Abstract
In this study, we employed geometric morphometrics (GMs) to analyze the shell shape differences among two mass-selected strains of bay scallops (red shell strain (RS) and black shell strain (BS)) and a control group (CG). The results revealed distinct shell shape differences corresponding to shell coloration, with the black shell strain displaying a more elliptical shell compared to the red shell strain. Additionally, the umbonal angle of the red shell strain was smaller than that of the black shell strain, indicating that the black shell strain had a more favorable jet direction that could enhance swimming capabilities. To evaluate the model’s performance in practical applications, leave-one-out cross-validation was carried out on the two shell strains and one control group. The results demonstrated discrimination accuracy rates of 67.44%, 47.62%, and 68.18% for the BS strain, RS strain, and CG, respectively. Similarly, for the right valves, the discrimination accuracy rates were 62.79%, 50.00%, and 75.00% for the BS strain, RS strain, and CG, respectively.
Key Contribution:
The shell color in bay scallops correlates with various heritable and adaptive traits associated with growth. Geometric morphometric analysis techniques prove more adept at capturing subtle variations in shell shape compared to conventional methods.
1. Introduction
Shell morphology is a central feature of bivalve biology in the fields of taxonomy, evolution, and functional anatomy and is a common phenomenon in mollusks [1,2,3,4,5,6,7]. The diversity of shell color has been a major issue in evolutionary biology research for half a century [8,9] because it is a very important feature in camouflage, warning, and immunity [10,11,12,13]. Although environmental conditions and feeding habits sometimes affect shell color [14,15], many studies have shown that shell color traits are genetically controlled [16,17,18,19,20,21]. Adamkewicz and Castagna employed the self-fertilization mating technique with Argopecten irradians, revealing that the shell background coloration in A. irradians is controlled by one allelic gene at one locus, with orange and yellow being dominant over white [22].
The biological form has four main elements: shape, size, physical properties, and orientation. Geometric morphometrics (GMs) can eliminate the influence of other factors, such as size, orientation, and physical properties, to obtain more accurate comparative results [23]. Lai et al. [24] employed GMs to analyze the developmental stages of Patinopecten yessoensis. Márquez et al. [25] reported that during five years, the shell phenotype of the scallop changed from symmetrical to asymmetrical in the anterior and posterior auricle, and from slender to elliptical in shape, in order to adapt to the habit of swimming as opposed to attaching themselves.
Shell colors in marine bivalves are not only diverse but also integral to biological adaptations within species [18,26,27,28,29]. Moreover, these colors are linked to quantitative characteristics such as growth rates and survival chances [19,30,31,32].
The bay scallop (A. irradians) is native to the Atlantic coast and was introduced to China in 1982, where it is one of the main economic scallops [33]. A. irradians grows fast and can reach market size within a year [34,35], generating great economic value [36].
The bay scallop is currently experiencing germplasm degradation, and researchers have cultivated many excellent scallop strains [37,38]. Improving germplasm quality is a common and effective method for genetic improvement in aquaculture.
The bay scallop’s shell color demonstrates polymorphism, particularly in its northern subspecies, where both shells exhibit a range of colors [22]. Research on the genetics of shell color has concentrated on this subspecies [22,32,39,40]. Studies have revealed that the shell color of the bay scallop is a heritable qualitative trait that is genetically controlled and not affected by environmental factors. It is theoretically feasible to develop strains of bay scallops with different shell colors through selective breeding.
Zheng et al. conducted statistical analyses on the shell color and growth characteristics of bay scallops in the northern aquaculture zones of Hebei, Shandong, and Liaoning province in China. The findings revealed that, with the exception of one group from Liaoning, the prevalence of black shells was around 50%; both purple and white shells were rare, occurring at frequencies of 3.8% and 1.1%, respectively. Scallop individuals with brown and black shells were found to have a growth advantage [41]. During 2018–2023, we performed individual selection on the shell length of two shell color strains of bay scallops. The shell length of the RS strain increased by 11.71%, while the shell length of the BS strain increased by 13.23% [42]. However, previous studies focused on the benefits of morphological evaluation using traditional methods that do not explore the subtle changes in shell shape and adaptive changes in scallops with different shell strains.
In this study, GMs were applied to analyze and compare the morphological differences between different strains of bay scallops and provide a theoretical basis for breeding and ecological research of bay scallops.
2. Materials and Methods
2.1. Source and Material Treatment
In 2018, two distinct strains known for their rapid growth were identified and selected: one with red shell and the other with black shell [42]. In 2020, a random sample of 50 scallops was collected from each of the black shell strain (BS), red shell strain (RS), and control group (CG) in Yuanbaotuozi (123.09° E, 39.50° N), Dalian, Liaoning, China (Figure 1). The samples were collected from the same area with raft cultivation. The samples are illustrated in Figure 2.
Figure 1.
Sampling location of BS, RS, and CG scallops.

Figure 2.
Examples of A. irradians shells. (A) Depicts a shell from BS strain, (B) depicts a shell from RS strain, and (C) depicts a shell from CG.
