Exposure of Zebrafish Embryos to Morphine and Cocaine Induces Changes in the Levels of Dopamine and of Proteins Related to the Reward Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish Care and Breeding
2.3. Experimental Design
2.4. Determination of Morphine, Cocaine and Benzoylecgonine Levels
2.5. Western Blot Analysis
2.6. Dopamine (DA) Assay
2.7. In Silico Determination of Binding Sites for Transcription Factors (TFBSs) at the Regulatory Promoter
2.8. Statistical Analysis
3. Results
3.1. Amount of Morphine and Cocaine Absorbed by Zebrafish Embryos
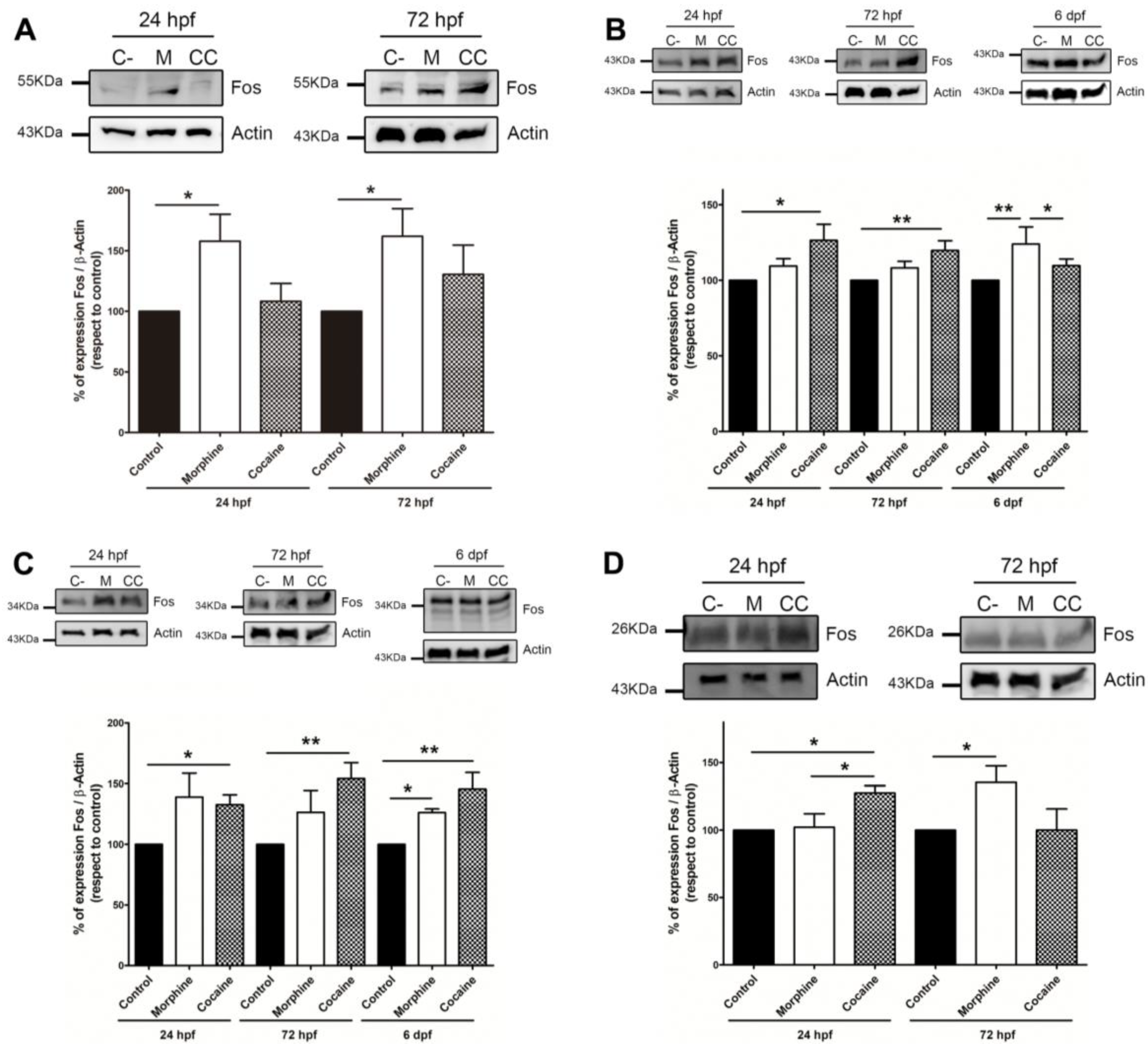
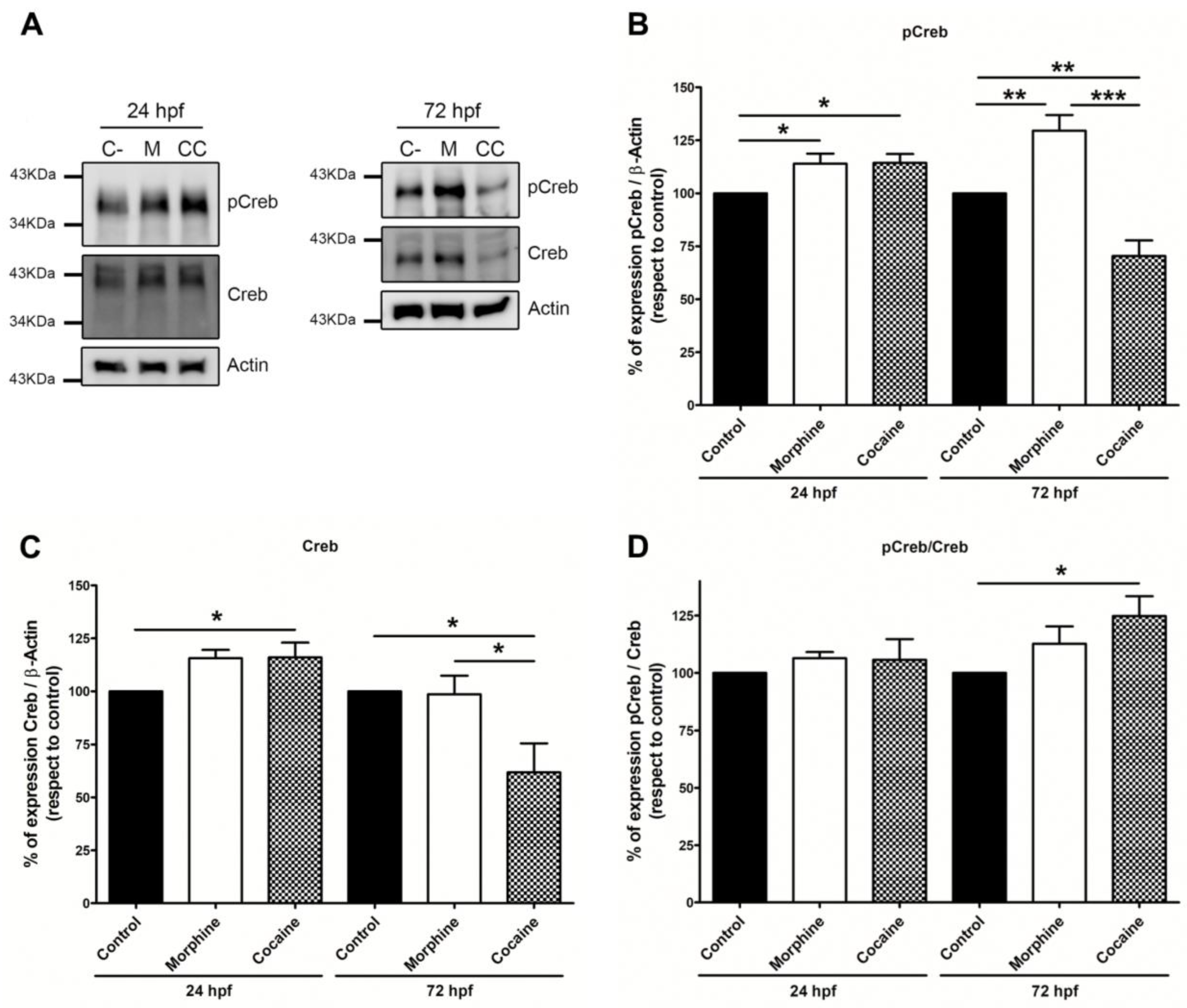
3.2. Expression of IEGs, Fos and Creb after Morphine or Cocaine Treatment
3.2.1. Fos Expression Analysis
3.2.2. pCreb and Creb Expression Analysis
3.3. Fos and Creb TFBSs in Target Genes
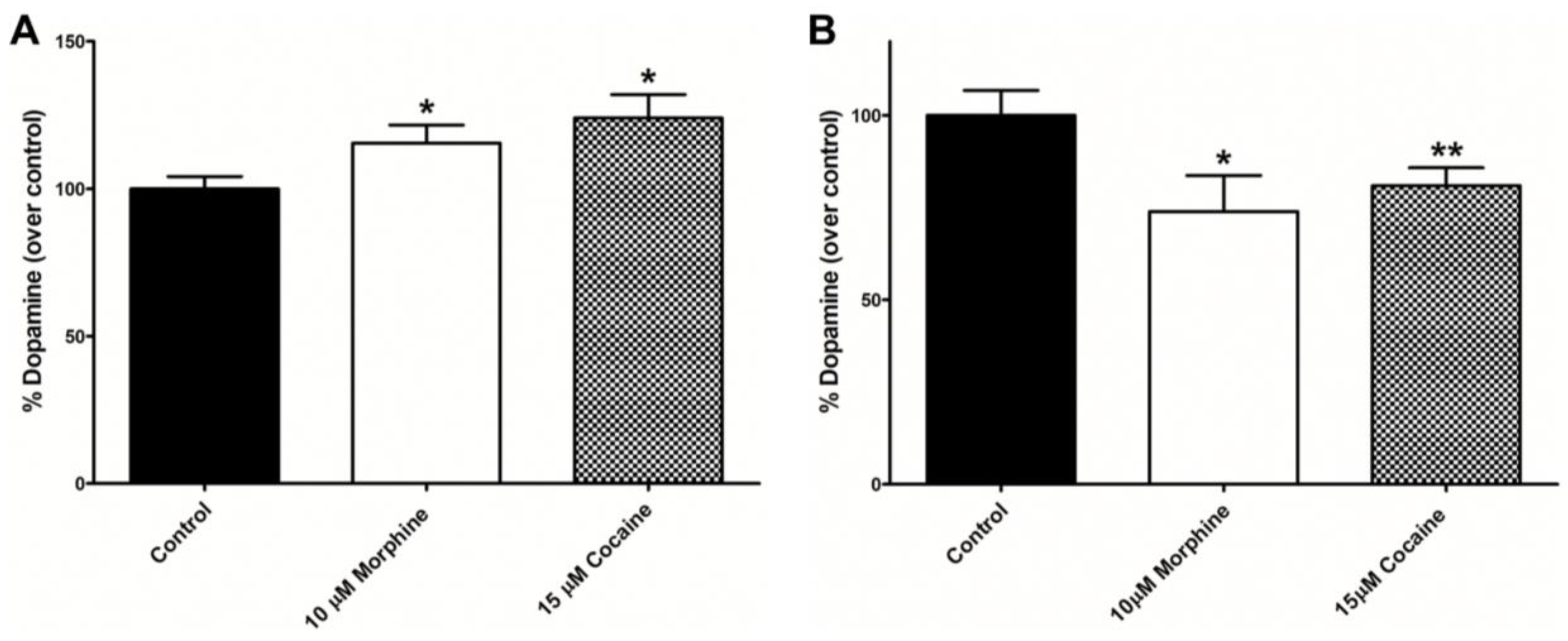
3.4. Dopamine (DA) Assays
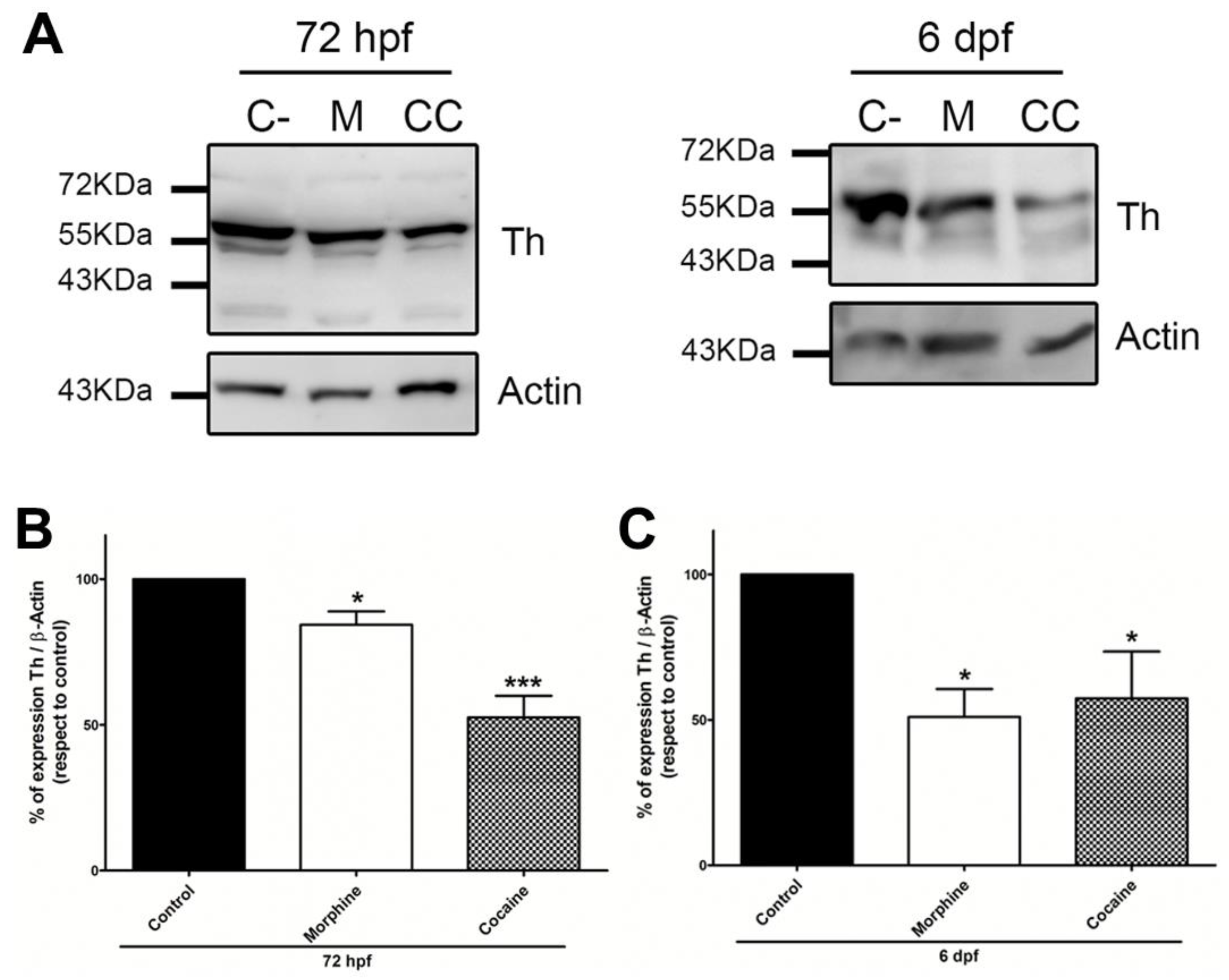
3.5. Analysis of the Effect of Morphine and Cocaine on the Expression of Tyrosine Hydroxylase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devereaux, A.L.; Mercer, S.L.; Cunningham, C.W. DARK Classics in Chemical Neuroscience: Morphine. ACS Chem. Neurosci. 2018, 9, 2395–2407. [Google Scholar] [CrossRef]
- Stein, C.; Schäfer, M.; Machelska, H. Why Is Morphine Not the Ultimate Analgesic and What Can Be Done to Improve It? J. Pain 2000, 1, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.R. Pharmacology of Opioids. Pharmacol. Rev. 1984, 35, 283–323. [Google Scholar]
- Pacifici, G.M. Metabolism and Pharmacokinetics of Morphine in Neonates: A Review. Clinics 2016, 71, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Chang, A.; Liu, N.; Gintzler, A.R. HHS Public Access. J. Neurochem. 2016, 139, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Sverrisdóttir, E.; Lund, T.M.; Olesen, A.E.; Drewes, A.M.; Christrup, L.L.; Kreilgaard, M. A Review of Morphine and Morphine-6-Glucuronide’s Pharmacokinetic-Pharmacodynamic Relationships in Experimental and Clinical Pain. Eur. J. Pharm. Sci. 2015, 74, 45–62. [Google Scholar] [CrossRef]
