Abstract
The deep-sea Caribbean lobster (Metanephrops binghami) and the Norway lobster (Nephrops norvegicus) are Nephropidae species of high commercial interest. Although the first one still remains unexploited, the second is overexploited in the Mediterranean Sea. For effective fisheries management, size at sexual maturity is an essential indicator to protect immature individuals from exploitation. The estimation of this indicator can, however, be biased due to the difficulty of differentiating juveniles from adults by their size structure due to the natural process of molting. This study aims to estimate the size at sexual maturity of M. binghami and N. norvegicus females by comparing the effectiveness of the morphometric method versus the macroscopic evaluation of gonad maturity. Samples of M. binghami were collected from the Colombian Caribbean Sea in August and December 2009, March and May 2010, and August 2020 to May 2021. Samples of N. norvegicus were collected from the northwestern Mediterranean Sea from 2019 to 2022. Similar sizes at sexual maturity were found for M. binghami between the morphometric approach (ranging from 28.6 to 33.9 mm cephalothorax length, CL) and the gonadal staging approach (31.4 mm CL). Conversely, for N. norvegicus, the morphometric approach yielded higher measurements (between 27.2 and 30.4 mm CL) than the gonadal approach (26.0 mm CL). This discrepancy might stem from the intense fishing overexploitation conditions of N. norvergicus, leading to a physiological adaptation that enables earlier gonadal maturation at faster rates than morphometric adaptation. Further research is required to elucidate these discrepancies and the effect of overexploitation on physiological (i.e., mature gonads) and functional maturity (i.e., capacity to brood eggs at a larger size).
Key Contribution:
This research is the first comparative study on the morphological sexual maturity of Nephropidae species populations unexploited in the Colombian Caribbean and overexploited in the Mediterranean Sea.
1. Introduction
Deep-sea crustaceans are important constituents of demersal megafauna, holding substantial potential for global fisheries [1,2]. The deep-sea clawed Nephropidae species, the Caribbean lobster Metanephrops binghami and the Norway lobster Nephrops norvegicus, are of high commercial interest [3]. Nephrops norvegicus inhabits the Mediterranean Sea and the Atlantic Ocean [3] and faces significant overexploitation, exceeding three times the maximum sustainable yield in the northwestern (NW) Mediterranean [4] (STECF 2022). Differently, M. binghami in the Colombian Caribbean Sea still remains, to date, unexploited [5].
Morphologically, both species are relatively small compared to other Nephropids such as Homarus spp. The mean cephalothorax length (CL) of M. binghami is approximately 34 mm [5], while that of N. norvegicus is around 30 mm [6]. Both lobsters show a very similar ecological niche and inhabit areas of soft sediments within self-made burrows, around which strong territorial behavior is associated [5,7]. Life trait similarities make M. binghami a valuable reference species to understand the effects of fishery management on exploited N. norvegicus stocks. The species establishes an unexploited baseline scenario for N. norvegicus stock recovery, given that the unexploited baseline was lost in Europe decades ago.
For effective fisheries management, size at sexual maturity is an essential indicator for protecting immature individuals from exploitation while ensuring sustainable harvesting that aligns with the reproductive capacity of populations [8,9]. M. binghami has been poorly studied in the Colombian Caribbean Sea, while N. norvegicus has traditionally been an object of fishery studies relating size and reproductive cycle, especially in the Mediterranean Sea. The estimation of its average CL at sexual maturity has been made through analyses of the different stages of gonadal maturity and associations with size classes [10,11,12]. However, the estimation of size at sexual maturity based on gonadal status can be biased due to possible visual errors in assigning maturity stages. In crustaceans, there is difficulty in differentiating juveniles from adults by size structure due to the natural process of molting to shed their exoskeleton in order to grow larger [13,14].
Other methods, such as morphometric analyses, could describe allometric growth changes linked to maturity aspects, being less affected by temporal and demographic fluctuations [15]. The change in growth at the beginning of sexual reproduction in crustaceans can be identified by analyzing morphometric relationships with the aim of differentiating juveniles and adults, and thus size at sexual maturity [16]. Consequently, it is expected that patterns of morphometric variation indicate differences in growth, as the shape of the body is related to structural changes in the ontogeny of organisms; this is very important for implementing efficient fishery management strategies [8].
