The Effects of Water Flow Speed on Swimming Capacity and Energy Metabolism in Adult Amur Grayling (Thymallus grubii)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
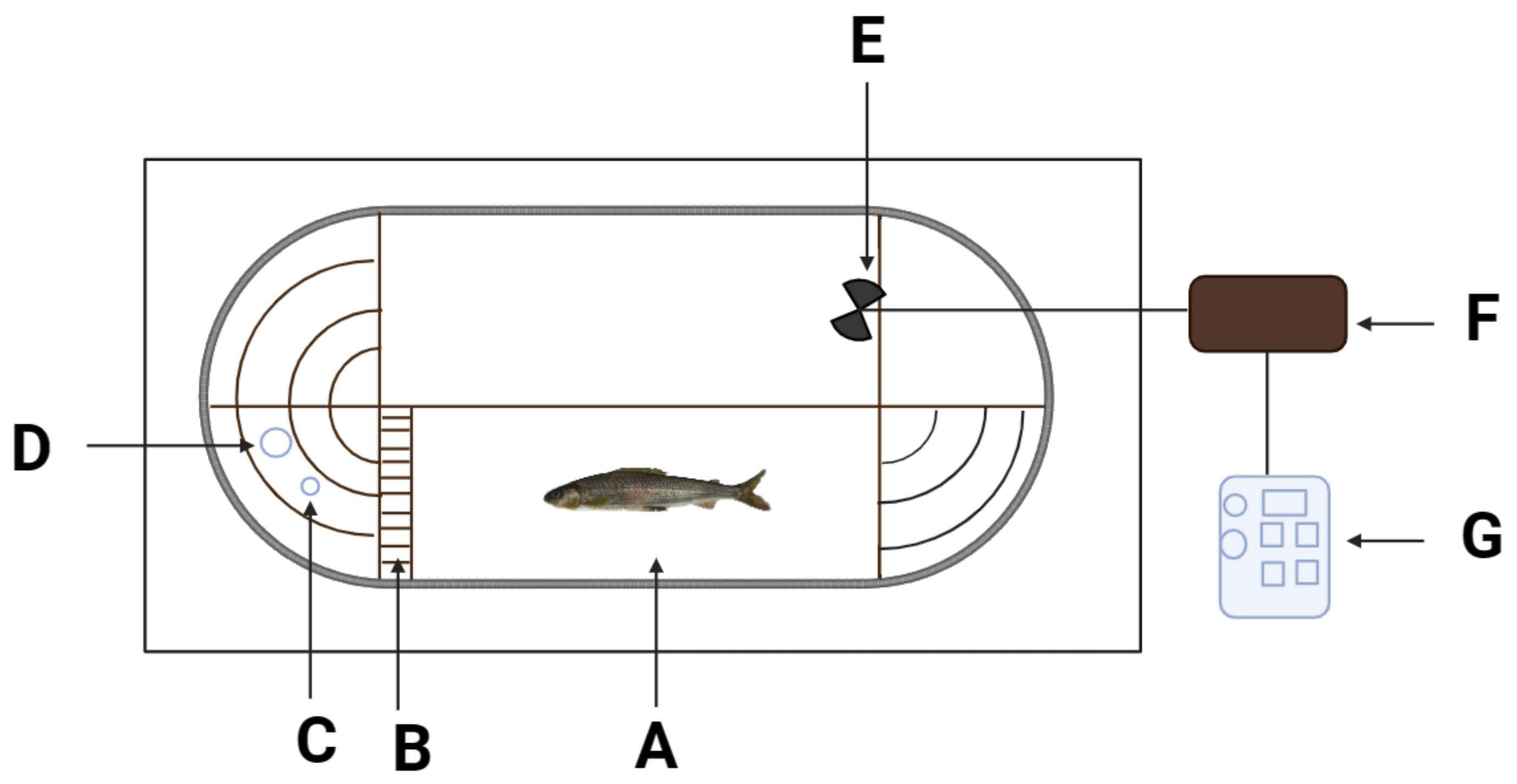
2.2. Experimental Facility
2.3. Measure for Swimming Speed and Oxygen Consumption
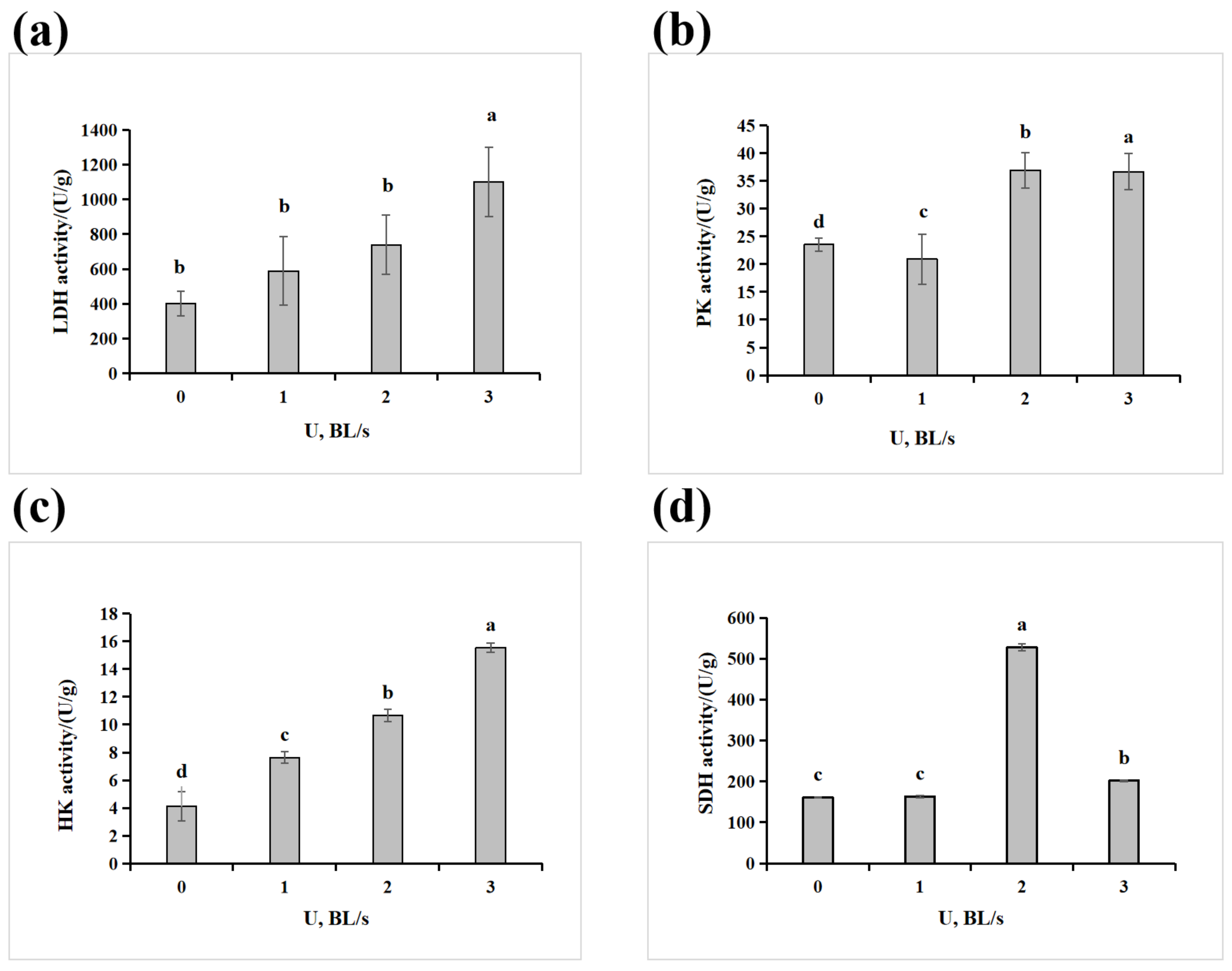
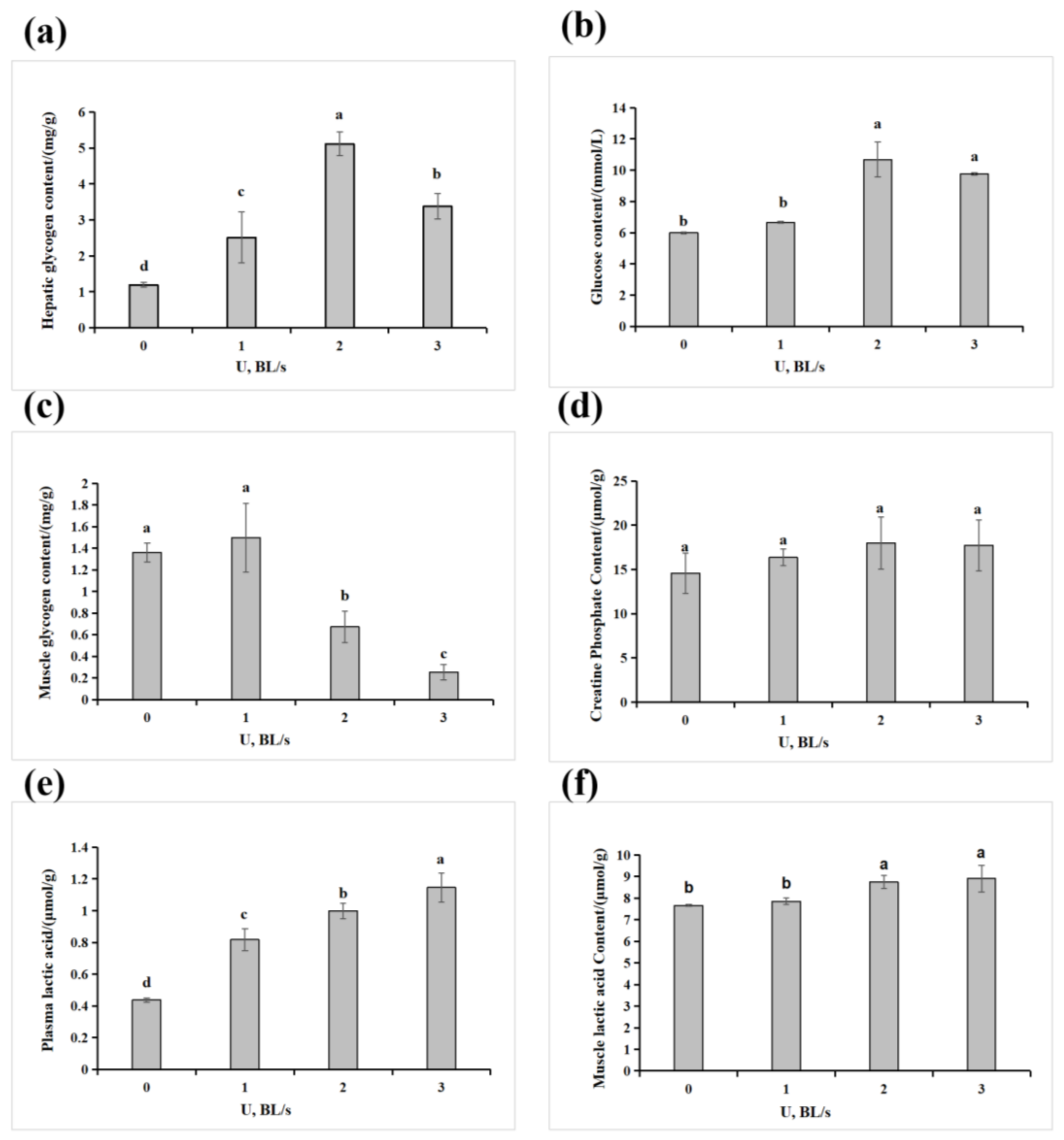
2.4. Glucose Metabolism Enzyme Activity and Energy Metabolism Substance Content Determination
2.5. Statistical Analysis
3. Results
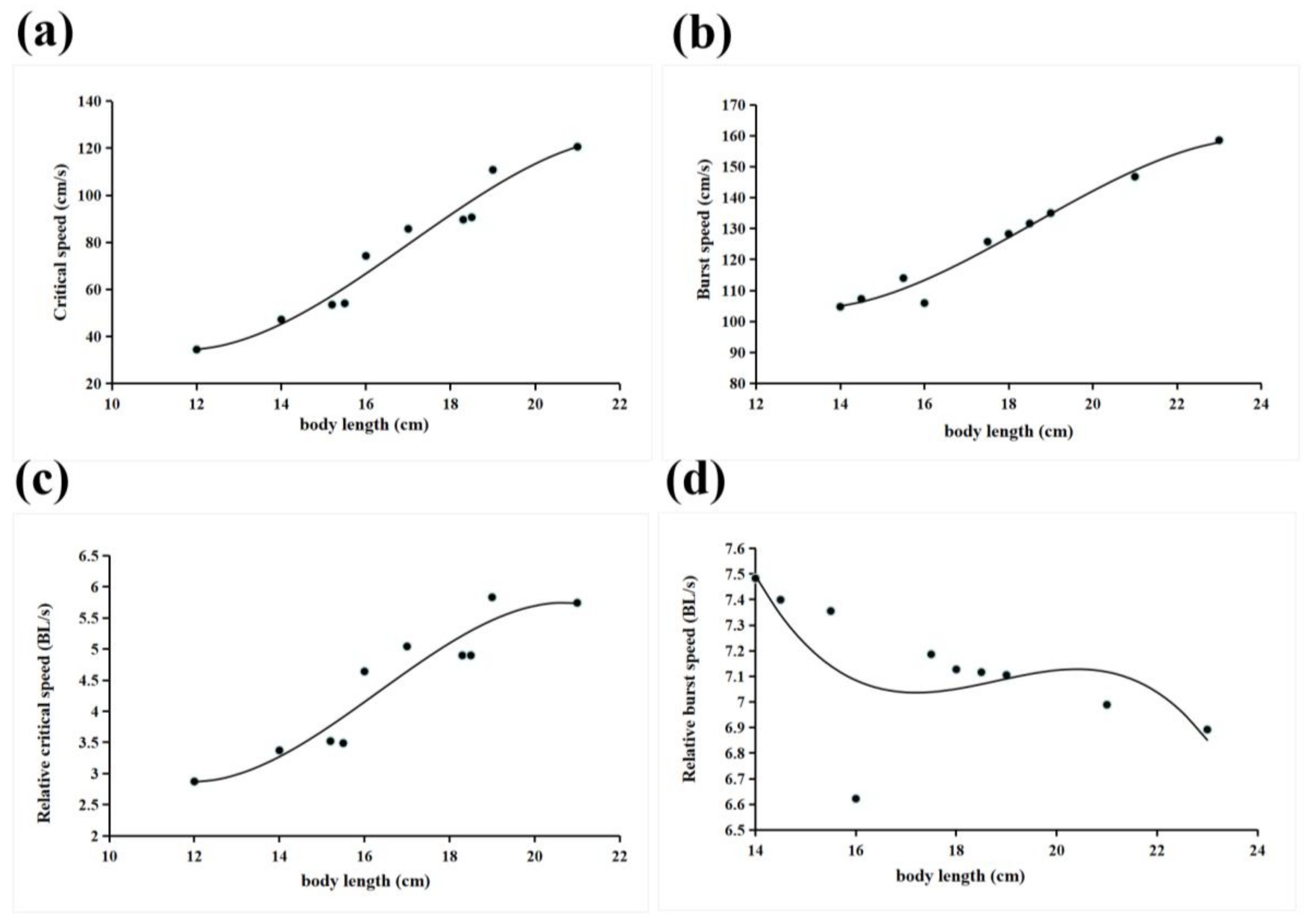
3.1. Critical and Burst Swimming Speed
3.2. Effect of Swimming Velocity on the Oxygen Consumption Rate of the Amur Grayling
3.3. Effect of Velocity on the Physiological and Biochemical Characteristics of the Amur Grayling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cano-Barbacil, C.; Radinger, J.; Argudo, M.; Rubio-Gracia, F.; Vila-Gispert, A.; García-Berthou, E. Key factors explaining critical swimming speed in freshwater fish: A review and statistical analysis for Iberian species. Sci. Rep. 2020, 10, 18947. [Google Scholar] [CrossRef] [PubMed]
- Downie, A.T.; Illing, B.; Faria, A.M.; Rummer, J.L. Swimming performance of marine fish larvae: Review of a universal trait under ecological and environmental pressure. Rev. Fish Biol. Fish. 2020, 30, 93–108. [Google Scholar] [CrossRef]
- Knaepkens, G.; Bruyndoncx, L.; Eens, M. Assessment of residency and movement of the endangered bullhead (Cottus gobio) in two Flemish rivers. Ecol. Freshw. Fish. 2004, 13, 317–322. [Google Scholar] [CrossRef]
- Tudorache, C.; Viaene, P.; Blust, R.; Vereecken, H.; De Boeck, G. A comparison of swimming capacity and energy use in seven European freshwater fish species. Ecol. Freshw. Fish. 2008, 17, 284–291. [Google Scholar] [CrossRef]
- Brett, J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish Res. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Hammer, C. Fatigue and exercise tests with fish. Comp. Biochem. Physiol. A 1995, 112, 1–20. [Google Scholar] [CrossRef]
