Ontogenetic, Spatial and Inter-Annual Variability in the Diet of European Hake Merluccius merluccius Linnaeus, 1758, in the North Aegean Sea
Abstract
1. Introduction
2. Materials and Methods
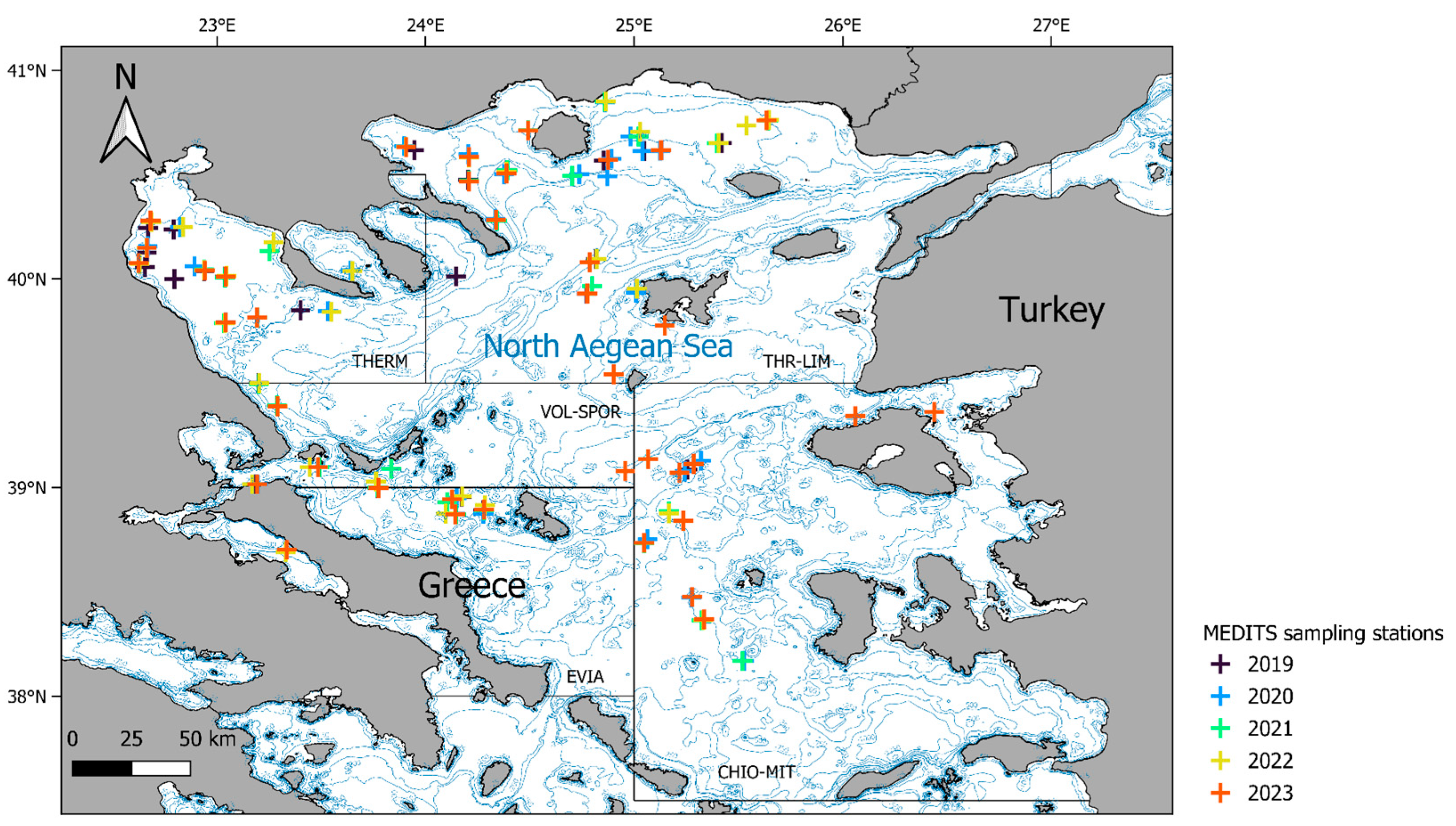
2.1. Study Area
2.2. Field Work
2.3. Lab Work
2.4. Data Analysis
3. Results
3.1. Sampling Effort
3.2. Feeding Intensity
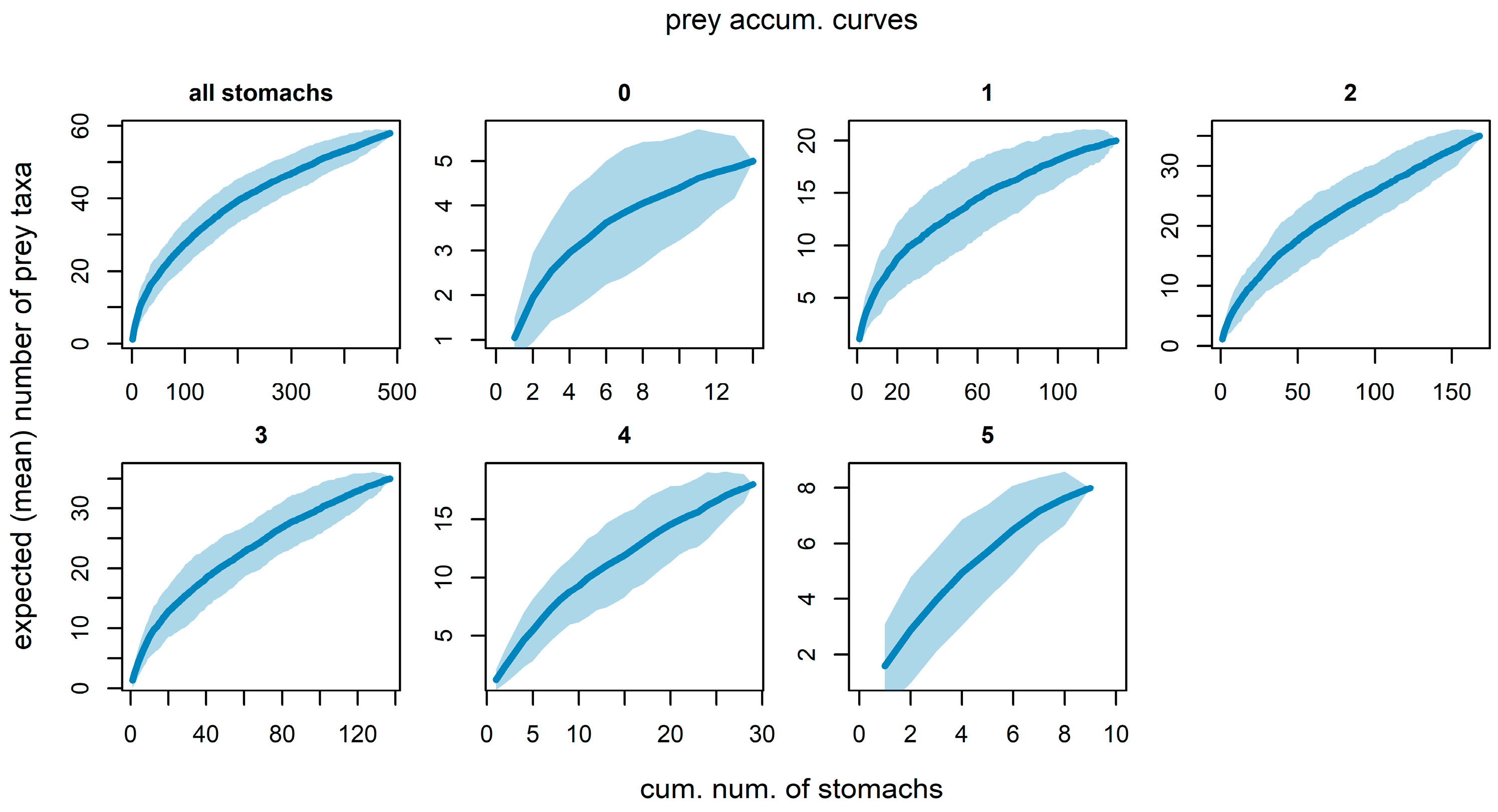
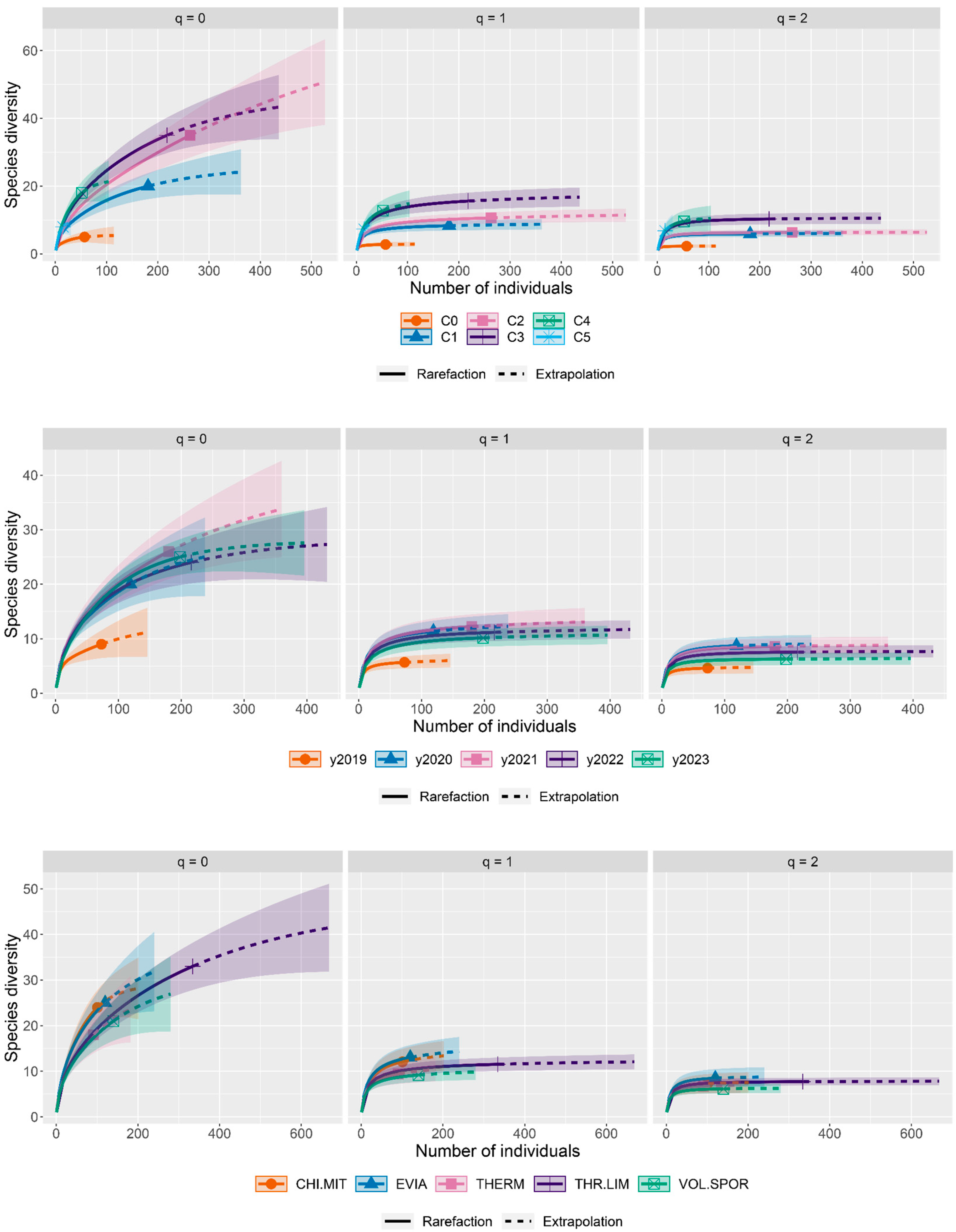
3.3. Sufficiency of the Number of Examined Fish Stomachs
3.4. Diet Composition
3.5. Importance of Prey Taxa in Hake Diet
3.6. Trophic Niche Breadth
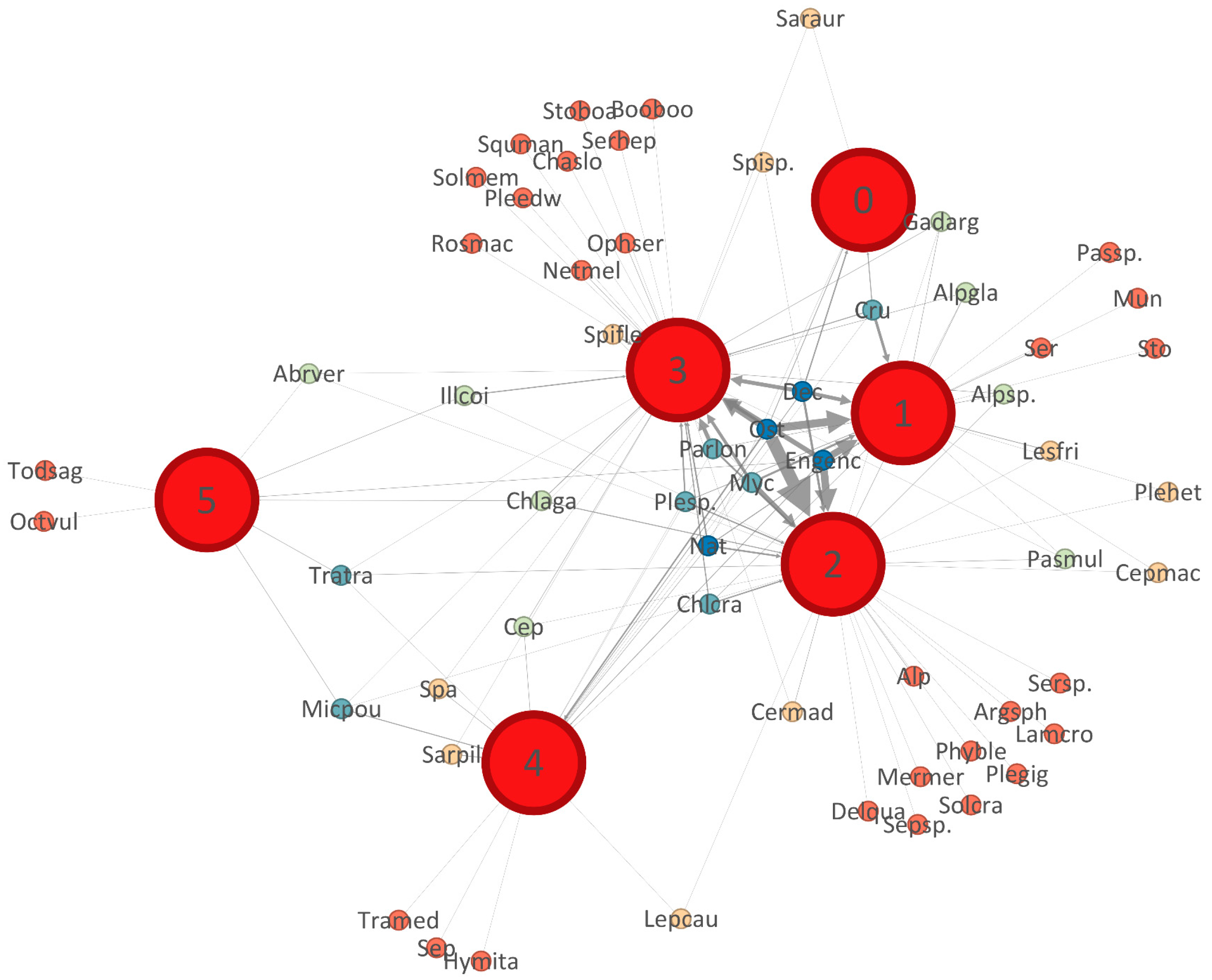
3.7. Diet Overlap
4. Discussion
4.1. Ontogenetic Shifts in the Diet of Hake
4.2. Spatial Variability in the Diet of Hake
4.3. Interannual Variability in the Diet of Hake
4.4. Cannibalism
4.5. Trophic Niche Breadth
4.6. Feeding Strategy
4.7. Study Shortcomings and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Diversity Accumulation Curves Methodology
References
- Lloris, D.; Matallanas, J.; Oliver, R. Hakes of the World (Family Merlucciidae): An Annotated and Illustrated Catalogue of Hake Species Known to Date; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; Available online: https://openknowledge.fao.org/items/19194d7a-7b27-4066-836a-d95b16e9bc46 (accessed on 29 April 2024).