In Nephrops norvegicus, size at sexual maturity onset in variable fishery pressure conditions can be attributed to reduced somatic growth, as energy is being used for the construction of gonads at smaller sizes [12,17,18,19,20]. In crustaceans, this process produces body changes that can be detected by discontinuities of growth at the onset of sexual maturity [18,19,21,22,23]. As a result, such discontinuities allow for analyzing morphometric relationships to differentiate juveniles from adults, and therefore to determine the size at sexual maturity [16]. In unexploited habitats, the size at maturity of N. norvegicus is unknown because unexploited areas in the Atlantic and Mediterranean are absent. In highly exploited fisheries, a decline in the onset of maturity is expected due to the selective removal of larger and more mature individuals from the populations [24]. This can lead to a shift in these populations toward smaller and younger individuals, which can result in a decline in the average size at sexual maturity. N. norvegicus, which has been overexploited for decades in NW Mediterranean fisheries, has had a decline in the size at maturity of females from around 30 mm CL to around 25.3 mm CL in the last 25 years [20]. Similarly, in the Irish Sea, a decline in female size at onset of maturity from 23.6 to 20.6 mm in two decades has been reported for N. norvegicus [25].
Here, the demographic analysis of the ecologically equivalent and unexploited Caribbean M. binghami has strategic value for European fishery management. The morphometric approach has been successfully used to establish a morphometric breakpoint for M. binghami [26] and M. rubellus, the latter in a partially exploited fishery in the Atlantic Sea off Sao Paulo, Brazil [19]. This study aimed to compare the effectiveness of the morphometric approach with the gonadal macroscopic approach to estimate size at sexual maturity for females of unexploited M. binghami and overexploited N. norvegicus.
2. Materials and Methods
2.1. Data Collection for M. binghami and N. norvegicus
Samples of M. binghami were collected from the Colombian Caribbean Sea, between Punta Gallinas and the Gulf of Urabá (Figure 1A), in August and December 2009, March and May 2010, and from August 2020 to May 2021. Considering that Pérez et al. [26] reported the reproductive season for M. binghami to take place in October, the sampling encompassed both the reproductive and non-reproductive seasons, which enabled individuals from the entire size range to be evaluated. Sampling was carried out onboard a commercial bottom trawler, with an opening of 11.6 m at the footrope and a cod-end mesh size of 44.5 mm from knot to knot. A total of 87 fishing trawls were performed in depths ranging from 200 to 550 m, with at least two hauls per 100 m depth stratum. Each haul lasted 30 min and was conducted at an average speed of 2.5 knots. Size at sexual maturity was obtained for 490 females through visual gonadal stage classification, and for 199 females through the morphometric approach.

Figure 1.
Sampling stations for M. binghami ((A), the Colombian Caribbean Sea) and N. norvegicus ((B), the NW Mediterranean Sea). Blue- and orange-colored dots are the trawl sampling events used to gather gonadal and morphometric data.
Samples of N. norvegicus were collected from deep-sea surveys in the northwestern Mediterranean Sea (Balearic Sea) along the Catalan coast (Figure 1B). ICATMAR [27] fishing monitoring program observers collected samples from 2019 to 2022, three times per month, on board bottom trawlers equipped with bottom-trawl nets with a cod-end mesh size of 40 mm squared. Surveys were carried out in slope areas where intensive fishing pressure has been occurring for decades [27]. A total of 197 fishing trawls were performed at 91 and 540 m on the Ebro Delta shelf and between 537 and 373 m on the slope off Blanes (see Figure 1B). The hauls lasted about 1.5 h at an average speed of 2.2 knots. Size at sexual maturity obtained by macroscopic examination of the color of the ovaries, based on Rotllant et al.’s [28] histological examination, was calculated for a total of 3433 females. A total of 116 females covering all size ranges were randomly selected for morphometric analyses. Given that the selective extraction of crustaceans in fisheries exploitation may modify size at sexual maturity, we compared the effectiveness of two methods to detect this change.