2.2. Methods
2.2.1. Image Acquisition
The samples of BS, RS, and CG scallops were all measured, including with traditional methods which are used for evaluating morphology via traditional morphometric variables such as shell length, shell width, and shell height with a Vernier scale (0.01 mm) [43]. The detailed measurements of the BS, RS, and CG scallops are shown in Table 1. Following the cleaning process of the valves, the left and right valves (with the concave side facing upward) were photographed using a Canon G12 digital camera. The photographs were taken along the y-axis, aligned with the vertical line of the umbo. The discs of the valves were positioned on the same plane and parallel to the camera, ensuring they were at an equal distance [24].

Table 1.
Morphological characteristics of BS, RS, and CG scallops.
2.2.2. Standardized Processing of Data
Landmarks and semi-landmarks were used to determine the shape of the right and left valves of the bay scallops. The Cartesian coordinates for a set of eight landmarks and 30 semi-landmarks were defined as the (1) umbo; (2) top of posterior auricle edge; (3) inflection point between the shell disc and posterior auricle; (4) extreme points of the posterior; (5) extreme points of the ventral margin; (6) extreme points of the anterior; (7) inflection point between the disc and anterior auricle; (8) top of the anterior auricle; (9–13) semi-landmarks along the outline of the posterior auricle; (14–18) semi-landmarks along the outline between the inflection point of the posterior auricle and extremal points of the posterior margin; (19–23) semi-landmarks along the outline between the posterior and ventral; (24–28) semi-landmarks along the outline between the anterior and ventral; (29–33) semi-landmarks along the outline between the inflection point of anterior auricle and extreme points of the anterior margin; and (34–38) semi-landmarks along the outline of the anterior auricle (Figure 3; modified from Lai et al. [24]). The semi-landmarks slide equally between two adjacent landmarks. The centroid size was the Euclidean distances of the landmarks from geometrical center [44]. To analyze the variation in centroid size of the BS, RS, and CG scallops, the researchers employed the Welch ANOVA due to the non-homogeneous variance. Post hoc analysis was conducted using the Games–Howell test. Additionally, generalized Procrustes analysis (GPA) was utilized to eliminate non-morphological landmark data, including position, scale, and direction. The remaining morphological variables were then analyzed by superimposing landmarks [45].
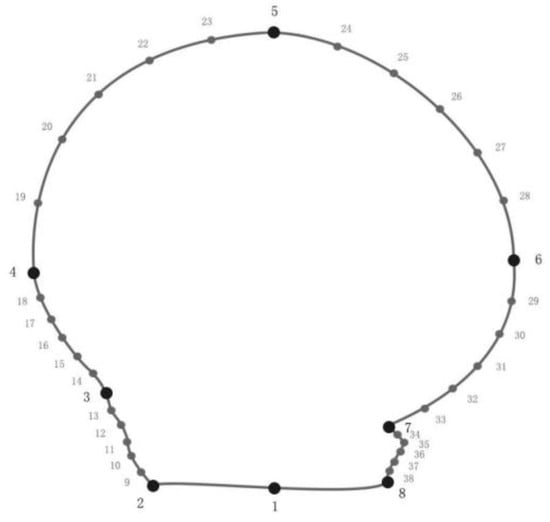
Figure 3.
Position of the 8 landmarks (black dots) and 30 semi-landmarks (grey dots) on the shell of scallops.
To further examine the differences in shell shape among the BS, RS, and CG scallops, principal components analysis (PCA) and canonical variate analysis (CVA) were performed. The data were processed and analyzed using Image J v1.53c and Past v4.03 software [46].
3. Results
There was a significant difference in centroid size among the left and right valves of BS, RS, and CG scallops based on Welch ANOVA analysis (F: 15.59, p < 0.001; F: 22.53, p < 0.001). The median was 3270.96 for the BS strain, 3302.15 for the RS strain, and 3101.45 for the CG in the left valves (Figure 4) and 3281.87 for the BS strain, 3364.05 for the RS strain, and 3093.35 for the CG in the right valves (Figure 5). There was no significant difference in the size of the left and right valves of the BS and RS strains (p > 0.05), but both were significantly larger than the CG (p < 0.001) based on the Games–Howell test.
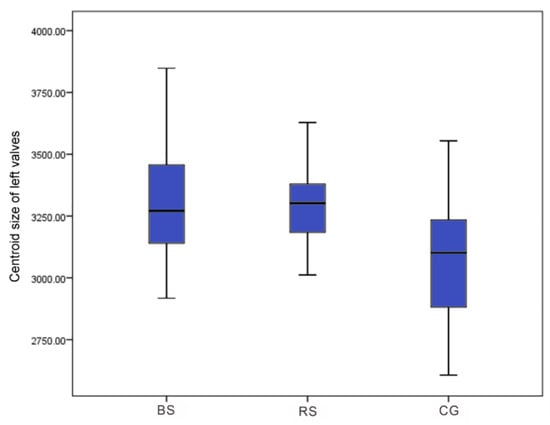
Figure 4.
The centroid size of left valves for the BS, RS, and CG scallops.