- Yanase, K.; Eayrs, S.; Arimoto, T. Influence of water temperature and fish length on the maximum swimming speed of sand flathead, Platycephalus bassensis: Implications for trawl selectivity. Fish. Res. 2007, 84, 180–188. [Google Scholar] [CrossRef]
- Blaxter, J.H.S. Swimming speeds of fish. In Proceedings of the FAO Conference on Fish Behavior in Relation to Fishing Techniques and Tactics, Bergen, Norway, 19–27 October 1967. [Google Scholar]
- Pavlov, D.S. Structures Assisting the Migrations of Non-Salmonid Fish: USSR; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1989; Volume 308, pp. 1–97. [Google Scholar]
- Beamish, F.W.H. Swimming capacity. In Fish Physiology, VII; Hoar, W.S., Randall, D.J., Eds.; Academic Press: New York, NY, USA, 1978; pp. 101–187. [Google Scholar]
- Vodianitskyi, O.M.; Potrokhov, O.S.; Zinkovskyi, O.G.; Khudiiash, Y.M.; Prychepa, M.V. Effects of increasing water temperature and decreasing water oxygen concentration on enzyme activity in developing carp embryos (Cyprinus carpio). Fish. Aquat. Life 2021, 29, 35–44. [Google Scholar] [CrossRef]
- Sims, D.W. The effect of body size on the standard metabolic rate of the lesser spotted dogfish. J. Fish Biol. 1996, 48, 542–544. [Google Scholar] [CrossRef]
- Caputo, F.; Oliveira, M.F.M.d.; Denadai, B.S.; Greco, C.C. Fatores intrínsecos do custo energético da locomoção durante a natação. Rev. Bras. Med. Esporte. 2006, 12, 399–404. [Google Scholar] [CrossRef][Green Version]
- Guo, T.T.; Yang, Y.; Meng, F.X.; Wang, S.D.; Xia, S.L.; Qian, Y.X.; Li, M.; Wang, R.X. Effects of low salinity on gill and liver glycogen metabolism of great blue-spotted mudskippers (Boleophthalmus pectinirostris). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 230, 108709. [Google Scholar] [CrossRef] [PubMed]
- Dyldin, Y.; Hanel, L.; Romanov, V.; Plesnik, J. A review of the genus thymallus (pisces: Salmoniformes, salmonidae, thymallinae) with taxonomic notes. Bull. Lampetra 2017, 8, 103–126. [Google Scholar]