2.2. Morphometric Analysis Approach
For the morphometric approach to determine size at sexual maturity for females of M. binghami and N. norvegicus, all specimens were measured using seven body descriptors to the nearest 0.01 mm [29,30,31]: total length (TL); cephalothorax length (CL); first abdominal segment length (FSL); first abdominal segment width (FSW); first abdominal segment height (FSH); hepatic spine width (HSW); antennal spine width (ASW) (Figure 2).

Figure 2.
Morphometric measures of a female of M. binghami: total length (TL); cephalothorax length (CL); first abdominal segment length (FSL); first abdominal segment width (FSW); first abdominal segment height (FSH); hepatic spine width (HSW); and antennal spine width (ASW).
2.3. Data Analysis
The morphometric approach to distinguish juveniles and adults was conducted by means of a principal component analysis (PCA) with two allometric independent variables—TL, CL—and six dependent variables—CL, FSL, FSW, FSH, HSW, ASW—all transformed in a log base. The individuals were assigned to the juvenile and adult groups using a hierarchical cluster. Individuals from both groups were then assigned based on their weight on the two axes of the PCA [32]. Then, a discriminant analysis was performed to assign individuals to the juvenile or adult classes on the basis of the X and Y allometric variables. Size at 50% maturity (L50%) was estimated using a logistic regression, meaning the length at which a randomly chosen specimen had a 50% chance of being mature [31,32,33,34]. In the regression analysis, X (independent explanatory variable) and Y (the dependent response variable) had two alternative statuses (binomial): juveniles: 0; adults: 1. To evaluate differences in linear relationships between the juveniles and adults, an analysis of co-variance was performed (ANCOVA) [35].
2.4. Gonadal Maturity Analysis Approach
Size at sexual maturity was estimated via the classification of female stages of gonadal maturity based on the macroscopic examination of gonadal coloring. Maturity was evaluated only in female individuals of both species, as the maturity of males cannot be ascertained by the macroscopic inspection of gonads [28,36]. For M. binghami, we used five macroscopic stages, which were validated through histological observation [26]: 1, white, immature; 2, opaque, in development; 3, yellow, maturing; 4, green, mature; and finally, 5, ovigerous, carrying eggs on its pleopods. For N. norvegicus, we also used five maturity stages supported by previous histological analyses [37]: 1, white, immature, slender and thin ovaries; 2, resting, cream-yellowish; 3, beginning of maturation, small, light green; 4, big, thick, light green; and finally, 5, dark green, with advanced maturations in pre-spawning or spawning phases, also referred to as berried females.
The estimation of size at 50% maturity was carried out by equating the criteria defining maturity stages for both species. We defined mature females of M. binghami as those within stages 3 to 5, excluding stage 1 representing immature juvenile individuals and stage 2, representing individuals not yet mature, but in development. For N. norvegicus, mature individuals were defined from stage 2 to 5, with stage 1 representing immature juvenile individuals and those in development but not yet mature. The gonadal size at sexual maturity (L50%, CL and TL) was estimated using a logistic regression of the Bayesian Generalized Linear Model (GLM) (R package). We calculated the parameters a and b of the logistic function [16], where P(L) is the mature female proportion. The size at 50% maturity was obtained by L50% = (−a/b) [11].
3. Results
The total length (TL) of females of M. binghami ranged from 63.80 to 160.18 mm (122.87 ± standard deviation (SD) 22.97 mm) and the cephalothorax length (CL) ranged from 18.42 to 46.05 mm (35.25 ± 6.63 mm). Nephrops norvegicus females showed TL between 75.70 and 140.50 mm (103.46 ± 15.92 mm), and CL ranging from 22.26 to 44.36 mm (31.23 ± 5.13 mm). The morphometric relationships of M. binghami and N. norvegicus for both juveniles and adults were highly correlated between TL vs. CL, FSL, FSW, FSH, HSW, and ASW (Table 1 and Table 2). The ANCOVA showed statistically significant differences between parameter a (intercept) of females for both species in all linear relationships, as well as in parameter b (slope) between TL vs. FSW and FSH, and CL vs. TL, FSW, FSH, and HSW for M. binghami (Figure 3; Table 1). In contrast, there was no significant difference in parameter b for N. norvegicus (Figure 3; Table 2) or M. binghami between TL vs. CL, FSL, HSW and ASW, or CL vs. FSL and ASW (Figure 3; Table 1). Juveniles were distinguished from adults for M. binghami and N. norvegicus (Figure 3 and Figure 4; Table 1 and Table 2) through morphometric relationships using discriminant functions.