- Korsgaard, H.O.; Torgersen, S.; Wentzel-Larsen, T.; Ulberg, R. Substance Abuse and Personality Disorder Comorbidity in Adolescent Outpatients: Are Girls More Severely Ill than Boys? Child Adolesc. Psychiatry Ment. Health 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fridell, M.; Bäckström, M.; Hesse, M.; Krantz, P.; Perrin, S.; Nyhlén, A. Prediction of Psychiatric Comorbidity on Premature Death in a Cohort of Patients with Substance Use Disorders: A 42-Year Follow-Up. BMC Psychiatry 2019, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Park, T. Acute and Chronic Effects of Cocaine on Cardiovascular Health. Int. J. Mol. Sci. 2019, 20, 584. [Google Scholar] [CrossRef]
- Drake, L.R.; Scott, P.J.H. DARK Classics in Chemical Neuroscience: Cocaine. ACS Chem. Neurosci. 2018, 9, 2358–2372. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Vranjkovic, O.; Twining, R.C.; Gasser, P.J.; Mcreynolds, J.R.; Blacktop, J.M. Neurobiological Mechanisms That Contribute to Stress-Related Cocaine Use. Neuropharmacology 2014, 76, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, I.; Fiore, C.; De Carlo, D.; Pascale, N.; Neri, M.; Turillazzi, E.; Fineschi, V. Side Effects of Cocaine Abuse: Multiorgan Toxicity and Pathological Consequences. Curr. Med. Chem. 2012, 19, 5624–5646. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Ferro, J.M. Drug Abuse and Stroke. Curr. Neurol. Neurosci. Rep. 2013, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Buttner, A. Neuropathological Alterations in Cocaine Abuse. Curr. Med. Chem. 2012, 19, 5597–5600. [Google Scholar] [CrossRef] [PubMed]
- Spronk, D.B.; van Wel, J.H.P.; Ramaekers, J.G.; Verkes, R.J. Characterizing the Cognitive Effects of Cocaine: A Comprehensive Review. Neurosci. Biobehav. Rev. 2013, 37, 1838–1859. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Kringelbach, M.L. Pleasure Systems of the Brain. Neuron 2016, 86, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Yager, L.M.; Garcia, A.F.; Wunsch, A.M.; Ferguson, S.M. The Ins and Outs of the Striatum: Role in Drug Addiction. Neuroscience 2015, 301, 529–541. [Google Scholar] [CrossRef]
- Adinoff, B. Neurobiologic Processes in Drug Reward and Addiction. Harv. Rev. Psychiatry 2004, 12, 305–320. [Google Scholar] [CrossRef]