- Nelson, J.S. Fishes of the World; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; 601p. [Google Scholar]
- Junge, C.; Vøllestad, L.; Barson, N.; Haugen, T.O.; Otero, J.; Sætre, G.-P.; Leder, E.H.; Primmer, C.R. Strong gene flow and lack of stable population structure in the face of rapid adaptation to local temperature in a spring-spawning salmonid, the European grayling (Thymallus thymallus). Heredity 2011, 106, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.C.; Zhang, L.N.; Lv, X.N.; Kuang, Y.Y.; Tong, G.X.; Xue, S.Q.; Han, Y.; Yin, J.S. Development of 21 Microsatellite Loci and Diversity Analysis of Amur Grayling in Amur River. Thalassas 2020, 36, 165–170. [Google Scholar] [CrossRef]
- Liu, L.W.; Luo, Y.L.; Liang, X.F.; Ma, B.; Song, D. Occurrence of Amur grayling (Thymallus grubii grubii Dybowski, 1869) in the Amur River. J. Appl. Ichthyol. 2013, 29, 666–667. [Google Scholar] [CrossRef]
- Wu, H.P.; Chen, J.; Xu, J.J.; Zeng, G.M.; Sang, L.H.; Liu, Q.; Yin, Z.J.; Dai, J.; Yin, D.C.; Lang, J.; et al. Effects of dam construction on biodiversity: A review. J. Clean. Prod. 2019, 221, 480–489. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, G.M.; Wang, Q.S. China Red Data Book of Endangered Animals; Science Press: Beijing, China, 1998; pp. 204–206. [Google Scholar]
- Ma, B.; Lui, T.T.; Zhang, Y.; Chen, J.P. Phylogeography and Population Genetic Structure of Amur grayling Thymallus grubii in the Amur Basin. Anim. Biosci. 2012, 25, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J.; Gonçalves, D.V.; Secci-Petretto, G.; Englmaier, G.K.; Gomes-Dos-Santos, A.; Denys, G.P.J.; Persat, H.; Antonov, A.; Hahn, C.; Taylor, E.B.; et al. Global systematic diversity, range distributions, conservation and taxonomic assessments of graylings (Teleostei: Salmonidae; Thymallus spp.). Org. Divers. Evol. 2021, 21, 25–42. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.H.; Johnson, D.; Tu, Z.Y.; Huang, Y.P. Effect of body length on swimming capability and vertical slot fishway design. Glob. Ecol. Conserv. 2020, 22, e00990. [Google Scholar] [CrossRef]