Table 1.
Parameters of morphometric relationships in females of M. binghami: total length (TL), cephalothorax length (CL), first abdominal segment length (FSL), first abdominal segment width (FSW), first abdominal segment height (FSH), hepatic spine width (HSW), and antennal spine width (ASW). N: number of specimens; r2: determination coefficient. The numbers marked in bold denote significant differences from intercept and slope of morphometric relationships.

Table 2.
Parameters of morphometric relationships in females of N. norvegicus: total length (TL), cephalothorax length (CL), first abdominal segment length (FSL), first abdominal segment width (FSW), first abdominal segment height (FSH), hepatic spine width (HSW), and antennal spine width (ASW). N: number of specimens; r2: determination coefficient. The numbers marked in bold denote significant differences from intercept and slope of morphometric relationships.
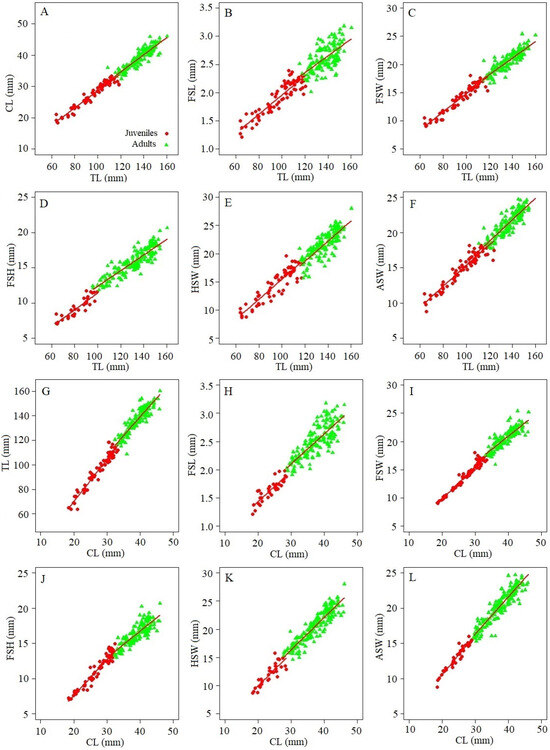
Figure 3.
Morphometric relationships using discriminant functions for juvenile and adult females of M. binghami. (A): TL vs. CL; (B): TL vs. FSL; (C): TL vs. FSW; (D): TL vs. FSH; (E): TL vs. HSW; (F): TL vs. ASW; (G): CL vs. TL; (H): CL vs. FSL; (I): CL vs. FSW; (J): CL vs. FSH; (K): CL vs. HSW; (L): CL vs. ASW.
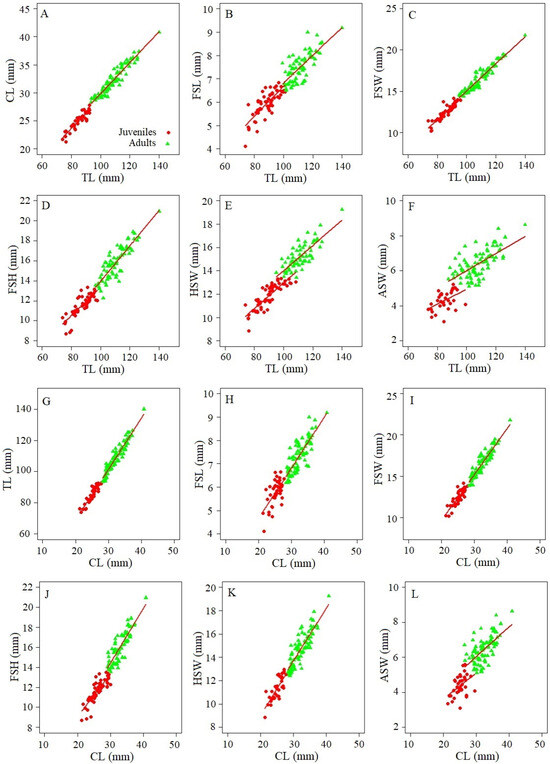
Figure 4.