- Nestler, E.J. Historical Review: Molecular and Cellular Mechanisms of Opiate and Cocaine Addiction. Trends Pharmacol. Sci. 2004, 25, 210–218. [Google Scholar] [CrossRef]
- Lau, B.; Bretaud, S.; Huang, Y.; Lin, E.; Guo, S. Dissociation of Food and Opiate Preference by a Genetic Mutation in Zebrafish. Genes Brain Behav. 2006, 5, 497–505. [Google Scholar] [CrossRef]
- Wenzel, J.M.; Rauscher, N.A.; Cheer, J.F.; Oleson, E.B. A Role for Phasic Dopamine Release within the Nucleus Accumbens in Encoding Aversion: A Review of the Neurochemical Literature. ACS Chem. Neurosci. 2014, 6, 16–26. [Google Scholar] [CrossRef]
- Musacchio, J.M. Enzymes Involved in the Biosynthesis and Degradation of Catecholamines. In Biochemistry of Biogenic Amines; Springer: New York, NY, USA, 2013; pp. 1–35. ISBN 978-1-4684-3171-1. [Google Scholar]
- Koob, G.F.; Ahmed, S.H.; Boutrel, B.; Chen, S.A.; Kenny, P.J.; Markou, A.; Dell, L.E.O.; Parsons, L.H.; Sanna, P.P. Neurobiological Mechanisms in the Transition from Drug Use to Drug Dependence. Neurosci. Biobehav. Rev. 2004, 27, 739–749. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous Opiates and Behavior: 2014. Peptides 2016, 124, 18–70. [Google Scholar] [CrossRef]
- Johnson, S.W.; North, R.A. Opioids Excite Dopamine Neurons by Hyperpolarization Interneurons. J. Neurosci. 1992, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.M.; Nadal, R.; Vignes, M.; Ortiz, J. Chronic Cocaine Self-Administration Modulates ERK1/2 and CREB Responses to Dopamine Receptor Agonists in Striatal Slices. Addict. Biol. 2012, 17, 565–575. [Google Scholar] [CrossRef]
- Haghparast, A.; Fatahi, Z.; Alamdary, S.Z.; Reisi, Z.; Khodagholi, F. Changes in the Levels of P-ERK, p-CREB, and c-Fos in Rat Mesocorticolimbic Dopaminergic System after Morphine-Induced Conditioned Place Preference: The Role of Acute and Subchronic Stress. Cell. Mol. Neurobiol. 2014, 34, 277–288. [Google Scholar] [CrossRef]
- Moratalla, R. Neurobiología de La Cocaína. Trastor. Adict. 2008, 10, 143–150. [Google Scholar] [CrossRef][Green Version]
