads) expressed Olig-2. (E,F) Rodlet cells (arrowheads) expressed iba1.
3.4. Immunohistochemical Characterization of the Telocytes
Furthermore, telocytes (TCs) have emerged as a significant component of the atrium. These unique interstitial cells were characterized by a cell body containing an oval nucleus and long cell processes called telopodes. TCs and their telopodes expressed Ach (Figure 5A), S100 protein (Figure 5B), CD68 (Figure 5C,D), and iba1 (Figure 5E,F).
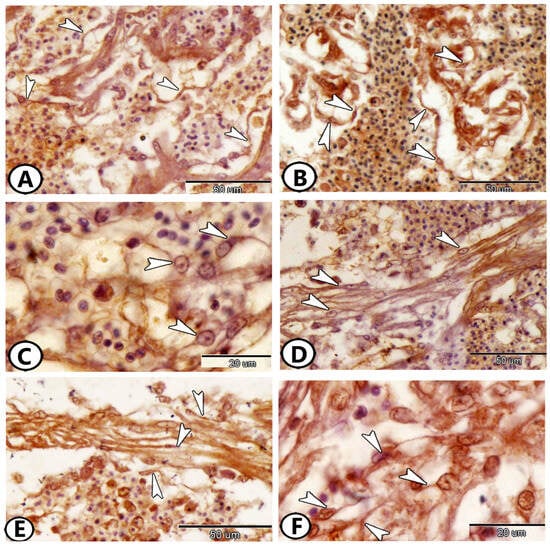
Figure 5.
Immunohistochemistry of telocytes (TCs) in the atrium. (A) TCs (arrowheads) expressed Ach. (B) TCs (arrowheads) expressed S100 Protein. (C,D) TCs (arrowheads) expressed CD68. (E,F) TCs (arrowheads) expressed iba1.
3.5. Immunohistochemical Features of the Cardiomyocytes
The ventricle consists of cardiac muscle fibers oriented in many directions. Proliferative features were notably observed in the cardiac muscles of the atria and ventricles. The myocardial cells exhibited an expression of myostatin (Figure 6A), Sox9 (Figure 6B), Nrf2 (Figure 6C), S100 protein (Figure 6D), Ach (Figure 6E), and IL-1β (Figure 6F). The relationship between cardiomyocytes and immune cells, particularly macrophages, is illustrated in Figure 7. This figure shows staining with Iba1 (Figure 7A,B), Ach (Figure 7C), and S100 protein (Figure 7D). Numerous processes of these immune cells are observed in direct contact with cardiac cells.
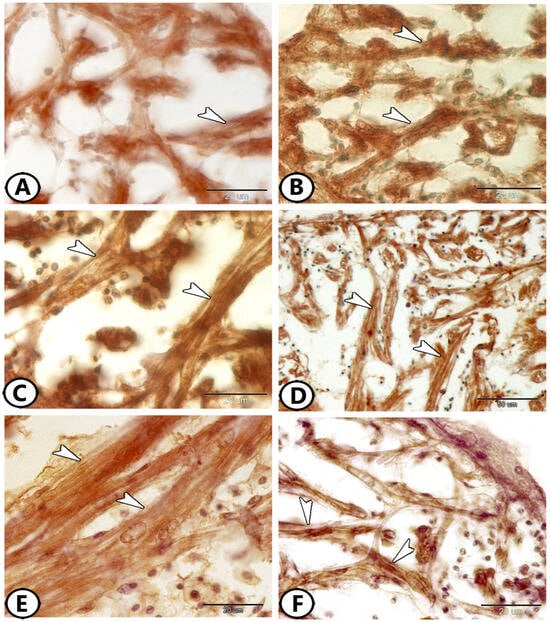
Figure 6.
Immunohistochemistry of cardiac muscle fibers. (A) Cardiac muscles (arrowheads) expressed myostatin. (B) Cardiac muscles (arrowheads) expressed SOX9. (C) Cardiac muscles (arrowheads) expressed Nrf2. (D) Cardiac muscles (arrowheads) expressed S100 protein. (E) Cardiac muscles (arrowheads) expressed Ach. (F) Cardiac muscles (arrowheads) expressed IL-1β.
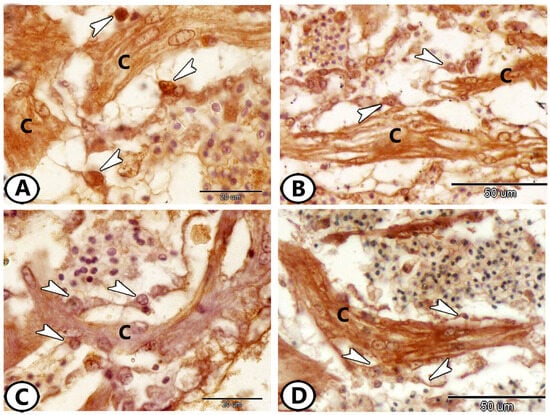
Figure 7.