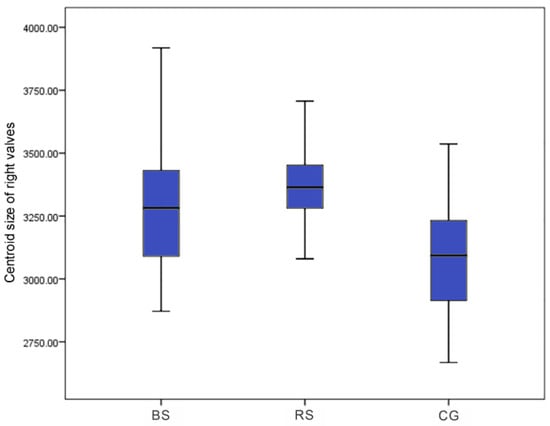
Figure 5.
The centroid size of right valves for the BS, RS, and CG scallops.
The first six principal components (PCs) of the left valves explained the morphological differences among the BS, RS, and CG strains. The cumulative contribution rate was 83.68% (Figure 6), and the contribution rates of the first six PCs were 38.11%, 16.36%, 9.80%, 9.02%, 6.82%, and 3.57%, respectively. The two PCs with the largest contribution were used as coordinates to create a two-dimensional distribution scatter plot (Figure 6). Taking the center point of the coordinate axis as the reference, in the positive direction of PC1, the auricle was inwardly contracted and the ventral margin was elongated, showing a more elliptical shell disc in the vertical direction. In the negative direction of PC1, the auricle was more lengthened outwards, the shell disc was more elliptical in the horizontal direction, and in the positive direction of PC2, the right contour of the shell was slightly stretched inward. The results in the negative direction of PC2 were opposite (Figure 6). Additionally, there is overlap between the BS, RS, and CG scallops; the changes in the BS and RS strains are within the change trend of the CG. The BS strain shifted towards the negative axis of PC1, and the RS strain shifted towards the positive axis of PC1 (Figure 6).
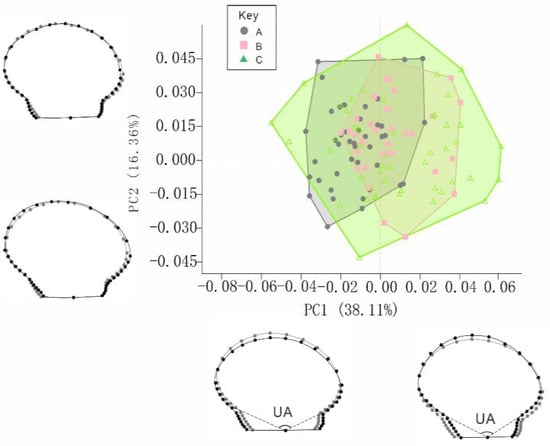
Figure 6.
Principal component analysis (PCA) of left valves of the BS, RS, and CG scallops. Notes: in the visualization, the grey dots represent the average shape of the BS, RS, and CG scallops. The black dots represent the scaling factor values of each principal component (PC), specifically with a scaling factor value of 0.05. A = black shell strain (BS), B = red shell strain (RS), and C = control group (CG).
The right valves’ morphologies were explained by the first six PCs, with a cumulative contribution rate of 81.40% (Figure 7). The contribution rates of the first six PCs were 42.07%, 11.44%, 10.27%, 8.12%, 5.98%, and 3.52%, respectively. The two PCs with the largest contributions were used as coordinates to create a two-dimensional distribution scatter plot. Using the center point of the coordinate axis as the reference, in the positive direction of PC1, the auricle was elongated, and the shell disc was more elliptical in the horizontal direction, while in the negative direction, the auricle was inwardly contracted, and the shape of the shell disc tended to be more elliptical in the vertical direction. In the positive direction of PC2, the left contour of the shell was lengthened outwards. PC2 was opposite in the negative direction. Additionally, the BS, RS, and CG scallops were all distributed on the positive and negative axis of PC1 and across the positive and negative axes of PC2. The BS strain shifted towards the positive axis of PC1, and the RS strain shifted towards the negative axis of PC1.
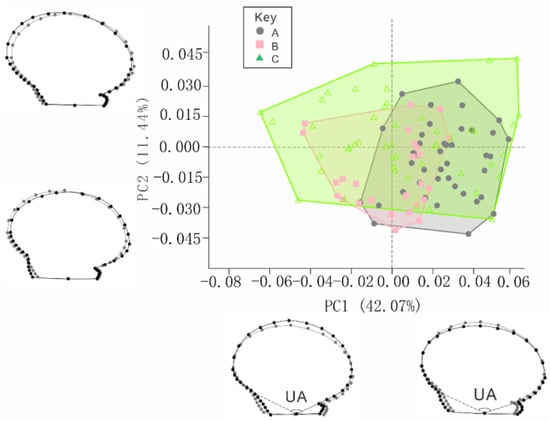
Figure 7.
Principal component analysis (PCA) diagram of right valves of the BS, RS, and CG scallops. Notes: The grey dots show the average shape of the scallops of BS, RS and CG scallops and the black dots show the 0.05 scaling factor values of each PC; A = black shell strain (BS), B = red shell strain (RS), and C = control group (CG).