- Kelz, M.B.; Nestler, E.J. ΔFosB: A Molecular Switch Underlying Long-Term Neural Plasticity. Curr. Opin. Neurol. 2000, 13, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Reflections on: “A General Role for Adaptations in G-Proteins and the Cyclic AMP System in Mediating the Chronic Actions of Morphine and Cocaine on Neuronal Function”. Brain Res. 2016, 1645, 71–74. [Google Scholar] [CrossRef]
- Corbett, A.D.; Henderson, G.; McKnight, A.T.; Paterson, S.J. 75 Years of Opioid Research: The Exciting but Vain Quest for the Holy Grail. Br. J. Pharmacol. 2006, 147, 153–162. [Google Scholar] [CrossRef]
- Waldhoer, M.; Bartlett, S.E.; Whistler, J.L. Opioid Receptors. Annu. Rev. Biochem. 2004, 73, 953–990. [Google Scholar] [CrossRef] [PubMed]
- Macey, T.A.; Lowe, J.D.; Chavkin, C. Mu Opioid Receptor Activation of ERK1/2 Is GRK3 and Arrestin Dependent in Striatal Neurons. J. Biol. Chem. 2006, 281, 34515–34524. [Google Scholar] [CrossRef] [PubMed]
- Blendy, J.A.; Maldonado, R. Genetic Analysis of Drug Addiction: The Role of CAMP Response Element Binding Protein. J. Mol. Med. 1998, 76, 104–110. [Google Scholar] [CrossRef]
- Nestler, E.J. Molecular Basis of Long-Term Plasticity Underlying Addiction. Nat. Rev. Neurosci. 2001, 2, 119–128. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.A.; Nestler, E.J. Regulation of Gene Expression and Cocaine Reward by CREB and ΔFosB. Nat. Neurosci. 2003, 6, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garcia, A.A.; Perez-Fernandez, M.; Curto-Aguilera, D.; Rodriguez-Martin, I.; Sánchez-Barba, M.; Gonzalez-Nunez, V. Exposure to Morphine and Cocaine Modify the Transcriptomic Landscape in Zebrafish Embryos. Neuroscience 2022, 507, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.J.; Norton, W.H.J.; Trümbach, D.; Meijer, A.H.; Ninkovic, J.; Topp, S.; Heck, D.; Marr, C.; Wurst, W.; Theis, F.J.; et al. Zebrafish Reward Mutants Reveal Novel Transcripts Mediating the Behavioral Effects of Amphetamine. Genome Biol. 2009, 10, R81. [Google Scholar] [CrossRef] [PubMed]
- Broos, S.; Soete, A.; Hooghe, B.; Moran, R.; van Roy, F.; De Bleser, P. PhysBinder: Improving the Prediction of Transcription Factor Binding Sites by Flexible Inclusion of Biophysical Properties. Nucleic Acids Res. 2013, 41, 531–534. [Google Scholar] [CrossRef]