- Pitcher, T.J.; Alheit, J. What makes a hake? A review of the critical biological features that sustain global hake fisheries. In Hake; Alheit, J., Pitcher, T.J., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 1–14. [Google Scholar]
- Cardinale, M.; Osio, G.C.; Scarcella, G. Mediterranean Sea: A failure of the European fisheries management system. Front. Mar. Sci. 2017, 4, 72. [Google Scholar] [CrossRef]
- FAO. The State of the Mediterranean and Black Sea Fisheries 2023; General Fisheries Commission for the Mediterranean: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/items/3869b6d2-c06c-45d1-9f4a-899865dfe817 (accessed on 29 April 2024).
- Tsagarakis, K.; Carbonell, A.; Brčić, J.; Bellido, J.M.; Carbonara, P.; Casciaro, L.; Edridge, A.; García, T.; González, M.; Šifner, S.K.; et al. Old info for a New Fisheries Policy: Discard Ratios and Lengths at Discarding in EU Mediterranean Bottom Trawl Fisheries. Front. Mar. Sci. 2017, 4, 99. [Google Scholar] [CrossRef]
- EC. Council Regulation No 1967/2006 of 21 December 2006, Concerning Management Measures for the Sustainable Exploitation of Fishery Resources in the Mediterranean Sea, Amending Regulation (EEC) no 2847/93 and Repealing Regulation (EC) no 1626/94. Official Journal of the European Union, 30-12-2006, L 409/11. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1967 (accessed on 29 April 2024).