Immunohistochemistry of the relationship of cardiomyocytes (C) with immune cells (macrophages, arrowheads). (A,B) Stained with Iba1. (C) Stained with Ach. (D) Stained with s100 protein.
4. Discussion
The heart of modern teleosts is traditionally described as consisting of four segments arranged in series: the sinus venosus, atrium, ventricle, and bulbus arteriosus. However, other studies have indicated that in addition to the four chambers, all teleosts also possess a conus arteriosus “situated between the ventricle and the bulbus arteriosus” [29,30] and a distinct atrioventricular segment [31]. The bulbus arteriosus is an elastic chamber that expands during ventricular ejection to store a significant portion of the cardiac stroke volume. In addition, its gradual elastic recovery ensures a steady flow of blood toward the gills to protect the delicate gill vasculature from damage [1].
Interestingly, previous studies have shown that the structure of the bulbus arteriosus is nearly species-specific [32,33,34]. In all studied teleosts, the atrioventricular region is identifiable as a distinct morphological segment situated between the atrium and the ventricle, and the morphological characteristics of this region appear to be species-specific [31]. The atrioventricular region of Molly fish consists of a compact myocardium ring encircled by a connective tissue ring. The myocardium contains vessels in most species with fully trabeculated ventricles [31].
Telocytes (TCs) represent a significant component of the atrium of Molly fish. They are specialized interstitial cells that interact with various cell types and play crucial roles in numerous biological processes across different tissues and organs [35]. Furthermore, they deliver microvesicles and macromolecules, such as RNAs and proteins, to other cells by secreting various types of extracellular vesicles, including exosomes, ectosomes, and multivesicular vesicles [36,37]. TCs have been detected in the gonads, gills, and liver of fish [38]. In silver carp, they establish a network in the dermis of the upper lip and extend to the epidermal cells through connection with fibroblasts [39]. Recently, the communication of TCs with stem cells, myoblasts, and skeletal muscles has been reported in common carp [40]. The expression of the various studied markers suggests that TCs are involved in multiple signaling pathways that contribute to the architecture of the heart.
The results of the present study revealed that Iba1 expression was observed in macrophages, rodlet cells, TCs, and their telopodes of the atrium. Iba1 expression has been detected in the brain and ovaries of Molly fish [41,42]. Iba1, an actin-cross-linking protein, stands out as a recently unveiled EF-hand protein that is exclusively present in cells of monocytic lineages, notably microglia. Its expression extends to macrophages and plays a crucial role in inducing membrane ruffling mediated by the macrophage colony-stimulating factor [43,44,45,46]. Furthermore, researchers have suggested that Iba1 participates in calcium signaling pathways, boosting its status as a pivotal molecule in the mechanisms governing membrane ruffling and phagocytosis within macrophages [44].
The external layer of the bulbus arteriosus, macrophages, lymphocytes of the AV valves, and dendritic-like cells of the atrium express Ach. Moreover, Ach expression was detected in the rodlet cells, TCs, and their telopodes in the atrium, in addition to the myocardial cells of the ventricle. Recently, Ach expression has been observed in the ovaries of Molly fish [42]. It serves as a key receptor in the cholinergic anti-inflammatory pathway and is widely distributed among diverse non-neuronal cells including macrophages, endothelial cells, and dendritic cells [47,48,49,50]. It is well-established that acetylcholine, released from efferent fibers of the vagus nerve, suppresses the secretion of various pro-inflammatory cytokines by engaging with Ach present in diverse immune cells and macrophages. The activation of Ach has been extensively documented to mitigate inflammatory reactions in peripheral tissues and rejuvenate compromised immune cell function [51,52,53]. Furthermore, the inability of the activated cholinergic system to attenuate inflammatory responses has been observed in mice lacking Ach [54].
Interestingly, CD68 expression was observed in atrial macrophages, TCs, and their telopodes. CD68 is a myeloid-specific surface marker extensively expressed in cells of the mononuclear phagocyte lineage, including macrophages and myeloid dendritic cells [55]. Its primary association lies within the endosomal/lysosomal compartment [56], where CD68′s preferential localization within late endosomes suggests its involvement in peptide transport and antigen processing [57]. Moreover, macrophages expressing CD68 are acknowledged as essential components of the foreign body reaction [58].