- Khan, A.; Fornes, O.; Stigliani, A.; Gheorghe, M.; Castro-Mondragon, J.A.; Van Der Lee, R.; Bessy, A.; Chèneby, J.; Kulkarni, S.R.; Tan, G.; et al. JASPAR 2018: Update of the Open-Access Database of Transcription Factor Binding Profiles and Its Web Framework. Nucleic Acids Res 2018, 46, D260–D266. [Google Scholar] [CrossRef]
- Sadat-shirazi, M.; Monfared, N.; Matloob, M. Possible Involvement of Nucleus Accumbens D1-like Dopamine Receptors in the Morphine-Induced Condition Place Preference in the Offspring of Morphine Abstinent Rats. Life Sci. 2019, 233, 116712. [Google Scholar] [CrossRef]
- Schaefer, C.P.; Arkwright, N.B.; Jacobs, L.M.; Jarvis, C.K.; Hunn, C.; Largent-milnes, T.M.; Tome, M.E.; Davis, T.P. Chronic Morphine Exposure Potentiates P-Glycoprotein Trafficking from Nuclear Reservoirs in Cortical Rat Brain Microvessels. PLoS ONE 2018, 13, e0192340. [Google Scholar] [CrossRef]
- Rubio, F.J.; Quintana-Feliciano, R.; Warren, B.L.; Li, X.; Witonsky, K.F.R.; del Valle, F.S.; Selvam, P.V.; Caprioli, D.; Venniro, M.; Bossert, J.M.; et al. Prelimbic Cortex Is a Common Brain Area Activated during Cue-Induced Reinstatement of Cocaine and Heroin Seeking in a Polydrug Self-Administration Rat Model. Eur. J. Neurosci. 2019, 49, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Mersereau, E.J.; Boyle, C.A.; Poitra, S.; Espinoza, A.; Seiler, J.; Longie, R.; Delvo, L.; Szarkowski, M.; Maliske, J.; Chalmers, S.; et al. Longitudinal Effects of Embryonic Exposure to Cocaine on Morphology, Cardiovascular Physiology, and Behavior in Zebrafish. Int. J. Mol. Sci. 2016, 17, 847. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Ghilardi, A.; Della Torre, C.; Magni, S.; Prosperi, L.; Calvagno, M.; Del Giacco, L.; Binelli, A. Environmental Concentrations of Cocaine and Its Main Metabolites Modulated Antioxidant Response and Caused Cyto-Genotoxic Effects in Zebrafish Embryo Cells. Environ. Pollut. 