- Huang, Y.P.; Malik, A.H.; Tu, Z.Y.; Johnson, D.; Li, W.M.; Yuan, X. Effect of repeated exercise fatigue on the swimming performance of juvenile rock carp (Procypris rabaudi, Tchang). J. Appl. Ichthyol. 2020, 36, 811–816. [Google Scholar] [CrossRef]
- Li, X.M.; Pang, X.; Zheng, H.; Li, X.J.; Fu, S.J.; Zhang, Y.G. Effects of prolonged exercise training and exhaustive chasing training on the swimming performance of an endangered bream Megalobrama pellegrini. Aquat. Biol. 2017, 26, 125–135. [Google Scholar] [CrossRef]
- Peake, S.J.; Beamish, F.W.; McKinley, R.S.; Scruton, D.A.; Katopodis, C. Relating swimming performance of lake sturgeon, Acipenser fulvescens, to fishway desing. Can. J. Fish Aquat. Sci. 1997, 54, 1361–1366. [Google Scholar] [CrossRef]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenolinduced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Granchi, C.; Bertini, S.; Macchia, M.; Minutolo, F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr. Med. Chem. 2010, 17, 672–697. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, T.T.; Agudo, J.P.; Mosquera, L.P.; Gonz’alez, E.P. Evaluating vertical-slot fishway designs in terms of fish swimming capabilities. Ecol. Eng. 2006, 27, 37–48. [Google Scholar] [CrossRef]
- Warren, M.L.; Burr, B.M.; Walsh, S.J.; Bart, H.L.; Cashner, R.C.; Etnier, D.A.; Freeman, B.J.; Kuhajda, B.R.; Mayden, R.L.; Robison, H.W.; et al. Diversity, distribution, and conservation status of the native freshwater fishes of the southern United States. Fisheries 2000, 25, 7–31. [Google Scholar] [CrossRef]
- Noonan, M.J.; Grant, J.W.A.; Jackson, C.D. A quantitative assessment of fish passage efficiency. Fish Fish. 2012, 13, 450–464. [Google Scholar] [CrossRef]
- Braaten, P.J.; Elliott, C.M.; Rhoten, J.C.; Fuller, D.B.; McElroy, B.J. Migrations and swimming capabilities of endangered pallid sturgeon (Scaphirhynchus albus) to guide passage designs in the fragmented Yellowstone River. Restor. Ecol. 2015, 23, 186–195. [Google Scholar] [CrossRef]