CVA was performed on the left and right valves’ data from the BS, RS, and CG scallops. Together, the CVA clearly distinguished between the left and right valves sample morphologies in the original discriminant analysis (Figure 8 and Figure 9; Table 2 and Table 3). In addition, the CVA maps of the left valves show that the BS strain is distributed in the first and fourth quadrants, the RS strain is distributed in the first and second quadrants, and the CG is distributed in the second and third quadrants (Figure 8). The CVA maps of the right valves show that the BS strain is distributed in the second and third quadrants, the RS strain is distributed in the third and fourth quadrants, respectively, and the CG is distributed in the first and fourth quadrants (Figure 9). In the original discrimination analysis of the left valves, three BS strain left-valve samples were misjudged as belonging to the RS strain, and one RS strain sample was misjudged as belonging to the BS strain. The successful discrimination rate of the CG was 100%, and the total successful discrimination rate was 96.90% (Table 2). For the analysis of the right valves, one sample in the BS strain was misjudged as belonging to the RS strain, and one sample in the CG was misjudged as belonging to the RS strain. The successful discrimination rate of the RS strain was 100%, and the total success discrimination rate was 98.45% (Table 3). In the jack-knifed discrimination analysis of the left valves, 13 samples in the BS strain were misjudged as belonging to the RS strain, 1 sample in the BS strain was misjudged as belonging to the CG, 11 samples in the RS strain were misjudged as belonging to the BS strain, 11 samples in the RS strain were misjudged as belonging to the CG, 1 sample in the CG strain was misjudged as belonging to the BS strain, 13 samples in the CG were misjudged as belonging to the RS strain, and the total successful discrimination rate was 61.24% (Table 2). For the right valves’ analysis, 12 samples in the BS strain were misjudged as belonging to the RS strain, 4 samples in the BS strain were misjudged as belonging to the CG, 9 samples in the RS strain were misjudged as belonging to the BS strain, 12 samples in the RS strain were misjudged as belonging to the CG strain, 6 samples in the CG were misjudged as belonging to the BS strain, 5 samples in the CG were misjudged as belonging to the RS strain, and the total successful discrimination rate was 62.79% (Table 3).
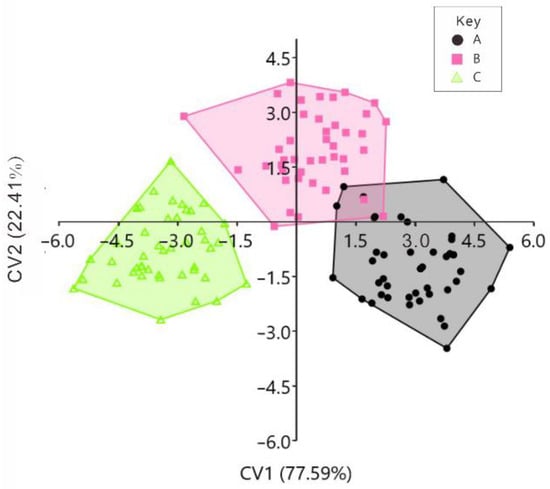
Figure 8.
Canonical variate analysis (CVA) scattergram of left valves of the BS, RS, and CG scallops. Notes: A = black shell strain (BS), B = red shell strain (RS), and C = control group (CG).
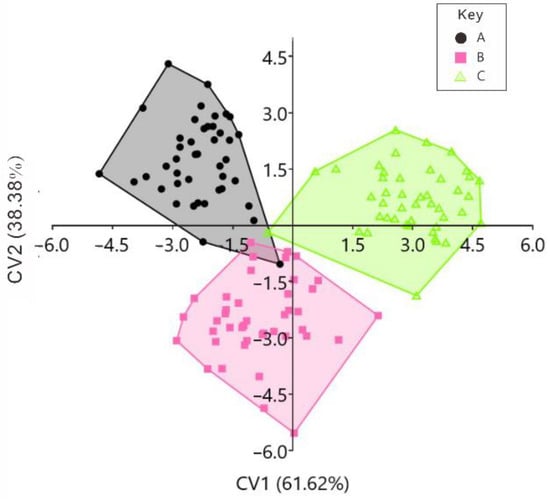
Figure 9.
Canonical variate analysis (CVA) scattergram of right valves of the BS, RS, and CG scallops. Notes: A = black shell strain (BS), B = red shell strain (RS), and C = control group (CG).

Table 2.
Left valves’ discriminant analysis results for the BS, RS, and CG scallops.

Table 3.
Right valves’ discriminant analysis results for the BS, RS, and CG scallops.
4. Discussion
In this study, shellfish morphology was analyzed using traditional morphological and geometric morphology measurements. Traditional morphology is the main method for measuring the morphological traits among species (e.g., shell length, width, and height). These measurements are widely used in the study of shellfish such as A. irradians [47], Argopecten. ventricosus [48], Chlamys nobilis [49], Mizuhopecten yessoensis [50], Ruditapes philippinarum [51], Mytilus edulis [52], Meretrix meretrix [53], Cyclina sinensis [53], and Pinctada martensii [54]. The morphological traits of shellfish are correlated with economic traits, such as total weight, adductor muscle weight, and edible weight. This morphological index is simple to measure and can directly select morphological traits for the selection of target economic traits. Therefore, it is an effective and practical method for informing the breeding of shellfish strains.