- GFCM. Recommendation GFCM/33/2009/2 on the Minimum Mesh Size in the Codend of Demersal Trawl Nets. Available online: https://gfcm.sharepoint.com/CoC/_layouts/15/guestaccess.aspx?docid=0f0a719040a464e78ad76682d9858b5ff&authkey=ASSjcmI7_zXp75a_mgie4P8 (accessed on 29 April 2024).
- Stergiou, K.I.; Moutopoulos, D.K.; Tsikliras, A.C. Spatial and temporal variability in Hellenic marine fisheries landings. In State of Hellenic Fisheries; Papaconstantinou, C., Zenetos, A., Vassilopoulou, V., Tserpes, G., Eds.; Hellenic Centre for Marine Research: Athens, Greece, 2007; pp. 141–150. [Google Scholar]
- Stergiou, K.I.; Moutopoulos, D.K.; Tsikliras, A.C.; Papaconstantinou, C. Hellenic marine fisheries: A general perspective from the national statistical service data. In State of Hellenic Fisheries; Papaconstantinou, C., Zenetos, A., Vassilopoulou, V., Tserpes, G., Eds.; Hellenic Centre for Marine Research: Athens, Greece, 2007; pp. 132–140. [Google Scholar]
- Papaconstantinou, C.; Stergiou, K.I. Biology and fisheries of eastern Mediterranean hake (M. merluccius). In Hake; Alheit, J., Pitcher, T.J., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 149–180. [Google Scholar]
- FAO. FishStatJ—Software for Fishery and Aquaculture Statistical Time Series. Available online: https://www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 29 April 2024).