- Katopodis, C.; Gervais, R. Fish Swimming Performance Database and Analyses; Canadian Science Advisory Secretariat, Fisheries and Oceans Canada: Ottawa, ON, Canada, 2016; 550p. [Google Scholar]
- Plaut, I. Critical swimming speed: Its ecological relevance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 131, 41–50. [Google Scholar] [CrossRef]
- Cooke, S.J.; Cech, J.J.; Glassman, D.M.; Simard, J.; Louttit, S.; Lennox, R.J.; Cruz-Font, L.; O’Connor, C.M. Water resource development and sturgeon (Acipenseridae): State of the science and research gaps related to fish passage, entrainment, impingement and behavioural guidance. Rev. Fish Biol. Fish. 2020, 30, 219–244. [Google Scholar] [CrossRef]
- Poletto, J.B.; Cocherell, D.E.; Ho, N.; Cech, J.J.J.; Klimley, A.P.; Fangue, N.A. The effect of size on juvenile green sturgeon (Acipenser medirostris) behavior near water-diversion fish screens. Environ. Biol. Fish. 2018, 101, 67–77. [Google Scholar] [CrossRef]
- Tan, J.; Li, H.; Guo, W.; Tan, H.; Ke, S.; Wang, J.; Shi, X. Swimming Performance of Four Carps on the Yangtze River for Fish Passage Design. Sustainability 2021, 13, 1575. [Google Scholar] [CrossRef]
- Katopodis, C.; Cai, L.; Johnson, D.M. Sturgeon survival: The role of swimming performance and fish passage research. Fish. Res. 2019, 212, 162–171. [Google Scholar] [CrossRef]
- Cai, L.; Taupier, R.; Johnson, D.; Tu, Z.Y.; Liu, G.Y.; Huang, Y.P. Swimming capability and swimming behavior of juvenile Acipenser schrenckii. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2013, 319, 149–155. [Google Scholar] [CrossRef]
- Gallaugher, P.E.; Thorarensen, H.; Kiessling, A.; Farrell, A.P. Effects of high intensity exercise training on cardiovascular function, oxygen uptake, internal oxygen transport and osmotic balance in chinook salmon (Oncorhynchus tshawytscha) during critical speed swimming. J. Exp. Biol. 2001, 204 Pt 16, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- Wardle, C.S.; Soofiani, N.M.; O’Neill, F.G.; Glass, C.W.; Johnstone, A.D.F. Measurements of aerobic metabolism of a school of horse mackerel at different swimming speeds. J. Fish Biol. 1996, 49, 854–862. [Google Scholar] [CrossRef]