The present study showed that the atrial granulocytes, rodlet cell capsule, TCs, and their telopodes, in addition to the myocardial cells of the ventricle, expressed S100 proteins. The S100 protein is a crucial player in defense mechanisms across various species, exhibiting both extracellular and intracellular functions. S100 protein expression has been detected in various Molly fish tissues [41,42,59,60], where it plays a fundamental role in pro-inflammatory stimulation and cytoskeleton rearrangement, and it acts as a scavenger for free radicals [61]. Furthermore, S100 proteins are implicated in diverse regulatory mechanisms that govern cellular functions. These processes include cell proliferation and differentiation, apoptosis, invasion, enzyme activation, and energy metabolism [61,62,63]. In Zebrafish, three S100 proteins have been described: S100S, S100T, and S100Z. The expression of S100T has been observed in non-sensory tissues, including the heart [64].
NF-ĸB and Olig-2 expressions have been observed in the atrial rodlet cells cytoplasm and capsules, respectively. NF-κB is a responsive element to inflammatory and immune stimuli, orchestrating a wide array of cellular processes including proliferation, adhesion, invasion, apoptosis, and angiogenesis across multiple cell types [65]. Its expression has been demonstrated in many tissues of Molly fish, including the spleen, pseudobranch, liver, pancreas, ovary, intestinal bulb, and gills [42,59,60,66,67,68,69]. NF-κB signaling within epithelial cells is particularly vital for preserving immune homeostasis in barrier tissues [70]. As a critical transcription factor in the innate immune response, NF-κB regulates the production of numerous pro-inflammatory cytokines and participates in a myriad of signaling pathways [71,72]. Olig-2 is a crucial transcription factor necessary for the specification and differentiation of oligodendrocytes, astrocytes, and neurons during development [73]. Furthermore, the upregulation of Olig-2 in oligodendrocyte progenitor cells enhances their migration and differentiation, resulting in precocious myelination in prenatal mice [74]. In zebrafish, Olig2 is essential for the development of primary motor neurons and oligodendrocytes [75]. In addition, Olig-2 has been identified as an upstream regulator of SOX10, which is another key transcription factor involved in oligodendrocyte development [76].
The myocardial cells of the ventricle exhibited an expression of myostatin and Sox9. Myostatin, a member of the TGF-β superfamily, was initially identified as a potent negative regulator of skeletal muscle growth [77]. Moreover, Sox9, a member of the SOX family, plays a crucial role in regulating cell proliferation and cell fate during embryogenesis with mutations leading to abnormal cell growth [78]. The SOX family as a whole has critical functions in stem cell maintenance, embryonic development, and lineage commitment. Specifically, Sox9 governs stem and progenitor cells in adult tissues [79,80]. Interestingly, myostatin and Sox9 expressions have been extensively observed in various Molly fish tissues [41,42,59,60,66,67,68,69]. A previous study reported the expression of sox9b, one of the two mammalian Sox9 homologs, in the developing zebrafish heart ventricle, where its signaling is essential for epicardium formation. Additionally, zebrafish lacking Sox9b exhibited an elongated heart and pericardial edema, and they failed to form valve cushions and leaflets [81].
The myocardial cells of the ventricle exhibited the expression of Nrf2 and IL-1β. Nrf2 has been reported to play a role in antioxidation, immunopotentiation, and osmoregulation in fish under salinity stress in addition to its established functions in toxicity and oxidative stress [82,83]. It plays a fundamental role in the regulation of cell inflammatory responses and lipid metabolism [84]. Furthermore, many Nrf2-regulated enzymes play a crucial role in the pathogenesis of cardiovascular diseases [85]. Additionally, Nrf2 can prolong graft survival and modulate both innate and adaptive immune responses following heart transplantation [86]. Nrf2 knockout has been reported in mice to induce structural and functional cardiac alterations [84,87]. IL-1β is secreted by activated endothelial cells, tissue macrophages, blood monocytes, activated T lymphocytes, granulocytes, and other cell types [88], where it plays a major role in initiating local and systemic responses to stimuli through the activation of T and B lymphocytes, macrophages, and natural killer cells [89,90]. Previous studies have shown that the expression patterns of IL-1β vary across different organs in various organisms under normal conditions. For example, in sea bream, a lower expression of IL-1β mRNA has been detected in the kidney, gill, spleen, and intestine, while its expression was absent in the liver, heart, and muscles [91,92]. In Molly fish, the expressions of Nrf2 and IL-1β have been detected in most of the studied tissues [41,42,59,60,66,67,69].