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Quaas, M.; Matz-Lück, N. Status and rebuilding of European fisheries. Mar. Pol. 2018, 93, 159–170. [Google Scholar] [CrossRef]
- Mahe, K.; Amara, R.; Bryckaert, T.; Kacher, M.; Brylinski, J.M. Ontogenetic and spatial variation in the diet of hake (Merluccius merluccius) in the Bay of Biscay and the Celtic Sea. ICES J. Mar. Sci. 2007, 64, 1210–1219. [Google Scholar] [CrossRef]
- Murua, H. The Biology and Fisheries of European Hake, Merluccius merluccius, in the North-East Atlantic. Adv. Mar. Biol. 2010, 58, 97–154. [Google Scholar] [CrossRef]
- Cartes, J.E.; Rey, J.; Lloris, D.; Gil de Sola, L. Influence of environmental variables on the feeding and diet of European hake (Merluccius merluccius) on the Mediterranean Iberian coasts. J. Mar. Biol. Ass. UK 2004, 84, 831–835. [Google Scholar] [CrossRef]
- Ferraton, F.; Harmelin-Vivien, M.; Mellon-Duval, C.; Souplet, A. Spatio-temporal variation in diet may affect condition and abundance of juvenile European hake in the Gulf of Lions (NW Mediterranean). Mar. Ecol. Prog. Ser. 2007, 337, 197–208. [Google Scholar] [CrossRef]
- Stagioni, M.; Montanini, S.; Vallisneri, M. Feeding habits of European hake, Merluccius merluccius (Actinopterygii: Gadiformes: Merlucciidae), from the Northeastern Mediterranean Sea. Acta Ichthyol. Piscat. 2011, 41, 277–284. [Google Scholar] [CrossRef]
- Philips, A.E. Feeding behavior of the European hake Merluccius merluccius Linnaeus, 1758 (Family: Gadidae) from Egyptian Mediterranean waters off Alexandria. Egypt. J. Aquat. Res. 2012, 38, 39–44. [Google Scholar] [CrossRef]
- D’Iglio, C.; Porcino, N.; Savoca, S.; Profeta, A.; Perdichizzi, A. Ontogenetic shift and feeding habits of the European hake (Merluccius merluccius L., 1758) in Central and Southern Tyrrhenian Sea (Western Mediterranean Sea): A comparison between past and present data. Ecol. Evol. 2022, 12, e8634. [Google Scholar] [CrossRef] [PubMed]
- D’Iglio, C.; Famulari, S.; Albano, M.; Giordano, M.; Rinelli, P.; Capillo, G.; Spanò, N.; Savoca, S. Time-Scale Analysis of Prey Preferences and Ontogenetic Shift in the Diet of European Hake Merluccius merluccius (Linnaeus, 1758) in Southern and Central Tyrrhenian Sea. Fishes 2022, 7, 167. [Google Scholar] [CrossRef]
- Riccioni, G.; Stagioni, M.; Piccinetti, C.; Libralato, S. A metabarcoding approach for the feeding habits of European hake in the Adriatic Sea. Ecol. Evol. 2018, 8, 10435–10447. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Stagioni, M.; Manfredi, C.; Tinti, F.; Piccinetti, C.; Libralato, S. DNA metabarcoding suggests dietary niche partitioning in the Adriatic European hake. Sci. Rep. 2022, 12, 1343. [Google Scholar] [CrossRef] [PubMed]
- Gül, G. Feeding ecology of European hake: Insights from stomach content and stable isotope analyses. Reg. Stud. Mar. Sci. 2024, 69, 103314. [Google Scholar] [CrossRef]
- Caragitsou, E.; Tsimenidis, N. The food of hake (Merluccius merluccius) in the Saronikos Gulf. Thalassographica 1977, 1, 232–244. [Google Scholar]
- Papaconstantinou, C.; Caragitsou, E. The food of hake (Merluccius merluccius) in Greek seas. Vie Milieu 1987, 37, 21–29. [Google Scholar]
- Labropoulou, M.; Markakis, G. Morphological-dietary relationships within two assemblages of marine demersal fishes. Environ. Biol. Fishes 1998, 51, 309–319. [Google Scholar] [CrossRef]
- Karachle, P. Feeding Ecology of the Most Important Fish Stock in the North Aegean Sea. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2008. [Google Scholar]
- Yannopoulos, C. The feeding niche of Merluccius merluccius L. and its influence on Length-Weight relationship. Rapp. Comm. Int. Mer Médit. 1976, 24, 69–71. [Google Scholar]
- Papaconstantinou, C.; Caragitsou, E. Diet of juvenile hake (M. merluccius) on the nursery grounds. In Proceedings of the 1st World Fisheries Congress, Athens, Greece, January 1992; pp. 341–350. [Google Scholar]
- Link, J.S. What does ecosystem-based fisheries management mean. Fisheries 2002, 27, 18–21. [Google Scholar]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B.; et al. Ecosystem-based fishery management. Science 2004, 305, 346–347. [Google Scholar] [CrossRef]
- EU. Regulation (EU) No 1380/2013 of the European Parliament and the Council of 11 December 2013 on the Common Fisheries Policy, Amending Council Regulations (EC) No 1954/2003 and (EC) No 1224/2009 and repealing Council Regulations (EC) No 2371/2002 and (EC) No 639/2004 and Council Decision 2004/585/EC. Official Journal of the European Union, 28.12.2013, L 354/22. Available online: https://eur-lex.europa.eu/eli/reg/2013/1380/oj (accessed on 29 April 2024).
- EC. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Official Journal of the European Union, 25-6-2008, L 164/19. Available online: https://eur-lex.europa.eu/eli/dir/2008/56/oj (accessed on 29 April 2024).
- EU. Directive 2014/89/EU of the European Parliament and of the Council of 23 July 2014 Establishing a Framework for Maritime Spatial Planning. Official Journal of the European Union, 28-8-2014, L 257/135. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014L0089 (accessed on 29 April 2024).
- Tsagarakis, K.; Coll, M.; Giannoulaki, M.; Somarakis, S.; Papaconstantinou, C.; Machias, A. Food-web traits of the North Aegean Sea ecosystem (Eastern Mediterranean) and comparison with other Mediterranean ecosystems. Estuar. Coast. Shelf Sci. 2010, 88, 233–248. [Google Scholar] [CrossRef]
- ELSTAT. Hellenic Statistical Authority. Sea Fisheries Statistics. Available online: https://www.statistics.gr/en/statistics/-/publication/SPA03/- (accessed on 29 April 2024).
- RCG Med&BS. Strengthening regional cooperation in the area of fisheries data collection in the Mediterranean and Black Sea, D0.2. Final Report: Deliverable 3.3. Protocols and guidelines for sampling, processing and analysing the stomach contents. Agreement number MARE/2014/19–SI2.705484.
- RCG Med&BS. Workshop on Sampling, Processing and Analysing the Stomach Contents (WKSTCON). WKSTCON Report 2018, 24–27 April 2018, Palma de Mallorca, Spain.
- RCG Med&BS. Strengthening Regional Cooperation in the Area of Fisheries Data Collection in the Mediterranean and Black Sea (STREAM), D0.3 Final Report: Deliverable, D. 4.1. Updated Protocols and Guidelines for Collection, Processing and Analysis of Stomach Contents. Agreement Number MARE/2016/22–SI2.770115. Available online: https://datacollection.jrc.ec.europa.eu/documents/d/dcf/med-and-bs_stream_mare-2016-22 (accessed on 29 April 2024).
- RCG NA, NS & EA, RCG Baltic. ISSG Regionally Coordinated Stomach Sampling. Intersessional Subgroup (ISSG) 2022–2023 Reports. Available online: https://datacollection.jrc.ec.europa.eu/documents/d/dcf/2023_rcg_nansea-rcg-baltic_tm-rpt_part-iii (accessed on 29 April 2024).