- Norin, T.; Canada, P.; Bailey, J.A.; Gamperl, A.K. Thermal biology and swimming performance of Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus). PeerJ 2019, 7, e7784. [Google Scholar] [CrossRef] [PubMed]
- Peake, S.J. Swimming and respiration. Sturgeons Paddlefish N. Am. 2005, 27, 147–166. [Google Scholar]
- Liu, H.; Yin, X.A.; Qiu, X.T.; Qin, J.L.; Yang, W.; Zhang, J. Coupled influence of flow velocity and water temperature on grass carp swimming behavior and gonad development. Hydrol. Process. 2021, 35, e14052. [Google Scholar] [CrossRef]
- Christensen, E.A.F.; Svendsen, M.B.S.; Steffensen, J.F. The combined effect of body size and temperature on oxygen consumption rates and the size-dependency of preferred temperature in European perch Perca fluviatilis. J. Fish Biol. 2020, 97, 794–803. [Google Scholar] [CrossRef]
- Claireaux, G.; Couturier, C.; Groison, A. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 2006, 209, 3420–3428. [Google Scholar] [CrossRef]
- Brown, D.R.; Thompson, J.; Chernick, M.; Hinton, D.E.; Di Giulio, R.T. Later life swimming performance and persistent heart damage following subteratogenic PAH mixture exposure in the Atlantic killifish (Fundulus heteroclitus). Environ. Toxicol. Chem. 2017, 36, 3246–3253. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Vashi, R.T. Chapter 4—Batch adsorption treatment of textile wastewater. In Characterization and Treatment of Textile Wastewater; Patel, H., Vashi, R.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 111–125. [Google Scholar]
- Domenici, P.; Herbert, N.A.; Lefrançois, C.; Steffensen, J.F.; McKenzie, D.J. The effect of hypoxia on fish swimming performance and behaviour. In Swimming Physiology of Fish; Palstra, A.P., Planas, J.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 129–159. [Google Scholar]