Compared to traditional morphometry, geometric morphometry retains shape variables during the analysis of shell shape and obtains non-size-related shape differences through coordinate transformation analysis [34]. Landmarks and semi-landmarks were selected in the auricle and ventral margin because shell shape is related to shellfish swimming capabilities [6,55]. The preference for analyzing the right valve in previous geometric morphology (GM) studies of bivalves was likely due to practical considerations [24,25,43] such as ease of access and fewer anatomical variations between the right valves of different individuals. However, the current study’s discrimination results indicate that both valves of bay scallops exhibit similar geometric morphology changes, which is consistent with the conclusion that the shell shape of A. irradians is almost symmetrical [6,56,57]. Both valves can be used in the GM method in scallop research. At the same time, the visualization results of the PCA indicate that there are subtle differences in shape between the left valves and right valves, with the LM34-38 of right valve showing a curved and protruding outward shape, while the LM34-38 of the left valve showed a straight shape. It seems that it is not enough to rely on traditional morphology to study the morphology of bivalves, and it is more comprehensive and accurate to quantify and study shell shape changes by combining geometric morphometry techniques [5,58].
Our study found that the centroid size of both the left and right valves in the BS and RS strains was significantly larger than that of the CG, aligning with the traditional measurements reported by Wang et al. [42]. This agreement supports the consistency of results between traditional and geometric morphometric assessments for the BS, RS, and CG scallops. Principal component analysis (PCA) revealed a distinct morphological divergence: the BS strain tended towards the negative axis of the first principal component (PC1), whereas the RS strain shifted towards the positive axis. This separation suggests variations in shell shape among the different strains. Specifically, the BS strain showed a trend towards elliptical valves with a wider umbonal angle compared to the RS strain. Additionally, canonical variates analysis (CVA) effectively differentiated between the BS, RS, and CG scallops, confirming the presence of three distinct morphologies. This research underscores the morphometric differences in shell shape and size among bay scallops with distinct coloration, with geometric morphometrics offering a deeper understanding of their morphological characteristics beyond what is provided by traditional measurement methods.
In exploring the disparities in growth rates and shell morphology between two colored bay scallops of the same genetic background, we are considering multiple potential explanations. Conversely, it could be that the black or brown coloration is inherently more conducive to growth in the northern climate, a trait we have observed in other shellfish species. Additionally, we have gathered insights from studies on scallop morphology. Our research noted that the BS strain possesses a larger umbonal angle and an increased shell dimension that aligns orthogonality with their direction of movement. Márquez et al. [25] revealed that the juvenile anterior and posterior auricles transitioned from a symmetrical to an asymmetrical form in scallops, and the shell shape evolved from slender to elliptical over a five-year growth period. This transformation was observed as an adaptation from an attachment lifestyle to a swimming lifestyle. Stanley [6] also discovered that a large umbonal angle enhances the swimming ability of free-living species. In comparison to the RS strain, the BS strain had a larger umbonal angle and an increased shell dimension perpendicular to the movement direction. The expanded umbonal angle provides a more advantageous orientation for directional jetting, while the larger shell dimension, perpendicular to their movement, helps reduce hydrodynamic resistance during swimming [6,59,60]. This suggests that the shell shape changes in the BS strain, which exhibited a more stronger swimming capability, suggesting a better adaptation to a lifestyle characterized by frequent swimming.
In the present study, we selected eight shell landmarks and 30 semi-landmarks from the scallops of the BS, RS, and CG scallops for analysis. The discrimination results for the left valves were 93.02% for the BS strain, 97.62% for the RS strain, and 100% for the CG, indicating a high level of accuracy. For the right valves, the discrimination rates were 97.67% for the BS strain, 100.00% for the RS strain, and 97.73% for the CG, confirming the effectiveness of the method in distinguishing right valves as well. The discrimination analysis achieved an average accuracy exceeding 90% for both left and right valves, with the jack-knifed discrimination method enhancing this to an average of 60%. This study validates the high accuracy of the landmark method for morphological analysis of both left and right scallop valves.
5. Conclusions
This investigation has highlighted that both the RS and BS varieties of bay scallops show significantly better growth patterns than those in the control group. Notably, both types have demonstrated improved growth attributes, suggesting they could be more beneficial for farming purposes. The study reinforces the value of selective breeding techniques in enhancing the yield and sustainability of bay scallop farming operations. Through geometric morphometric analysis of the left and right valves from bay scallops, we achieved a high degree of accuracy (over 90%) in distinguishing between two distinct strains. Visual analysis using grid patterns exposed pronounced disparities between the RS and BS strains. The BS strain was found to have a greater umbonal angle and larger shell measurements orthogonally related to its direction of movement as opposed to the RS strain. These characteristics point to the BS strain’s adaptation for swimming, with its shell shape reflecting changes that are advantageous for such an active lifestyle. These discoveries lay the groundwork for more targeted breeding efforts and support the development of sustainable bay scallop aquaculture practices.