- Ferry, L.A.; Cailliet, G.M. Sample size and data analysis: Are we characterizing and comparing diet properly? In GUTSHOP ’96, Feeding Ecology and Nutrition in Fish, Proceedings of the International Congress on the Biology of Fishes, San Francisco, CA, USA, 14–18 July 1996; American Fisheries Society: Washington, DC, USA, 1996; pp. 71–80. [Google Scholar]
- Tiralongo, F.; Messina, G.; Cazzolla Gatti, R.; Tibullo, D.; Lombardo, B.M. Some biological aspects of juveniles of the rough ray, Raja radula Delaroche, 1809 in Eastern Sicily (central Mediterranean Sea). J. Sea Res. 2018, 142, 174–179. [Google Scholar] [CrossRef]
- Hureau, J.-C. Biologie comparee de quelques poissons antarctiques (Nototheniidae). Bull. Mus. Oceanogr. Monaco 1970, 68, 1–244. [Google Scholar]
- Graham, B.S.; Grubbs, D.; Holland, K.; Popp, B.N. A rapid ontogenetic shift in the diet of juvenile yellowfin tuna from Hawaii. Mar. Biol. 2007, 150, 647–658. [Google Scholar] [CrossRef]
- Anderson, M. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Hyslop, J. Stomach content analysis: A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Pinkas, L.; Oliphant, M.; Iverson, K. Food habits of albacore, bluefin tuna and bonito in California waters. Calif. Dep. Fish Game Fish. Bull. 1971, 152, 1–150. [Google Scholar]
- Hacunda, S. Trophic relationships among demersal fishes in a coastal area of the Gulf of Maine. Fish. Bull. 1981, 79, 775–788. [Google Scholar]
- Cortes, E. A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 1997, 54, 726–738. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Van Valen, L. Morphological variation and width of ecological niche. Am. Nat. 1965, 99, 377–389. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanback, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Yang, L.H.; Fordyce, J.A.; Davis, J.M.; Svanback, R. Measuring individual-level resource specialization. Ecology 2002, 83, 2936–2941. [Google Scholar] [CrossRef]
- Roughgarden, J. Evolution of niche width. Am. Nat. 1972, 106, 683–718. [Google Scholar] [CrossRef]
- Costello, M.J. Predator feeding strategy and prey importance: A new graphical analysis. J. Fish Biol. 1990, 36, 261–263. [Google Scholar] [CrossRef]
- Amundsen, P.-A.; Gabler, H.-M.; Staldvik, F.J. A new method for graphical analysis of feeding strategy from stomach contents data. J. Fish Biol. 1996, 48, 607–614. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 3rd ed.; Addison-Wesley Educational Publishers, Inc.: Boston, MA, USA, 2014. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 30 January 2024).
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open-source software for exploring and manipulating networks. In Proceedings of the Third International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Werner, E.E.; Gilliam, J.F. The ontogenetic niche and species interactions in size-structured populations. Ann. Rev. Ecol. Syst. 1984, 15, 393–425. [Google Scholar] [CrossRef]
- Nakazawa, T. Ontogenetic niche shifts matter in community ecology: A review and future perspectives. Popul. Ecol. 2015, 57, 347–354. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Nunn, A.D.; Adams, C.E.; Amundsen, P.-A. Causes and consequences of ontogenetic dietary shifts: A global synthesis using fish models. Biol. Rev. 2019, 94, 539–554. [Google Scholar] [CrossRef]
- Amundsen, P.-A.; Bøhn, T.; Popova, O.A.; Staldvik, F.J.; Reshetnikov, Y.S.; Kashulin, N.A.; Lukin, A. Ontogenetic niche shifts and resource partitioning in a subarctic piscivore fish guild. Hydrobiologia 2003, 497, 109–119. [Google Scholar] [CrossRef]
- Kolasinski, J.; Frouin, P.; Sallon, A.; Rogers, K.; Bruggemann, H.J.; Potier, M. Feeding ecology and ontogenetic dietary shift of yellowstripe goatfish Mulloidichthys flavolineatus (Mullidae) at Reunion Island, SW Indian Ocean. Mar. Ecol. Prog. Ser. 2009, 386, 181–195. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Prati, S.; Henriksen, E.H.; Smalås, A.; Knudsen, R.; Klemetsen, A.; Amundsen, P.-A. Exploring temporal patterns in fish feeding ecology: Are ontogenetic dietary shifts stable over time? Rev. Fish Biol. Fish. 2022, 32, 1141–1155. [Google Scholar] [CrossRef]