2017, 226, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gonzalez, A.; García-Concejo, A.; López-Benito, S.; Gonzalez-Nunez, V.; Arévalo, J.C.; Rodriguez, R.E. Role of Morphine, MiR-212/132 and Mu Opioid Receptor in the Regulation of Bdnf in Zebrafish Embryos. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Braunbeck, T.; Kais, B.; Lammer, E.; Otte, J.; Schneider, K.; Stengel, D. The Fish Embryo Test (FET): Origin, Applications, and Future. Environ. Sci. Pollut. Res. Int. 2015, 22, 16247–16261. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, J.; Bai, Y.; Zheng, X.; Alizamini, M.M.; Shang, W.; Yang, Q.; Li, M.; Li, Y.; Sui, N. Melanin-Concentrating Hormone in Rat Nucleus Accumbens or Lateral Hypothalamus Differentially Impacts Morphine and Food Seeking Behaviors. J. Psychopharmacol. 2020, 34, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang-james, Y.; Lloyd, D.R.; James, M.L.; Yang, L.; Richards, J.B.; Faraone, S.V. Oral Methylphenidate Treatment of an Adolescent ADHD Rat Model Does Not Alter Cocaine-Conditioned Place Preference during Adulthood: A Negative Report. J. Psychiatr. Brain Sci. 2019, 4, e190021. [Google Scholar] [CrossRef] [PubMed]
- NIDA. How Does Cocaine Produce Its Effects? Available online: https://www.drugabuse.gov/publications/research-reports/cocaine/how-does-cocaine-produce-its-effects (accessed on 2 March 2024).
- Wydra, K.; Golembiowska, K.; Zaniewska, M.; Kamińska, K.; Ferraro, L.; Fuxe, K.; Filip, M. Accumbal and Pallidal Dopamine, Glutamate and GABA Overflow during Cocaine Self-Administration and Its Extinction in Rats. Addict. Biol. 2013, 18, 307–324. [Google Scholar] [CrossRef]
- Womersley, J.S.; Kellaway, L.A.; Stein, D.J.; Gerhardt, G.A.; Russell, V.A. Effect of Cocaine on Striatal Dopamine Clearance in a Rat Model of Developmental Stress and Attention-Deficit/Hyperactivity Disorder. Stress 2016, 19, 78–82. [Google Scholar] [CrossRef]
- Dickson, P.W.; Briggs, G.D. Tyrosine Hydroxylase. Regulation by Feedback Inhibition and Phosphorylation, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 68, ISBN 9780124115125. [Google Scholar]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine Hydroxylase and Regulation of Dopamine Synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, P.R.; Bobrovskaya, L.; Graham, M.E.; Von Nagy-Felsobuki, E.I.; Dickson, P.W. Tyrosine Hydroxylase Phosphorylation: Regulation and Consequences. J. Neurochem. 