The relationship between cardiomyocytes and immune cells has been detected in Molly fish. Previous studies have described the relationship between immune cells and cardiac remodeling and regeneration in zebrafish, in which the activation of immune cells, in particular macrophages and T cells, is essential for successful regeneration [15,16]. Furthermore, a recent study reported that the ablation of macrophage in zebrafish larvae blocks cardiomyocyte proliferation following cardiac injury [93]. In this study, macrophages were detected to synapse with epicardial cells and induce their proliferation.
5. Conclusions
This study investigated the immunohistochemical characteristics and proliferative capacity of cardiomyocytes and immune cells in the hearts of Molly fish. The findings revealed the expression of various studied immunohistochemical markers in myocardial cells and various cardiac cells such as telocytes, rodlet cells, and immune cells. The data presented in this study suggest that telocytes have a potential role in cellular therapies. However, further studies are required to substantiate this hypothesis and explore their therapeutic applications more comprehensively. Furthermore, the study highlights the relationship between cardiomyocytes and innate immune cells in Molly fish, providing insights into potential cardiac regeneration processes. Nevertheless, future studies should be performed to investigate the regenerative capacity of the cardiomyocytes of Molly fish using cell culture.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fishes9070283/s1, Figure S1: (A–C) Negative control sections where primary antibodies including (A) S100 protein, (B) Nrf2, and (C) SOX9, were omitted, and instead, tissue specimens were incubated with buffer.
Author Contributions
Conceptualization, G.Z. and D.M.M.; investigation, A.A., G.C., M.A. (Marco Albano), M.T.H., M.A. (Marialuisa Aragona), E.R.L. and R.K.A.S.; data curation, A.A., G.C., M.A. (Marco Albano), M.T.H., M.A. (Marialuisa Aragona), E.R.L. and R.K.A.S.; writing—original draft preparation, G.Z. and D.M.M.; writing—review and editing, A.A., G.C., M.A. (Marco Albano), M.T.H., M.A. (Marialuisa Aragona), A.G., E.R.L. and R.K.A.S.; visualization, A.A., G.C., M.A. (Marco Albano), M.T.H., M.A. (Marialuisa Aragona), A.G., E.R.L. and R.K.A.S.; supervision, A.A., G.C., M.A. (Marco Albano), M.T.H., M.A. (Marialuisa Aragona), A.G., E.R.L. and R.K.A.S.; project administration, G.Z. and D.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the National Ethical Committee of the Faculty of Veterinary Medicine, Assiut University, Egypt. The Ethical Number is 04/0015-aun/vet.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study are available from the corresponding author, upon responsible request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Farrell, A.P.; Jones, D.R. The heart. In Fish physiology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 40, pp. 91–172. [Google Scholar]
- Buzete Gardinal, M.V.; Rocha Ruiz, T.F.; Estevan Moron, S.; Oba Yoshioka, E.T.; Uribe Gonçalves, L.; Franceschini Vicentini, I.B.; Vicentini, C.A. Heart structure in the Amazonian teleost Arapaima gigas (Osteoglossiformes, Arapaimidae). J. Anat. 2019, 234, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Icardo, J. The teleost heart: A morphological approach. In Ontogeny and Phylogeny of the Vertebrate Heart; Sedmera, D., Wang, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Zaccone, G.; de Oliveira Fernandes, J.; Icardo, J.M.; Guerrera, M.C.; Capillo, G.; Alesci, A. Control of cardiovascular function. In Encyclopedia of Fish Physiology, Alderman, S.L., Gillis, T.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 87–99. [Google Scholar]
- Sherrill, J.; Weber III, E.S.; Marty, G.D.; Hernandez-Divers, S. Fish cardiovascular physiology and disease. Vet. Clin. North Am. Exot. Anim. Pract. 2009, 12, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Kimoto, M.; Hanashima, A.; Hashimoto, K.; Mohri, S. Cardiac hemodynamics and ventricular stiffness of sea-run cherry salmon (Oncorhynchus masou masou) differ critically from those of landlocked masu salmon. PLoS ONE 2022, 17, e0267264. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.G.; Santer, R.; Benjamin, M.; Norman, D. Heart structure of some deep-sea fish (Teleostei: Macrouridae). J. Zool. 1985, 205, 75–89. [Google Scholar] [CrossRef]