- Loutrage, L.; Brind’Amour, A.; Chouvelon, T.; Spitz, J. Ontogenetic shift or not? Different foraging trade-offs within the meso- to bathypelagic fish community. Ecol. Evol. 2023, 14, e11129. [Google Scholar] [CrossRef]
- Hjelm, J.; Persson, L.; Christensen, B. Growth, morphological variation and ontogenetic niche shifts in perch (Perca fluviatilis) in relation to resource availability. Oecologia 2000, 122, 190–199. [Google Scholar] [CrossRef]
- Yasuno, N.; Fujimoto, Y.; Shimada, T.; Shikano, S.; Kikuchi, E. Ontogenetic dietary shifts of largemouth bass do not increase trophic position in a shallow eutrophic lake in Japan. Ann. Limnol. Int. J. Lim. 2016, 52, 355–364. [Google Scholar] [CrossRef]
- García-Berthou, E. Ontogenetic Diet Shifts and Interrupted Piscivory in Introduced Largemouth Bass (Micropterus salmoides). Internat. Rev. Hydrobiol. 2002, 4, 353–363. [Google Scholar] [CrossRef]
- Du Buit, M.H. Diet of hake (Merluccius merluccius) in the Celtic Sea. Fish. Res. 1996, 28, 381–394. [Google Scholar] [CrossRef]
- Punt, A.T.; Leslie, R.W.; Duplessis, S.E. Estimation of the annual consumption food by cape hake, Merluccius capensis and M. paradoxus, off the South African west coast. S. Afr. J. Mar. Sci. 1992, 12, 611–634. [Google Scholar] [CrossRef][Green Version]
- Lloret-Lloret, E.; Navarro, J.; Giménez, J.; López, N.; Albo-Puigserver, M.; Grazia Pennino, M.; Coll, M. The Seasonal Distribution of a Highly Commercial Fish Is Related to Ontogenetic Changes in Its Feeding Strategy. Front. Mar. Sci. 2020, 7, 566686. [Google Scholar] [CrossRef]
- Perdichizzi, A.; Pirrera, L.; Giordano, D.; Perdichizzi, F.; Busalacchi, B.; Profeta, A.; Bottari, T.; Rinelli, P. Distribution patterns and population structure of Illex coindetii (Cephalopoda: Ommastrephidae) in the Southern Tyrrhenian Sea: Historical series of 14 years trawl survey. Fish. Res. 2011, 109, 342–350. [Google Scholar] [CrossRef]
- Smale, M.J. Cephalopods as prey. IV. Fishes. Phil. Trans. R. Soc. Lond. B 1996, 351, 1067–1081. [Google Scholar] [CrossRef]
- Fanelli, E.; Rumolo, P.; Barra, M.; Basilone, G.; Genovese, S.; Bonnano, A. Mesoscale variability in the trophic ecology of the European hake Merluccius merluccius in the Strait of Sicily. Hydrobiologia 2018, 821, 57–72. [Google Scholar] [CrossRef]
- Scharf, F.S.; Juanes, F.; Rountree, R.A. Predator size—Prey size relationships of marine fish predators: Interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Prog. Ser. 2000, 208, 229–248. [Google Scholar] [CrossRef]
- Pyke, G.H. Optimal foraging theory: A critical review. Ann. Rev. Ecol. Syst. 1984, 15, 523–575. [Google Scholar] [CrossRef]
- Dill, L.M. Adaptive Flexibility in the Foraging Behavior of Fishes. Can. Fish. Aquat. Sci. 1983, 40, 398–408. [Google Scholar] [CrossRef]
- Preciado, I.; Punzón, A.; Velasco, F. Spatio-temporal variability in the cannibalistic behaviour of European hake Merluccius merluccius: The influence of recruit abundance and prey availability. J. Fish. Biol. 2015, 86, 1319–1334. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Clare, E.L.; Hayden, B.; Brett, M.T.; Kratina, P. Diet tracing in ecology: Method comparison and selection. Methods Ecol. Evol. 2017, 9, 278–291. [Google Scholar] [CrossRef]
- Carpentieri, P.; Colloca, F.; Cardinale, M.; Belluscio, A.; Ardizzone, G.D. Feeding habits of European hake (Merluccius merluccius) in the central Mediterranean Sea. Fish. Bull. 2005, 103, 411–416. [Google Scholar]
- Hurst, T.P.; Duffy, T.A. Activity patterns in northern rock sole are mediated by temperature and feeding history. J. Exp. Mar. Biol. Ecol. 2005, 325, 201–213. [Google Scholar] [CrossRef]
- Peck, M.A.; Buckley, L.J.; Bengtson, D.A. Effects of temperature and body size on the swimming speed of larval and juvenile Atlantic cod (Gadus morhua): Implications for individual based modelling. Environ. Biol. Fishes 2006, 75, 419–429. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef]
- Free, C.M.; Thorson, J.T.; Pinsky, M.L.; Oken, K.L.; Wiedenmann, J.; Jensen, O.P. Impacts of historical warming on marine fisheries production. Science 2019, 363, 979–983. [Google Scholar] [CrossRef]