Morphometric relationships using discriminant functions for juvenile and adult females of N. norvegicus. (A): TL vs. CL; (B): TL vs. FSL; (C): TL vs. FSW; (D): TL vs. FSH; (E): TL vs. HSW; (F): TL vs. ASW; (G): CL vs. TL; (H): CL vs. FSL; (I): CL vs. FSW; (J): CL vs. FSH; (K): CL vs. HSW; (L): CL vs. ASW.
The size at sexual maturity of M. binghami, as estimated by the morphometric relationships, varied between 110.49 and 118.56 mm TL50% (r2 = 0.85–0.96), and 28.60 and 33.89 mm CL50% (r2 = 0.95–1.00) (Table 3). In contrast, the size at sexual maturity of N. norvegicus estimated by morphometric relationships varied between 92.85 and 100.65 mm TL50% (r2 = 0.87–1.00), and 27.20 and 30.40 mm CL50% (r2 = 0.869–0.98) (Table 3).

Table 3.
Sizes at sexual maturity of M. binghami and N. norvegicus estimated by morphometric relationships using the discriminant functions method. C.I.: lower and upper confidence (95%); r2: determination coefficient.
The sexual maturity of females of M. binghami (N = 490; Figure 5) obtained by gonadal visual classification showed a majority of them in a mature condition, with 71.0% mature and 29.0% immature. The size at sexual maturity (TL50%) of females was 108.98 mm TL (95% CI = 106.10–111.60) and 31.40 mm CL (95% CI = 30.60–32.20) (Figure 5). The logistic model parameters for TL50% were specified as a = −15.06 and b = 0.14, and for CL50% as a = −15.57 and b = 0.50; r2 = 0.75 in both cases.
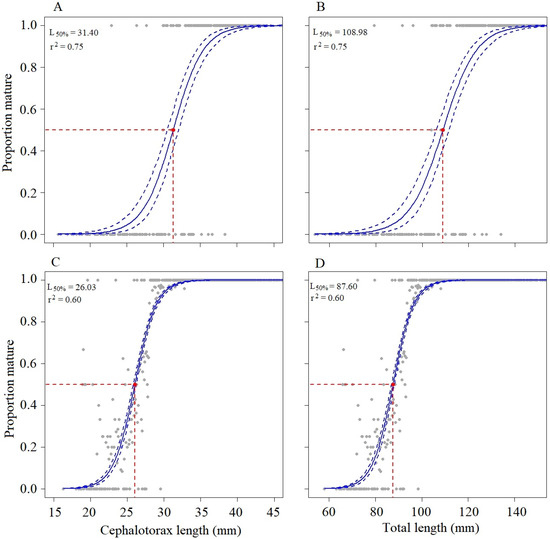
Figure 5.
Size at maturity from the visual classification of gonadal maturity stages of females of M. binghami and N. norvegicus. Grey solid symbols indicate the points included in the estimation of size at sexual maturity. Red-dashed line represents length at which 50% of individuals reach maturity (L50%), red point. The blue line is the fitted logistic function and blue dotted line are the confidence intervals. (A): CL50% M. binghami; (B): TL50% M. binghami; (C): CL50% N. norvegicus; (D): TL50% N. norvegicus.
The sexual maturity obtained by gonadal visual classification for N. norvegicus females (N = 3433; Figure 5) indicated a nearly equal distribution, with 50.4% classified as mature and 49.6% as immature. The size at sexual maturity (TL50%) of females was 87.60 mm TL (95% CI = 87.00–88.10) and 26.03 mm CL (95% CI = 25.80–26.20) (Figure 5). The logistic model parameters for TL50% were specified as a = −19.66 and b = 0.22, and for CL50% as a = −17.81 and b = 0.69; r2 = 0.60 in both cases (Figure 5).