- Potts, H.G.; Stockdale, W.T.; Mommersteeg, M.T. Unlocking the secrets of the regenerating fish heart: Comparing regenerative models to shed light on successful regeneration. J. Cardiovasc. Dev. Dis. 2021, 8, 4. [Google Scholar] [CrossRef]
- Matrone, G.; Tucker, C.S.; Denvir, M.A. Cardiomyocyte proliferation in zebrafish and mammals: Lessons for human disease. Cell. Mol. Life Sci. 2017, 74, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Belmonte, J.C.I. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef]
- González-Rosa, J.M.; Martín, V.; Peralta, M.; Torres, M.; Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 2011, 138, 1663–1674. [Google Scholar] [CrossRef]
- González-Rosa, J.M.; Burns, C.E.; Burns, C.G. Zebrafish heart regeneration: 15 years of discoveries. Regeneration 2017, 4, 105–123. [Google Scholar] [CrossRef]
- Bevan, L.; Lim, Z.W.; Venkatesh, B.; Riley, P.R.; Martin, P.; Richardson, R.J. Specific macrophage populations promote both cardiac scar deposition and subsequent resolution in adult zebrafish. Cardiovasc. Res. 2020, 116, 1357–1371. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.P.; Sheng, D.Z.; Sugimoto, K.; Gonzalez-Rajal, A.; Nakagawa, S.; Hesselson, D.; Kikuchi, K. Zebrafish regulatory T cells mediate organ-specific regenerative programs. Dev. Cell 2017, 43, 659–672.e655. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Morejon, A.; Garcia-Redondo, A.B.; Reuter, H.; Marques, I.J.; Bates, T.; Galardi-Castilla, M.; Große, A.; Manig, S.; Langa, X.; Ernst, A. Wilms tumor 1b expression defines a pro-regenerative macrophage subtype and is required for organ regeneration in the zebrafish. Cell Rep. 2019, 28, 1296–1306.e1296. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Lelek, S.; Spanjaard, B.; El-Sammak, H.; Simões, M.G.; Mintcheva, J.; Aliee, H.; Schäfer, R.; Meyer, A.M.; Theis, F. Origin and function of activated fibroblast states during zebrafish heart regeneration. Nat. Genet. 2022, 54, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Holdway, J.E.; Major, R.J.; Blum, N.; Dahn, R.D.; Begemann, G.; Poss, K.D. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 2011, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.; Karra, R.; Dickson, A.L.; Poss, K.D. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife 2015, 4, e05871. [Google Scholar] [CrossRef]
- Ito, K.; Morioka, M.; Kimura, S.; Tasaki, M.; Inohaya, K.; Kudo, A. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev. Dyn. 2014, 243, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, W.T.; Lemieux, M.E.; Killen, A.C.; Zhao, J.; Hu, Z.; Riepsaame, J.; Hamilton, N.; Kudoh, T.; Riley, P.R.; van Aerle, R. Heart regeneration in the Mexican cavefish. Cell Rep. 2018, 25, 1997–2007.e1997. [Google Scholar] [CrossRef]
- Long, D.W.; Webb IV, C.H.; Wang, Y. Persistent fibrosis and decreased cardiac function following cardiac injury in the Ctenopharyngodon idella (grass carp). Anat. Rec. 2022, 305, 66–80. [Google Scholar] [CrossRef]
- Grivas, J.; Haag, M.; Johnson, A.; Manalo, T.; Roell, J.; Das, T.L.; Brown, E.; Burns, A.R.; Lafontant, P.J. Cardiac repair and regenerative potential in the goldfish (Carassius auratus) heart. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 163, 14–23. [Google Scholar] [CrossRef]
- Meffe, G.K.; Snelson, F. An ecological overview of poeciliid fishes. Ecol. Evol. Livebearing Fishes (Poeciliidae) 1989, 12, 13–31. [Google Scholar]
- Schartl, M. Beyond the zebrafish: Diverse fish species for modeling human disease. Dis. Models Mech. 2014, 7, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Márquez, J.L.; Peña-Mendoza, B.; Guzmán-Santiago, J.L. Reproductive biology of Poecilia sphenops valenciennes, 1846 (Cyprinidontiformes: Poeciliidae) at the Emiliano Zapata reservoir in Morelos, Mexico. Neotrop. Ichthyol. 2016, 14, e140127. [Google Scholar] [CrossRef]
- Zutshi, B.; Singh, A. Interrelationship of photoperiod and feed utilization on growth and reproductive performance in the Red eyed orange molly (Poecilia sphenops). BioRxiv 2017, 209346. [Google Scholar]
- Hussein, M.T.; Sayed, R.K.; Mokhtar, D.M. Neuron mapping in the Molly fish optic tectum: An emphasis on the adult neurogenesis process. Microsc. Res. Tech. 2024. [Google Scholar] [CrossRef] [PubMed]