- Zhou, S.; Smith, A.D.M.; Punt, A.E.; Richardson, A.J.; Gibbs, M.; Fulton, E.; Pascoe, S.; Bulman, C.; Bayliss, P.; Sainsbury, K. Ecosystem-based fisheries management requires a change to the selective fishing philosophy. Proc. Natl. Acad. Sci. USA 2010, 107, 9485–9489. [Google Scholar] [CrossRef]
- Punt, A.E.; Ortiz, I.; Aydin, K.Y.; Hunt, G.L., Jr.; Wiese, F.K. End-to-end modeling as part of an integrated research program in the Bering Sea. Deep-Sea Res. II Top. Stud. Oceanogr. 2016, 134, 413–423. [Google Scholar] [CrossRef]
- Zhou, S.; Kolding, J.; Garcia, S.M.; Plank, M.J.; Bundy, A.; Charles, A.; Hansen, C.; Heino, M.; Howell, D.; Jacobsen, N.S.; et al. Balanced harvest: Concept, policies, evidence, and management implications. Rev. Fish Biol. Fish. 2019, 29, 711–733. [Google Scholar] [CrossRef]
- Sun, R.; Sun, P.; Fu, C.; Liu, G.; Liang, Z.; Shin, Y.-J.; Barrier, N.; Tian, Y. Exploring balanced harvest as a potential strategy for highly exploited multispecies fisheries. ICES J. Mar. Sci. 2023, 80, 897–910. [Google Scholar] [CrossRef]
- Thompson, R.M.; Hemberg, M.; Starzomski, B.M.; Shurin, J.B. Trophic levels and trophic tangles: The prevalence of omnivory in real food webs. Ecology 2007, 88, 612–617. [Google Scholar] [CrossRef]
- Pinnegar, K.; Trenkel, V.M.; Tidd, A.N.; Dawson, W.A.; Du Buit, M.H. Does diet in Celtic Sea fishes reflect prey availability? J. Fish Biol. 2003, 63, 197–212. [Google Scholar] [CrossRef]
- Roswell, M.; Dushoff, J.; Winfree, R. A conceptual guide to measuring species diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 39–54. [Google Scholar]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Shannon, C. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]

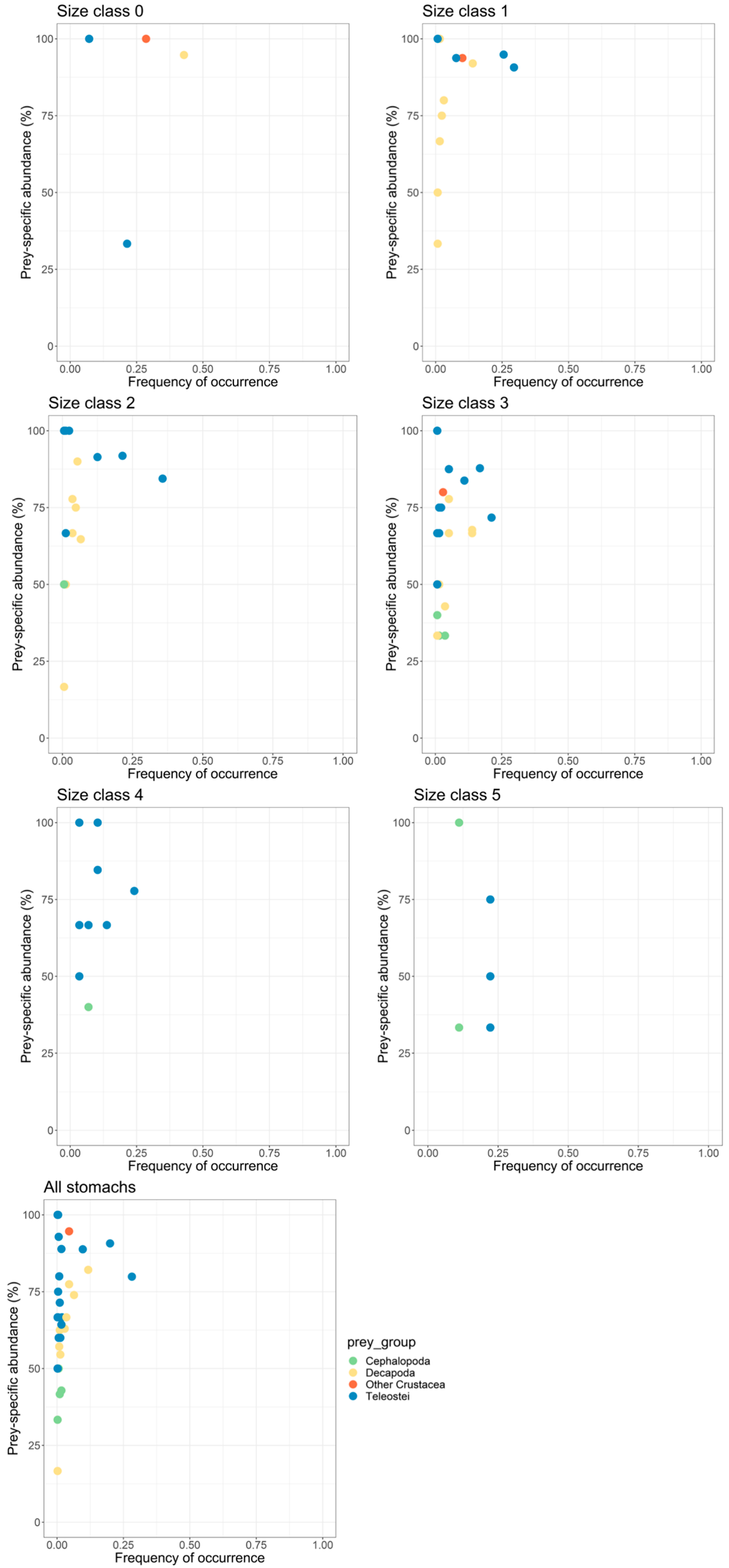
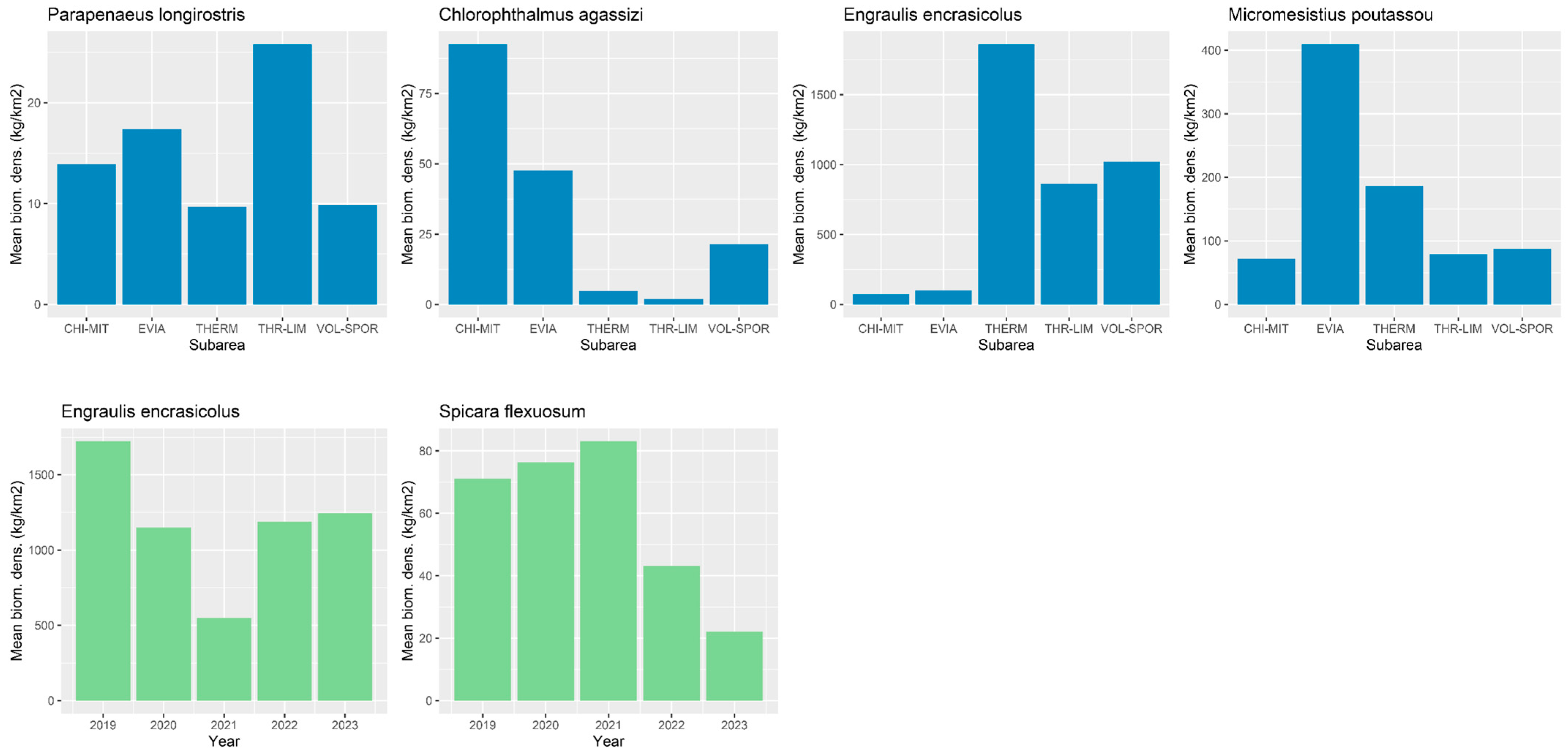
| Stomachs (n) | VI (%) | Mean RI (‰) | |
|---|---|---|---|
| Body-size class | |||
| 0 | 26 | 30.77 | 30.80 |
| 1 | 225 | 32.44 | 43.50 |
| 2 | 261 | 33.33 | 34.67 |
| 3 | 203 | 31.53 | 21.05 |
| 4 | 40 | 25.00 | 22.94 |
| 5 | 14 | 35.71 | 77.10 |
| Year | |||
| 2019 | 95 | 37.89 | 28.36 |
| 2020 | 138 | 36.23 | 36.48 |
| 2021 | 152 | 37.50 | 27.03 |
| 2022 | 232 | 33.62 | 34.21 |
| 2023 | 152 | 17.11 | 38.02 |
| Subarea | |||
| CHI-MIT | 90 | 24.44 | 38.94 |
| EVIA | 112 | 24.11 | 32.78 |
| THERM | 153 | 46.41 | 35.26 |
| THR-LIM | 303 | 30.36 | 36.15 |
| VOL-SPOR | 111 | 31.53 | 20.56 |
| Body-Size Classes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prey Taxa | Abbreviation | FG | 0 | 1 | 2 | 3 | 4 | 5 | All Stomachs |
| Cephalopoda | 0.04 | 0.76 | 0.75 | 36.44 | 0.91 | ||||
| Abralia veranyi | Abrver | 2 | 0.01 | 0.05 | 1.84 | 0.02 | |||
| Illex coindetii | Illcoi | 2 | 0.01 | 1.04 | 34.45 | 1.28 | |||
| Octopus vulgaris | Octvul | 1 | 2.82 | 0.01 | |||||
| Rossia macrosoma | Rosmac | 1 | 0.22 | 0.02 | |||||
| Sepiidae | Sep | 1 | 1.07 | 0.01 | |||||
| Sepiola sp. | Sepsp. | 1 | 0.02 | * | |||||
| Todarodes sagittatus | Todsag | 2 | 4.14 | 0.03 | |||||
| Cephalopoda | Cep | 0 | 0.01 | 0.08 | 1.28 | 0.04 | |||
| Decapoda | 46.74 | 12.06 | 6.50 | 19.20 | 5.52 | 11.40 | |||
| Alpheidae | Alp | 3 | 0.03 | 0.01 | |||||
| Alpheus glaber | Alpgla | 3 | 0.02 | 0.04 | 0.08 | 0.04 | |||
| Alpheus sp. | Alpsp. | 3 | 0.05 | 0.03 | 0.08 | 0.05 | |||
| Chlorotocus crassicornis | Chlcra | 3 | 0.07 | 0.39 | 0.67 | 0.73 | 0.39 | ||
| Munididae | Mun | 3 | 0.01 | * | |||||
| Natantia | Nat | 0 | 1.28 | 0.23 | 0.66 | 1.35 | 1.43 | 0.89 | |
| Parapenaeus longirostris | Parlon | 5 | 0.08 | 1.30 | 13.45 | 1.36 | 2.72 | ||
| Pasiphaea multidentata | Pasmul | 4 | 0.02 | 0.06 | 0.03 | 0.03 | |||
| Pasiphaea sp. | Passp. | 4 | 0.01 | * | |||||
| Plesionika edwardsii | Pleedw | 3 | 0.03 | * | |||||
| Plesionika giglioli | Plegig | 3 | 0.01 | * | |||||
| Plesionika heterocarpus | Plehet | 3 | 0.01 | 0.01 | 0.01 | ||||
| Plesionika sp. | Plesp. | 3 | 0.18 | 0.42 | 1.47 | 0.38 | 0.57 | ||
| Sergestidae | Ser | 4 | 0.14 | 0.01 | |||||
| Sergestes sp. | Sersp. | 4 | 0.01 | * | |||||
| Solenocera crassicornis | Solcra | 3 | 0.01 | * | |||||
| Solenocera membranacea | Solmem | 3 | 0.03 | * | |||||