2004, 91, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Zaragoza, J.; Ros-Simó, C.; Milanés, M.V.; Valverde, O.; Laorden, M.L. Binge Ethanol and MDMA Combination Exacerbates HSP27 and Trx-1 (Biomarkers of Toxic Cardiac Effects) Expression in Right Ventricle. Life Sci. 2019, 220, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Beitner-Johnson, D.; Guitart, X.; Nestler, E.J. Common Intracellular Actions of Chronic Morphine and Cocaine in Dopaminergic Brain Reward Regions. Ann. N. Y. Acad. Sci. 1992, 654, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Beitner-Johnson, D.; Guitart, X.; Nestler, E.J. Dopaminergic Brain Reward Regions of Lewis and Fischer Rats Display Different Levels of Tyrosine Hydroxylase and Other Morphine-and Cocaine-Regulated Phosphoproteins. Brain Res. 1991, 561, 147–150. [Google Scholar] [CrossRef]
- Han, M.; Bolaños, C.; Green, T.; Olson, V.; Neve, R.; Liu, R.; Aghajanian, G.; Nestler, E. Role of CAMP Response Element-Binding Protein in the Rat Locus Ceruleus: Regulation of Neuronal Activity and Opiate Withdrawal Behaviors. J. Neurosci. 2006, 26, 4624–4629. [Google Scholar] [CrossRef][Green Version]
- Sun, L.S.; Quamina, A. Perinatal Cocaine Exposure Stimulates the Expression and Activation of CREB in the Neonatal Rat Heart. Pediatr. Res. 2003, 53, 500–506. [Google Scholar] [CrossRef]
| Antibody (Reference n.) | Host Species | Company | lot n. | Dilution |
|---|---|---|---|---|
| Fos (K-25) (sc-253) | Rabbit | Santa Cruz Biotechnology ®, Dallas, TX, USA | J2809 | 1:500 |
| phospho-CREB Ser133(87G3) | Rabbit | Cell Signalling ®, Danvers, MA, USA | 14 | 1:1000 |
| CREB (48H2) | Rabbit | Cell Signalling ®, Danvers, MA, USA | 16 | 1:1000 |
| Tyrosine hydroxylase (AB152) | Rabbit | Millipore, Burlington, MA, USA | NG1830749 | 1:1000 |
| β-actin (4967) | Rabbit | Cell Signalling ®, Danvers, MA, USA | 10/2016 | 1:1000 |
| Anti-Rabbit-HRP (111-035-003) | Goat | Jackson InmunoResearch ®, West Grove, PA, USA | 131599 | 1:10,000 |