- Peake, S.J.; Farrell, A.P. Fatigue is a behavioural response in respirometer—Confined smallmouth bass. J. Fish Biol. 2006, 68, 1742–1755. [Google Scholar] [CrossRef]
- Shan, H.W.; Geng, Z.X.; Ma, S.; Wang, T. Comparative study of the key enzymes and biochemical substances involved in the energy metabolism of Pacific white shrimp, Litopenaeus vannamei, with different ammonia-N tolerances. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2019, 221, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Solstorm, F.; Solstorm, D.; Oppedal, F.; Fernö, A.; Fraser, T.W.K.; Olsen, R.E. Fast water currents reduce production performance of post-smolt Atlantic salmon Salmo salar. Aquac. Environ. Interact. 2015, 7, 125–134. [Google Scholar] [CrossRef]
- Newsholme, E.A.; William, T. The role of phosphoenolpyruvate carboxy-kinase in amino acid metabolism in muscle. Biochem. J. 1978, 176, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Hägerhäll, C. Succinate: Quinone oxidoreductases. Variations on a conserved theme. Biochim. Biophys. Acta 1997, 1320, 107–141. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Mustafa, T.; Jørgensen, J. Role of pyruvate kinase, phosphoenolpyruvate carboxykinase, malic enzyme and lactate dehydrogenase in anaerobic energy metabolism of Tubifex spec. J. Comp. Physiol. 1979, 130, 337–345. [Google Scholar] [CrossRef]
- Guillen, A.C.; Borges, M.E.; Herrerias, T.; Kandalski, P.K.; de Arruda Marins, E.; Viana, D.G.; de Souza, M.R.; Oliveira do Carmo Daloski, L.; Donatti, L. Effect of gradual temperature increase on the carbohydrate energy metabolism responses of the Antarctic fish Notothenia rossii. Mar. Environ. Res. 2019, 150, 104779. [Google Scholar] [CrossRef]

| Swimming Index | Sample Size | Age/years | Wet Wight/g | Body Length/cm | Water Temperature | Regression Equation | p | R2 |
|---|---|---|---|---|---|---|---|---|
| Critical swimming speed | 10 | 3+ | / | 16.65 ± 2.65 | 8.88 ± 0.069 | y = −0.1448x3 + 7.4003x2 − 113.4x + 579.91 | p < 0.05 | R2 = 0.9586 |