| Decapoda | Dec | 0 | 43.90 | 10.29 | 0.94 | 7.99 | 4.52 | 8.09 | |
| Other Crustacea | 30.91 | 0.76 | 0.09 | 0.05 | 0.30 | ||||
| Squilla mantis | Squman | 3 | 0.02 | * | |||||
| Crustacea | Cru | 0 | 39.78 | 2.25 | 0.35 | 0.35 | 1.59 | ||
| Teleostei | 22.36 | 87.18 | 93.46 | 79.95 | 93.68 | 63.56 | 87.40 | ||
| Argentina sphyraena | Argsph | 7 | 0.04 | * | |||||
| Boops boops | Booboo | 6 | 0.06 | * | |||||
| Cepola macrophthalma | Cepmac | 6 | 0.02 | 0.01 | 0.01 | ||||
| Ceratoscopelus maderensis | Cermad | 8 | 0.32 | 0.03 | 0.08 | ||||
| Chauliodus sloani | Chaslo | 8 | 0.08 | 0.01 | |||||
| Chlorophthalmus agassizi | Chlaga | 7 | 0.53 | 0.75 | 8.26 | 0.41 | |||
| Deltentosteus quadrimaculatus | Delqua | 6 | 0.01 | * | |||||
| Engraulis encrasicolus | Engenc | 10 | 54.68 | 32.87 | 25.56 | 9.08 | 8.60 | 32.18 | |
| Gadiculus argenteus | Gadarg | 10 | 0.08 | 0.02 | 0.19 | 0.07 | |||
| Hymenocephalus italicus | Hymita | 9 | 0.90 | * | |||||
| Lampanyctus crocodilus | Lamcro | 8 | 0.03 | * | |||||
| Lepidopus caudatus | Lepcau | 9 | 0.02 | 1.61 | 0.03 | ||||
| Lesueurigobius friesii | Lesfri | 6 | 0.16 | 0.01 | 0.03 | ||||
| Merluccius merluccius | Mermer | 6 | 0.03 | * | |||||
| Micromesistius poutassou | Micpou | 8 | 0.01 | 0.02 | 14.86 | 25.84 | 0.98 | ||
| Myctophidae | Myc | 8 | 3.55 | 14.71 | 10.24 | 0.32 | 9.09 | ||
| Nettastoma melanura | Netmel | 8 | 0.20 | 0.02 | |||||
| Ophisurus serpens | Ophser | 6 | 0.34 | 0.03 | |||||
| Phycis blennoides | Phyble | 9 | 0.03 | * | |||||
| Sardinella aurita | Saraur | 10 | 3.27 | 0.33 | 0.05 | ||||
| Sardina pilchardus | Sarpil | 10 | 0.10 | 30.38 | 0.32 | ||||
| Serranus hepatus | Serhep | 6 | 0.05 | * | |||||
| Sparidae | Spa | 6 | 0.03 | 2.69 | 0.03 | ||||
| Spicara flexuosum | Spifle | 6 | 0.04 | 6.08 | 0.58 | ||||
| Spicara sp. | Spisp. | 6 | 0.08 | 0.07 | 0.03 | ||||
| Stomiidae | Sto | 8 | 0.05 | * | |||||
| Stomias boa | Stoboa | 8 | 0.06 | * | |||||
| Trachurus mediterraneus | Tramed | 10 | 0.69 | * | |||||
| Trachurus trachurus | Tratra | 10 | 0.09 | 0.12 | 0.89 | 14.05 | 0.39 | ||
| Osteichthyes | Ost | 0 | 11.78 | 28.07 | 47.19 | 28.79 | 27.46 | 39.81 | |
| Body-Size Classes | Cxy | Years | Cxy | Subareas | Cxy | |||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 0.41 | 2019 | 2020 | 0.46 | CHI-MIT | EVIA | 0.70 |
| 0 | 2 | 0.13 | 2019 | 2021 | 0.53 | CHI-MIT | THERM | 0.46 |
| 0 | 3 | 0.21 | 2019 | 2022 | 0.41 | CHI-MIT | THR-LIM | 0.57 |
| 0 | 4 | 0.18 | 2019 | 2023 | 0.36 | CHI-MIT | VOL-SPOR | 0.40 |
| 0 | 5 | 0.00 | 2020 | 2021 | 0.57 | EVIA | THERM | 0.44 |
| 1 | 2 | 0.61 | 2020 | 2022 | 0.63 | EVIA | THR-LIM | 0.57 |
| 1 | 3 | 0.61 | 2020 | 2023 | 0.51 | EVIA | VOL-SPOR | 0.45 |
| 1 | 4 | 0.45 | 2021 | 2022 | 0.62 | THERM | THR-LIM | 0.60 |
| 1 | 5 | 0.13 | 2021 | 2023 | 0.54 | THERM | VOL-SPOR | 0.63 |
| 2 | 3 | 0.69 | 2022 | 2023 | 0.58 | THR-LIM | VOL-SPOR | 0.57 |
| 2 | 4 | 0.46 | ||||||
| 2 | 5 | 0.17 | ||||||
| 3 | 4 | 0.53 | ||||||
| 3 | 5 | 0.19 | ||||||
| 4 | 5 | 0.23 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evangelopoulos, A.; Geropoulos, A.; Kamidis, N.; Koutrakis, E. Ontogenetic, Spatial and Inter-Annual Variability in the Diet of European Hake Merluccius merluccius Linnaeus, 1758, in the North Aegean Sea. Fishes 2024, 9, 257. https://doi.org/10.3390/fishes9070257
Evangelopoulos A, Geropoulos A, Kamidis N, Koutrakis E. Ontogenetic, Spatial and Inter-Annual Variability in the Diet of European Hake Merluccius merluccius Linnaeus, 1758, in the North Aegean Sea. Fishes. 2024; 9(7):257. https://doi.org/10.3390/fishes9070257
Chicago/Turabian StyleEvangelopoulos, Athanasios, Antonios Geropoulos, Nikolaos Kamidis, and Emmanouil Koutrakis. 2024. "Ontogenetic, Spatial and Inter-Annual Variability in the Diet of European Hake Merluccius merluccius Linnaeus, 1758, in the North Aegean Sea" Fishes 9, no. 7: 257. https://doi.org/10.3390/fishes9070257
APA StyleEvangelopoulos, A., Geropoulos, A., Kamidis, N., & Koutrakis, E. (2024). Ontogenetic, Spatial and Inter-Annual Variability in the Diet of European Hake Merluccius merluccius Linnaeus, 1758, in the North Aegean Sea. Fishes, 9(7), 257. https://doi.org/10.3390/fishes9070257