| Gene | ENSEMBL ID | AP-1 Site | CRE Site | Synteny |
|---|---|---|---|---|
| apoc1 | ENSDARG00000092170 | PF0007.1 −2449 a −2442 | - | ✓ |
| apoea | ENSDARG00000102004 | PF0007.1 −1416 a −1406 −643 a −633 | - | ✓ |
| bdnf | ENSDARG00000018817 | PF0007.1 −1943 a −1933 | - | ✓ |
| cfos | ENSDARG00000031683 | PF0007.1 −2899 a −2893 | MA0018.2 −1098 a −1089 | ✓ |
| cxcl11.5 | ENSDARG00000092423 | - | MA0018.2 −232 a −223 | ✓ |
| cxcl11.7 | ENSDARG00000093779 | PF0007.1 −608 a −598 | - | ✓ |
| cyp1a | ENSDARG00000098315 | - | MA0018.2 −73 a −64 | ✓ |
| elf3 | ENSDARG00000077982 | - | MA0018.1 −1899 a −1893 | ✓ |
| fabp2 | ENSDARG00000006427 | PF0007.1 −1035 a −1028 | - | ✓ |
| gnb3a | ENSDARG00000004358 | HSA0000011.1 −1442 a −1434 | MA0018.3 −154 a −143 | ✓ |
| hbz | ENSDARG00000045142 | PF0007.1 −388 a −378 | - | ✓ |
| opn1sw1 | ENSDARG00000045677 | PF0007.1 −2886 a −2876 −2308 a −2298 −371 a −361 | ✓ | |
| rx3 | ENSDARG00000052893 | - | - | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderon-Garcia, A.A.; Sánchez-Barba, M.; Gonzalez-Nunez, V. Exposure of Zebrafish Embryos to Morphine and Cocaine Induces Changes in the Levels of Dopamine and of Proteins Related to the Reward Pathway. Fishes 2024, 9, 268. https://doi.org/10.3390/fishes9070268
Calderon-Garcia AA, Sánchez-Barba M, Gonzalez-Nunez V. Exposure of Zebrafish Embryos to Morphine and Cocaine Induces Changes in the Levels of Dopamine and of Proteins Related to the Reward Pathway. Fishes. 2024; 9(7):268. https://doi.org/10.3390/fishes9070268
Chicago/Turabian StyleCalderon-Garcia, Andres Angel, Mercedes Sánchez-Barba, and Veronica Gonzalez-Nunez. 2024. "Exposure of Zebrafish Embryos to Morphine and Cocaine Induces Changes in the Levels of Dopamine and of Proteins Related to the Reward Pathway" Fishes 9, no. 7: 268. https://doi.org/10.3390/fishes9070268
APA StyleCalderon-Garcia, A. A., Sánchez-Barba, M., & Gonzalez-Nunez, V. (2024). Exposure of Zebrafish Embryos to Morphine and Cocaine Induces Changes in the Levels of Dopamine and of Proteins Related to the Reward Pathway. Fishes, 9(7), 268. https://doi.org/10.3390/fishes9070268