- Grimes, A.C.; Stadt, H.A.; Shepherd, I.T.; Kirby, M.L. Solving an enigma: Arterial pole development in the zebrafish heart. Dev. Biol. 2006, 290, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Icardo, J.M. Conus arteriosus of the teleost heart: Dismissed, but not missed. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. Off. Publ. Am. Assoc. Anat. 2006, 288, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Icardo, J.M.; Colvee, E. The atrioventricular region of the teleost heart. A distinct heart segment. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2011, 294, 236–242. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Bulbus arteriosus of the Antarctic teleosts. II. The red-blooded Trematomus bernacchii. Anat. Rec. 1999, 256, 116–126. [Google Scholar] [CrossRef]
- Icardo, J.; Colvee, E.; Cerra, M.C.; Tota, B. The bulbus arteriosus of stenothermal and temperate teleosts: A morphological approach. J. Fish Biol. 2000, 57, 121–135. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Light and electron microscopy of the bulbus arteriosus of the European eel (Anguilla anguilla). Cells Tissues Organs 2000, 167, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Danisovic, L.; Kyselovic, J.; Gazova, A.; Musil, P.; Miko, M.; Polak, S. The functional morphology and role of cardiac telocytes in myocardium regeneration. Can. J. Physiol. Pharmacol. 2016, 94, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.; Gherghiceanu, M.; Cretoiu, D.; Radu, E. The connective connection: Interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells.: Electron microscope study in sity. J. Cell. Mol. Med. 2005, 9, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.; Faussone-Pellegrini, M.S. TELOCYTES—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell. Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M. Fish histology: From cells to organs; Apple Academic Press: Palm Bay, FL, USA, 2021. [Google Scholar]
- Sayed, R.K.; Abd-El Aziz, N.A.; Ibrahim, I.A.; Mokhtar, D.M. Structural, ultrastructural, and functional aspects of the skin of the upper lip of silver carp (Hypophthalmichthys molitrix). Microsc. Res. Tech. 2021, 84, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Massoud, D.; Abd-Elhafeez, H.H.; Emeish, W.F.; Fouda, M.; Shaldoum, F.; Alrashdi, B.M.; Hassan, M.; Soliman, S.A. A transmission electron microscopy investigation suggests that telocytes, skeletal muscles, myoblasts, and stem cells in common carp (Cyprinus carpio) respond to salinity challenges. BMC Vet. Res. 2024, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Sayed, R.K.; Zaccone, G.; Albano, M.; Hussein, M.T. Ependymal and neural stem cells of adult Molly fish (Poecilia sphenops, Valenciennes, 1846) brain: Histomorphometry, Immunohistochemical, and ultrastructural studies. Cells 2022, 11, 2659. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.; Mokhtar, D.M.; Hashim, M.A.; Aly, A.S.; Zaccone, G.; Albano, M.; Alesci, A.; Abdellah, N. Immune Cell Profiling in the Ovarian Stroma of a Viviparous Fish during the Breeding Season: A Histological and Immunohistochemical Investigation. Fishes 2023, 9, 10. [Google Scholar] [CrossRef]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel geneiba1in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Kanazawa, H.; Sasaki, Y.; Kohsaka, S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J. Cell Sci. 2000, 113, 3073–3084. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ohsawa, K.; Kanazawa, H.; Kohsaka, S.; Imai, Y. Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem. Biophys. Res. Commun. 2001, 286, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Kumon, Y.; Watanabe, H.; Ohnishi, T.; Shudou, M.; Chuai, M.; Imai, Y.; Takahashi, H.; Tanaka, J. Accumulation of macrophage-like cells expressing NG2 proteoglycan and Iba1 in ischemic core of rat brain after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2008, 28, 149–163. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Li, J.; Mathieu, S.L.; Harris, R.; Ji, J.; Anderson, D.J.; Malysz, J.; Bunnelle, W.H.; Waring, J.F.; Marsh, K.C.; Murtaza, A. Role of α7 nicotinic acetylcholine receptors in regulating tumor necrosis factor-α (TNF-α) as revealed by subtype selective agonists. J. Neuroimmunol. 2011, 239, 37–43. [Google Scholar] [CrossRef]
- Chao, R.; Tong, Y.-L.; Li, J.-C.; Lu, Z.-Q.; Yao, Y.-M. The protective effect of alpha 7 nicotinic acetylcholine receptor activation on critical illness and its mechanism. Int. J. Biol. Sci. 2017, 13, 46. [Google Scholar]