Author Contributions
Conceptualization, Y.T.; methodology, Y.T.; investigation X.W.; resources, X.W.; funding acquisition, Y.T. and Z.H.; writing—original draft, X.H., Z.H. and Y.T.; writing—review and editing, J.M., L.W., Z.H., X.H. and Y.T.; supervision, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2022YFD2400302), the Central Government Subsidy Project for Liaoning Fisheries (2023), and the Science and Technology Foundation of Dalian (2021JB11SN035).
Institutional Review Board Statement
The experimental protocol was designed in accordance with the recommendations of the Regulations of the Laboratory Animal—Guideline for Ethical Review of Animal Welfare (National Standards of P. R. China, GB/T 35823—2018) and reviewed and approved by the animal care and use committee of Dalian Ocean University (DLOU-2023009).
Informed Consent Statement
Not applicable.
Data Availability Statement
Relevant information has been included in the article.
Acknowledgments
The authors are also grateful to the anonymous reviewers for the great elaboration of the manuscript through their critical reviewing and comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gaspar, M.B.; Santos, M.N.; Vasconcelos, P.; Monteiro, C.C. Shell morphometric relationships of the most common bivalve species (Mollusca: Bivalvia) of the Algarve coast (southern Portugal). Hydrobiologia 2002, 477, 73–80. [Google Scholar] [CrossRef]
- Manuel, J.L.; Dadswell, M.J. Swimming of juvenile sea scallops, Placopecten magellanicus (Gmelin): A minimum size for effective swimming? J. Exp. Mar. Biol. Ecol. 1993, 174, 137–175. [Google Scholar] [CrossRef]
- Márquez, F.; Robledo, J.; Peñalosa, G.E.; Molen, S.V.D. Use of different geometric morphometrics tools for the discrimination of phenotypic stocks of the striped clam Ameghinomya antiqua (Veneridae) in north Patagonia, Argentina. Fish. Res. 2010, 101, 127–131. [Google Scholar] [CrossRef]
- Márquez, F.; González-José, R.; Bigatti, G. Combined methods to detect pollution effects on shell shape and structure in Neogastropods. Ecol. Indic. 2011, 11, 248–254. [Google Scholar] [CrossRef]
- Serb, J.M.; Alejandrino, A.; Otárola-castillo, E.; Adams, D.C. Morphological convergence of shell shape in distantly related scallop species (Mollusca: Pectinidae). Zool. J. Linn. Soc. 2011, 163, 571–584. [Google Scholar] [CrossRef]
- Stanley, S.M. Relation of shell form to life habitats in the Bivalvia (Mollusca). Mem. Geol. Soc. Am. 1970, 125, 1–296. [Google Scholar] [CrossRef]
- Ubukata, T. A morphometric study on morphological plasticity of shell form in crevice-dwelling Pterioida (Bivalvia). Biol. J. Linn. Soc. Lond. 2003, 79, 285–297. [Google Scholar] [CrossRef]
- Clarke, B. Natural selection in mixed populations of two polymorphic snails. Heredity 1962, 17, 319–345. [Google Scholar] [CrossRef]
- Murray, J.; Clarke, B. The inheritance of polymorphic shell characters in partula (Gastropoda). Genetics 1966, 54, 1261–1277. [Google Scholar] [CrossRef]
- Cook, L.M.; Freeman, P.M. Heating properties of morphs of the mangrove snail Littoraria pallescens. Biol. J. Linn. Soc. 1986, 29, 295–300. [Google Scholar] [CrossRef]
- Rosin, Z.M.; Kwieciński, Z.; Lesicki, A.; Skórka, P.; Kobak, J.; Szymańska, A.; Osiejuk, T.S.; Kałuski, T.; Jaskulska, M.; Tryjanowski, P. Shell colour, temperature, (micro)habitat structure and predator pressure affect the behaviour of Cepaea nemoralis. Sci. Nat. 2018, 105, 35. [Google Scholar] [CrossRef]
- Savazzi, E. The colour patterns of cypraeid gastropods. Lethaia 2007, 31, 15–27. [Google Scholar] [CrossRef]
- Scheil, A.E.; Hilsmann, S.; Triebskorn, R.; Köhler, H.R. Shell colour polymorphism, injuries and immune defense in three helicid snail species, Cepaea hortensis, Theba pisana and Cornu aspersum maximum. Results Immunol. 2013, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ekendahl, A. Colour polymorphic prey (Littorina saxatilis Olivi) and predatory effects of a crab population (Carcinus maenas L.). J. Exp. Mar. Biol. Ecol. 1998, 222, 239–246. [Google Scholar] [CrossRef]
- Raffaelli, D. Colour polymorphism in the intertidal snail Littorina rudis Maton. Zool. J. Linn. Soc. 1979, 67, 65–73. [Google Scholar] [CrossRef]