| Burst swimming speed | 10 | / | 17.7 ± 2.85 | 9.53 ± 0.085 | y = −0.0865x3 + 4.8225x2 − 82.031x + 545.38 | p < 0.05 | R2 = 0.9731 | |
| Relative critical Swimming speed | 10 | / | 16.65 ± 2.65 | 8.88 ± 0.069 | y = −0.0085x3 + 0.4168x2 − 6.308x + 33.259 | p < 0.05 | R2 = 0.8937 | |
| Relative burst swimming speed | 10 | / | 17.7 ± 2.85 | 9.53 ± 0.085 | y = −0.0056x3 + 0.3132x2 − 5.8463x + 43.188 | p < 0.05 | R2 = 0.4689 | |
| Oxygen consumption rate (group 1) | 6 | 3+ | 50.43 ± 5.21 d | 16.42 ± 0.86 d | 9.57 ± 0.09 a | y = 0.04623 + 0.02452x0.39484 | p < 0.05 | R2 = 0.94803 |
| Oxygen consumption rate (group 2) | 4 | 4+ | 122.73 ± 6.14 b | 20.50 ± 0.71 b | 8.99 ± 0.06 a | y = 0.03514 + 0.01284x0.80175 | p < 0.05 | R2 = 0.91076 |
| Oxygen consumption rate (group 3) | 5 | 5+ | 165.81 ± 18.77 a | 23.70 ± 0.84 a | 9.28 ± 0.02 a | y = 0.03214 + 0.00487x1.83557 | p < 0.05 | R2 = 0.99464 |
| Biochemical indicator | 12 | 4+ | 111.91 ± 21.30 | 21.06 ± 1.65 | 9.55 ± 0.082 | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, C.; Li, Y.; Zhu, G.; Peng, W.; E, Q.; Zhang, Y.; Ma, B. The Effects of Water Flow Speed on Swimming Capacity and Energy Metabolism in Adult Amur Grayling (Thymallus grubii). Fishes 2024, 9, 272. https://doi.org/10.3390/fishes9070272
Zhai C, Li Y, Zhu G, Peng W, E Q, Zhang Y, Ma B. The Effects of Water Flow Speed on Swimming Capacity and Energy Metabolism in Adult Amur Grayling (Thymallus grubii). Fishes. 2024; 9(7):272. https://doi.org/10.3390/fishes9070272
Chicago/Turabian StyleZhai, Cunhua, Yutao Li, Guanyu Zhu, Wenjie Peng, Qiuxu E, Ying Zhang, and Bo Ma. 2024. "The Effects of Water Flow Speed on Swimming Capacity and Energy Metabolism in Adult Amur Grayling (Thymallus grubii)" Fishes 9, no. 7: 272. https://doi.org/10.3390/fishes9070272
APA StyleZhai, C., Li, Y., Zhu, G., Peng, W., E, Q., Zhang, Y., & Ma, B. (2024). The Effects of Water Flow Speed on Swimming Capacity and Energy Metabolism in Adult Amur Grayling (Thymallus grubii). Fishes, 9(7), 272. https://doi.org/10.3390/fishes9070272