- Zaccone, G.; Alesci, A.; Mokhtar, D.M.; Aragona, M.; Guerrera, M.C.; Capillo, G.; Albano, M.; de Oliveira Fernandes, J.; Kiron, V.; Sayed, R.K. Localization of acetylcholine, alpha 7-nAChR and the antimicrobial peptide piscidin 1 in the macrophages of fish gut: Evidence for a cholinergic system, diverse macrophage populations and polarization of immune responses. Fishes 2023, 8, 43. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yuan, R.; Rosas-Ballina, M.; Ashok, M.; Goldstein, R.S.; Chavan, S.; Pavlov, V.A. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 2007, 35, 2762–2768. [Google Scholar] [PubMed]
- Pavlov, V.A.; Ochani, M.; Yang, L.-H.; Gallowitsch-Puerta, M.; Ochani, K.; Lin, X.; Levi, J.; Parrish, W.R.; Rosas-Ballina, M.; Czura, C.J. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007, 35, 1139–1144. [Google Scholar] [CrossRef]
- van Maanen, M.A.; Stoof, S.P.; LaRosa, G.J.; Vervoordeldonk, M.J.; Tak, P.P. Role of the cholinergic nervous system in rheumatoid arthritis: Aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann. Rheum. Dis. 2010, 69, 1717–1723. [Google Scholar] [CrossRef]
- Betjes, M.G.; Haks, M.C.; Tuk, C.W.; Beelen, R.H. Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-term culture. Immunobiology 1991, 183, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Barois, N.; De Saint-Vis, B.; Lebecque, S.; Geuze, H.J.; Kleijmeer, M.J. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic 2002, 3, 894–905. [Google Scholar] [CrossRef]
- Klinge, U.; Dievernich, A.; Tolba, R.; Klosterhalfen, B.; Davies, L. CD68+ macrophages as crucial components of the foreign body reaction demonstrate an unconventional pattern of functional markers quantified by analysis with double fluorescence staining. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3134–3146. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Sayed, R.K.; Mokhtar, D.M. Structural and immunohistochemical characterization of pancreas of Molly fish (Poecilia sphenops), with a special reference to its immune role. Microsc. Res. Tech. 2023, 86, 1667–1680. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Hussein, M.M.; Zaccone, G.; Alesci, A.; Lauriano, E.R.; Sayed, R.K. Gills of molly fish: A potential role in neuro-immune interaction. Fishes 2023, 8, 195. [Google Scholar] [CrossRef]
- Singh, P.; Ali, S.A. Multifunctional role of S100 protein family in the immune system: An update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef]
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Q.; Guo, F.; Chen, M.; Tao, X.; Dong, D. S100 proteins in pancreatic cancer: Current knowledge and future perspectives. Front. Oncol. 2021, 11, 711180. [Google Scholar] [CrossRef]
- Kraemer, A.M.; Saraiva, L.R.; Korsching, S.I. Structural and functional diversification in the teleost S100 family of calcium-binding proteins. BMC Evol. Biol. 2008, 8, 48. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.-P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.; Zaccone, G.; Capillo, G.; Albano, M.; Mokhtar, D.M. Structural and functional aspects of the spleen in molly fish Poecilia sphenops (Valenciennes, 1846): Synergistic interactions of stem cells, neurons, and immune cells. Biology 2022, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Hussein, M.M.; Sayed, R.K. Novel identification and microscopy of the intestinal bulb of molly fish (Poecilia sphenops) with a focus on its role in immunity. Microsc. Microanal. 2022, 28, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Sayed, R.K.; Zaccone, G.; Alesci, A.; Hussein, M.M. The potential role of the pseudobranch of molly fish (Poecilia sphenops) in immunity and cell regeneration. Sci. Rep. 2023, 13, 8665. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Sayed, R.K.; Mokhtar, D.M. Structural and immunohistochemical analysis of the cellular compositions of the liver of molly fish (Poecilia sphenops), focusing on its immune role. Zool. Lett. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M. Role of NF-κB in epithelial biology. Immunol. Rev. 2012, 246, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Correa, R.G.; Tergaonkar, V.; Ng, J.K.; Dubova, I.; Izpisua-Belmonte, J.C.; Verma, I.M. Characterization of NF-κΒ/IκΒ proteins in zebra fish and their involvement in notochord development. Mol. Cell. Biol. 2004, 24, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, L.; Lai, C.; Hou, K.; Chen, J.; Guo, Y.; Sambangi, A.; Swaminathan, S.; Xie, C.; Wu, Z. Region-specific distribution of Olig2-expressing astrocytes in adult mouse brain and spinal cord. Mol. Brain 2021, 14, 36. [Google Scholar] [CrossRef]