- Winkler, F.M.; Estévez, B.F.; Jollán, L.B.; Garrido, J.P. Inheritance of the general shell color in the scallop Argopecten purpuratus (Bivalvia: Pectinidae). J. Hered. 2001, 92, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, P.P.; Gantsevich, M.M.; Basova, L.A. Shell color polymorphism in Macoma balthica L. (Bivalvia, Tellinidae) from the White and Barents Seas. Dokl. Biol. Sci. 2001, 376, 59–61. [Google Scholar] [CrossRef]
- Mitton, J.B. Shell color and pattern variation in Mytilus edulis and its adaptive significance. Chesap. Sci. 1977, 18, 387–390. [Google Scholar] [CrossRef]
- Newkirk, G.F. Genetics of shell color in Mytilus edulis L. and the association of growth rate with shell color. J. Exp. Mar. Biol. Ecol. 1980, 47, 89–94. [Google Scholar] [CrossRef]
- Innes, D.J.; Leslie, E.H. Inheritance of a shell-color polymorphism in the mussel. J. Hered. 1977, 68, 203–204. [Google Scholar] [CrossRef]
- Evans, S.; Camara, M.D.; Langdon, C.G. Heritability of shell pigmentation in the Pacific oyster, Crassostrea gigas. Aquaculture 2009, 286, 211–216. [Google Scholar] [CrossRef]
- Adamkewicz, L.; Castagna, M. Genetics of the shell color and pattern in the bay scallop Argopecten irradians. J. Hered. 1988, 79, 14–17. [Google Scholar] [CrossRef]
- Bai, M.; Yang, X.K.; Li, J.; Wang, W.C. Geometric morphometrics, a super scientific computing tool in morphology comparison. Chin. Sci. Bull. 2014, 59, 887–894. [Google Scholar] [CrossRef]
- Lai, S.Q.; Xie, Y.Y.; Huang, X.P.; Mao, J.X.; Wang, X.B.; Tian, Y.; Chang, Y.Q. Ontogenetic changes during the life span of the scallop Patinopecten yessoensis determined using a geometric morphometric method. J. Shellfish Res. 2023, 42, 259–264. [Google Scholar] [CrossRef]
- Márquez, F.; Amoroso, R.; Sainz, G.M.F.; Molen, S.V.D. Shell morphology changes in the scallop Aequipecten tehuelchus during its life span: A geometric morphometric approach. Aquat. Biol. 2010, 11, 149–155. [Google Scholar] [CrossRef]
- Cain, A.J. The colours of marine bivalve shells with special reference to Macoma balthica. Malacologia 1988, 28, 289–318. [Google Scholar]
- Yonge, C.M. On the habits and adaptations of Aloidis (corbula) gibba. J. Mar. Biol. Associ. UK 1946, 26, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.S. Polymorphism and selective predation in Donax faba Gmelin (Bivalvia: Tellinacea). J. Exp. Mar. Biol. Ecol. 1975, 17, 89–94. [Google Scholar] [CrossRef]
- Beukema, J.J.; Meehan, B.W. Latitudinal variation in linear growth and other shell characteristics of Macoma balthica. Mar. Bio. 1985, 90, 27–33. [Google Scholar] [CrossRef]
- Wolff, M.; Garrido, J. Comparative study of growth and survival of two color morphs of the Chilean scallop Argopecten purpuratus (Lamarck) in suspended culture. J. Shellfish Res. 1991, 10, 47–53. [Google Scholar]
- Alfonsi, C.; Perez, J.E. Growth and survival in the scallop Nodipecten nodosus as related to self-fertilization and shell colour. Bol. Inst. Oceanogr. Venez. 1998, 37, 69–73. [Google Scholar]
- Zheng, H.P.; Zhang, G.F.; Liu, X.; Que, H.Y. Establishment of different shell color lines of bay scallop Argopecten irradians irradians Lamarck (1819) and their development. Oceanol. Limnol. Sin. 2003, 34, 632–639. [Google Scholar]
- Hou, X.M.; Zhang, F.C.; Mu, Y.T. Characteristics, problems and development strategies of the bay scallop industry in Hebei. Chin. Fish. Econ. 2017, 35, 80–88. (In Chinese) [Google Scholar]
- Xiao, J.; Ford, S.E.; Yang, H.S.; Zhang, G.F.; Zhang, F.S.; Guo, X.M. Studies on mass summer mortality of cultured zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture 2005, 250, 602–615. [Google Scholar] [CrossRef]
- Milke, L.M.; Bricelj, V.M.; Parrish, C.C. Comparison of early life history stages of the bay scallop, Argopecten irradians: Effects of microalgal diets on growth and biochemical composition. Aquaculture 2006, 260, 272–289. [Google Scholar] [CrossRef]
- Wilbur, A.E.; Gaffney, P.M. A genetic basis for geographic variation in shell morphology in the bay scallop, Argopecten irradians. Mar. Bio. 1997, 128, 97–105. [Google Scholar] [CrossRef]
- Bao, Z.M.; Huang, X.T.; Xing, Q.; Wang, Y.T. The bay scallop Argopecten irradians “Haiyifeng 12”. Ch. Fish. 2017, 44, 70–73. (In Chinese) [Google Scholar]
- Wang, C.D.; Liu, B.; Ma, B.; Zhao, Y.M.; Zhao, X. The scallop “Bohai red”. Ch. Fish. 2016, 43, 72–77. (In Chinese) [Google Scholar]
- Kraeuter, J.; Adamkewicz, L.; Castagna, M.; Wall, R.; Karney, R. Rib number and shell color in hybridized subspecies of the Atlantic bay scallop, Argopecten irradians. Nautilus 1984, 98, 17–20. [Google Scholar]