For the case of M. binghami, the sizes at maturity from the morphometric approach that were close to the size at sexual maturity estimated by maturity stages (108.98 mm TL and 31.40 mm CL) were TL vs. FSW (112.05 mm TL), HSW (110.49 mm TL), ASW (112.33 mm TL), CL vs. TL (31.75 mm CL), FSW (29.45 mm CL), FSH (31.47 mm CL), and ASW (31.76 mm CL) (Figure 6; Table 3). For the case of N. norvegicus, the sizes at maturity from the morphometric approach that were close to the size at sexual maturity estimated by maturity stages (87.60 mm TL and 26.03 mm CL) were TL vs. CL (94.77 mm TL), FSL (92.85 mm TL), FSH (96.45 mm TL), CL vs. TL (28.77 mm CL), FSL (27.20 mm CL), and FSW (29.44 mm CL) (Figure 6; Table 3).
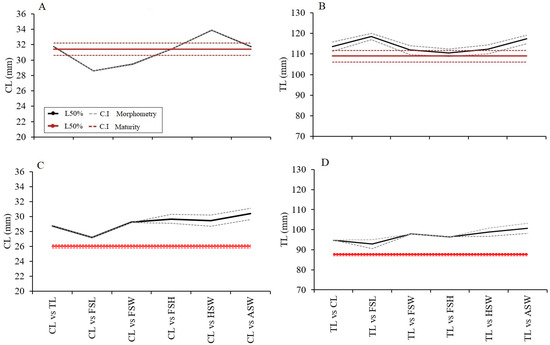
Figure 6.
Comparison of sizes at sexual maturity estimated by morphometric and gonadal maturity for M. binghami and N. norvegicus. Dashed lines represent boundaries of 95% confidence intervals. (A): CL50% M. binghami; (B): TL50% M. binghami; (C): CL50% N. norvegicus; (D): TL50% N. norvegicus.
4. Discussion
In this study, we observed a discrepancy in the size at maturity between the visual gonadal approach and the morphometric approach for N. norvegicus. Specifically, the results from the morphometric approach indicated values between 27.20 and 30.40 mm CL, which were higher than those obtained through the gonadal visual approach (i.e., 26.03 mm CL). In contrast, when assessing the size at maturity of M. binghami using the morphometric approach, we found values between 28.60 and 33.89 mm CL, which were comparable to the value obtained through the gonadal visual approach (i.e., 31.40 mm of CL). Consequently, employing both methodologies as complementary techniques effectively estimated size at maturity in the unexploited species M. binghami. However, for the overexploited species, N. norvegicus, both methodologies are not complementary. Instead, they serve to highlight the overexploited condition of the population.
Intense fishery overexploitation may drive changes in size at sexual maturity because phenotypic traits are related to physiology, which could be more sensitive to contingent ecological factors [38,39]. In contrast, the phenotypic changes at the morphological level are more conservative. In fact, in crustaceans, physiological traits are capable of rapid local adaptation and divergent evolution [40]. Therefore, some studies suggest that size at maturity may respond faster than morphometric changes to the selective pressures of accrued mortality rates as a result of fishery overexploitation, reducing population density and at the same time impoverishing the food web due to sediment resuspension and removal by towed nets [41,42,43]. Morphometric changes may take longer to show modifications according to external fishery selective pressures, as they depend on the accumulation of genetic variations and the inheritance of favorable traits which are also influenced by a larger set of selective environmental forces [44]. In particular, N. norvegicus populations may be showing a selective response to overfishing conditions by accelerating the reproductive age in smaller size classes (i.e., a reduction in gonadal maturity size), while the ontogeny process imposes size conservation of the abdominal segments given their slowed-down growth adjusted to a benthic lifestyle, the selective conditions (e.g., burrowing behavior) of which are invariant in the life history of the species today.
Nephrops norvegicus in Scottish waters show a larger CL size at the onset of maturity in females than that estimated from the gonadal maturity visual classification method [18]. Our results also confirm this observation for the deeper NW Mediterranean stock, and are in line with what has been reported in other decapod species. For example, the size at maturity of the crab Portunus sanguinolentus is smaller in heavily fished than less intensively harvested areas [45]. Also, the size at maturity of the crab Sesarma rectum is smaller in impacted mangrove areas [46]. However, for the latter species, the allometric growth of chelipeds was positive for both juveniles and adults, indicating no significant morphometric changes.