- Wegener, A.; Deboux, C.; Bachelin, C.; Frah, M.; Kerninon, C.; Seilhean, D.; Weider, M.; Wegner, M.; Nait-Oumesmar, B. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain 2015, 138, 120–135. [Google Scholar] [CrossRef]
- Park, H.-C.; Mehta, A.; Richardson, J.S.; Appel, B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. 2002, 248, 356–368. [Google Scholar] [CrossRef]
- Küspert, M.; Hammer, A.; Bösl, M.R.; Wegner, M. Olig2 regulates Sox10 expression in oligodendrocyte precursors through an evolutionary conserved distal enhancer. Nucleic Acids Res. 2011, 39, 1280–1293. [Google Scholar] [CrossRef]
- Rodgers, B.D.; Weber, G.M.; Sullivan, C.V.; Levine, M.A. Isolation and characterization of myostatin complementary deoxyribonucleic acid clones from two commercially important fish: Oreochromis mossambicus and Morone chrysops. Endocrinology 2001, 142, 1412–1418. [Google Scholar] [CrossRef][Green Version]
- Pritchett, J.; Athwal, V.; Roberts, N.; Hanley, N.A.; Hanley, K.P. Understanding the role of SOX9 in acquired diseases: Lessons from development. Trends Mol. Med. 2011, 17, 166–174. [Google Scholar] [CrossRef]
- Sarkar, A.; Hochedlinger, K. The sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell stem cell 2013, 12, 15–30. [Google Scholar] [CrossRef]
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014, 1, 149–161. [Google Scholar] [CrossRef]
- Hofsteen, P.; Plavicki, J.; Johnson, S.D.; Peterson, R.E.; Heideman, W. Sox9b is required for epicardium formation and plays a role in TCDD-induced heart malformation in zebrafish. Mol. Pharmacol. 2013, 84, 353–360. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Z. Nrf2 is involved in osmoregulation, antioxidation and immunopotentiation in Coilia nasus under salinity stress. Biotechnol. Biotechnol. Equip. 2019, 33, 1453–1463. [Google Scholar] [CrossRef][Green Version]
- Gutiérrez-Cuevas, J.; Galicia-Moreno, M.; Monroy-Ramírez, H.C.; Sandoval-Rodriguez, A.; García-Bañuelos, J.; Santos, A.; Armendariz-Borunda, J. The role of NRF2 in obesity-associated cardiovascular risk factors. Antioxidants 2022, 11, 235. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qiu, Q.; Wang, H.; Whitman, S.A.; Fang, D.; Lian, F.; Zhang, D.D. Nrf2 is crucial to graft survival in a rodent model of heart transplantation. Oxidative Med. Cell. Longev. 2013, 2013, 919313. [Google Scholar] [CrossRef][Green Version]
- Erkens, R.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Krause, L.; Weidenbach, M.; Dirzka, J.; Krenz, T.; Mergia, E.; Suvorava, T. Left ventricular diastolic dysfunction in Nrf2 knock out mice is associated with cardiac hypertrophy, decreased expression of SERCA2a, and preserved endothelial function. Free. Radic. Biol. Med. 2015, 89, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-y.; Nie, L.; Zhu, G.; Xiang, L.-x.; Shao, J.-z. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013, 39, 39–62. [Google Scholar] [CrossRef]
- Netea, M.G.; Simon, A.; van de Veerdonk, F.; Kullberg, B.-J.; Van der Meer, J.W.; Joosten, L.A. IL-1β processing in host defense: Beyond the inflammasomes. PLoS Pathog. 2010, 6, e1000661. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, D.; Li, J.; Liu, Z. Molecular characterization, recombinant expression and bioactivity analysis of the interleukin-1β from the yellowfin sea bream, Acanthopagrus latus (Houttuyn). Fish Shellfish. Immunol. 2008, 24, 323–336. [Google Scholar] [CrossRef]
- Lu, X.-J.; Chen, J.; He, Y.-Q.; Shi, Y.-H. Molecular characterization of an IL-1β gene from ayu, Plecoglossus altivelis. Fish Shellfish. Immunol. 2013, 34, 1253–1259. [Google Scholar] [CrossRef]
- Bruton, F.A.; Kaveh, A.; Ross-Stewart, K.M.; Matrone, G.; Oremek, M.E.; Solomonidis, E.G.; Tucker, C.S.; Mullins, J.J.; Lucas, C.D.; Brittan, M. Macrophages trigger cardiomyocyte proliferation by increasing epicardial vegfaa expression during larval zebrafish heart regeneration. Dev. Cell 2022, 57, 1512–1528.e1515. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).