- Elek, J.A.; Adamkewicz, S.L. Polymorphism for shell color in the Atlantic bay scallop Argopecten irradians irradians (Lamarck) (Mollusca; bivalvia) on Martha’s Vineyard Island. Am. Malac. Bull. 1990, 7, 117–126. [Google Scholar]
- Zheng, H.P.; Xu, F.; Zhang, G.F.; Liu, X.; Wang, L.S. Relationships between shell colors and quantitative traits in the bay scallop, Argopecten irradians irradians (Lamarck, 1819). Oceanol. Limnol. Sin. 2008, 39, 328–333. (In Chinese) [Google Scholar]
- Wang, X.B.; Ding, S.Q.; Yin, D.H.; Song, J.; Chang, Y.Q. Response to selection for growth in the second generation of two shell color lines of the bay scallop Argopecten irradians. Aquaculture 2020, 528, 735536. [Google Scholar] [CrossRef]
- Shu, Y.; Shi, L.; Lai, S.Q.; Tian, Y.; Chang, Y.Q. Morphometric study and comparison of scallops (Chlamys farreri) cultured in different water depth. Acta. Hydrobiol. Sin. 2021, 45, 132–139. (In Chinese) [Google Scholar]
- Bookstein, F.L. Distance measures. In Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1992; pp. 88–124. [Google Scholar]
- Rohlf, F.J.; Marcus, L.F. A revolution morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Xu, X.Y.; Hu, L.P.; He, J.B.; Jiang, L.M.; Yang, T.; Shi, W.K.; Zhang, Y.H.; Ll, W. Effects of morphometric traits of Argopecten irradians on its quality traits. Trans. Oceanol. Limnol. 2023, 45, 73–78. (In Chinese) [Google Scholar]
- Ibarra, A.M. Correlated responses at age 5 months and 1 year for a number of growth traits to selection for total weight and shell width in catarina scallop (Argopecten ventricosus). Aquaculture 1999, 175, 243–254. [Google Scholar] [CrossRef]
- Zheng, H.P.; Sun, Z.W.; Zhang, T.; Liu, H.; Li, Y.Y. Correlation and path analysis to quantitative traits of noble scallop Chlamys nobilis Reeve at one-year old. Chin. Agric. Sci. Bull. 2009, 25, 322–326. (In Chinese) [Google Scholar]
- Chang, Y.Q.; Zhang, C.S.; Cao, X.B.; Yang, X.G.; Li, Y.F. Effect of morphometrical traits on weight traits in one-year old yesso scallop Patinopecten yessoensis. J. Dalian Ocean. Univ. 2008, 23, 330–334. (In Chinese) [Google Scholar]
- Watanabe, S.; Katayama, S. Relationships among shell shape, shell growth rate, and nutritional condition in the Manila clam (Ruditapes philippinarum) in Japan. J. Shellfish. Res. 2010, 29, 353–359. [Google Scholar] [CrossRef]
- Alunno-Bruscia, M.; Bourget, E.; Fréchette, M. Shell allometry and length-mass-density relationship for Mytilus edulis in an experimental food-regulated situation. Mar. Ecol. Prog. Ser. 2001, 219, 177–188. [Google Scholar] [CrossRef]
- Zhang, A.G.; Wang, L.L.; Yang, X.L.; Hu, X.C.; Fu, Y.B.; Li, C.H.; Chen, A.H.; Yuan, X.T. Relationship between shell morphological traits and body weight in two estuarine clams, Meretrix meretrix and Cyclina sinensis in Shuangtaizi Estuary, Bohai Sea in China. J. Shellfish. Res. 2018, 37, 989–996. [Google Scholar] [CrossRef]
- Deng, Y.W.; Fu, S.; Du, X.D.; Wang, Q.H. Realized heritability and genetic gain estimates of larval shell length in the Chinese pearl oyster Pinctada martensii at three different salinities. N. Am. J. Aquacilt. 2009, 71, 302–306. [Google Scholar] [CrossRef]
- Ackerly, S.C. The structure of ontogenetic variation in the shell of Pecten. Palaeontology 1992, 35, 847–867. [Google Scholar]
- Minchin, D. Introductions: Some biological and ecological characteristics of scallops. Aquat. Living Resour. 2003, 16, 521–532. [Google Scholar] [CrossRef]
- Sherratt, E.; Serb, J.M.; Adams, D.C. Rates of morphological evolution, asymmetry and morphological integration of shell shape in scallops. BMC Evol. Biol. 2017, 17, 248. [Google Scholar] [CrossRef]
- Serb, J.M.; Sherratt, E.; Alejandrino, A.; Adams, D.C. Phylogenetic convergence and multiple shell shape optima for gliding scallops (Bivalvia: Pectinidae). J. Evolution. Biol. 2017, 30, 1736–1747. [Google Scholar] [CrossRef]
- Speiser, D.I.; Wilkens, L.A. Neurobiology and behaviour of the scallop. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 219–251. [Google Scholar] [CrossRef]
- Stanley, S.M. Adaptive morphology of the shell in bivalves and gastropods. In Form and Function; Elsevier: Amsterdam, The Netherlands, 1988; pp. 105–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).