The size at maturity of crustacean species has been extensively studied in exploited marine environments with well-developed fisheries, e.g., N. norvegicus in the Mediterranean and North seas, based on gonadal maturity [6,12]. Nevertheless, the size of this species at their maturity in unexploited environments is information we will never attain, because unexploited grounds in the Atlantic and Mediterranean are missing. Therefore, it would be interesting to derive this knowledge with Nephropidae ecologically equivalent species such as M. binghami which inhabit unexploited continental margin areas of the Caribbean Sea [29].
In this study, using the gonadal maturity approach, the size at 50% maturity of N. norvegicus was 26.0, which differs slightly from that estimated by Vigo et al. [20], of 25.3 mm CL. It is important to note that the females analyzed in that study were collected in the same NW Mediterranean area at similar depths between 2019 and 2021 during the spawning season. Differently, here, we considered females collected from 2019 to 2022 across all the different seasons. Estimates of the onset maturity are known to vary seasonally, which may explain the slight difference in the present case.
Marković et al. [47] suggested that overfishing was the cause of the decreased size at 50% maturity (25.7 mm CL) observed for N. norvegicus in the South Adriatic Sea, the same size as the one found in the NW Mediterranean. Indeed, the abrupt decline in population abundances, which is observed in the NW Mediterranean Sea, caused by overfishing [4], can be followed by shifts in size and age at first maturity [48,49,50]. The current estimates of size at sexual maturity using the gonadal maturity of N. norvegicus (26.0 CL) confirm a decline in size at sexual maturity of about 4 mm CL in two decades, consistent with that reported by Vigo et al. [20], and probably linked to relatively high fishing pressure [12]. The size at sexual maturity of N. norvegicus found in this study through the morphometric approach showed a slowdown in somatic growth predominantly at 28.05 mm CL, which is closer to the size at maturity values reported more than 20 years ago of around 30 mm CL [12]. The overfishing effect of reducing size at maturity has also been suggested by Abelló et al. [19], who associated higher fishing pressures with smaller cephalothorax length in individuals from different areas subject to different exploitation levels in the Balearic Sea, Algarve, and the Adriatic. The size at maturity of N. norvegicus has also been observed to decline in the Western Irish Sea, from 23.6 mm CL in 1997 to 20.6 mm in 2016 [25]. According to the authors, the species has been overexploited in the past, though currently the population density is stable, but the stock abundance remains declining.
Identifying factors that drive the decline in the size at maturity of crustaceans is useful for understanding fishing and environmental impacts. Aside from fishing pressure, water warming has also been claimed to be responsible for the early maturity of crustaceans. For example, a study on the American lobster (Homarus americanus) shows that females living in warmer waters mature at smaller sizes [51]. Increasing water temperature may have been impacting the redistribution of N. norvegicus in the Balearic Sea [52], particularly after an abrupt warming from 2015 [53]. To our best knowledge, no studies of the direct effect of changes in water temperature on crustacean decapods’ reproductive physiology or morphological changes have been reported. For the Caribbean Sea case, no reports are found on deep-sea water warming that may be impacting demersal communities.
This research is the first comparative study on the morphological sexual maturity of Nephropidae species populations in the Colombian Caribbean and Mediterranean Seas. The morphometric size at sexual maturity of M. binghami is similar to that obtained by gonadal maturity stages (CL50% = 30.6 mm and TL50% = 104.6 mm; Paramo and Saint-Paul [5]. In fact, Cusba and Paramo [29] estimated size at sexual maturity by morphometric measurements using the breakpoint approach [54] with similar results: the first abdominal segment height (FSH) in females resulted in TL50% = 106.03 mm; taking FSH vs. CL as the morphometric variable resulted in CL50% = 33.80 mm. Estimating the size at sexual maturity of N. norvegicus by the morphometric approach (CL vs. FSW, FSH, HSW, ASW and TL vs. FSW, FSH, HSW, ASW) resulted in similar results to those obtained by the gonadal approach in the Balearic Sea, Algarve, and Adriatic (CL50% = 30 mm) [12]. The estimates of size at sexual maturity in this study obtained through morphometry indicate that the measurements of FSW, FSH, HSW, and ASW where the gonads are located are very similar to the estimates using gonadal stages. These morphological descriptors are the best variables for the determination of sexual maturity in both Nephropidae species. Furthermore, the size variability in the abdominal segment can be related to the beginning of sexual maturity, since at this life cycle stage, the onset of sexual maturity occurs, requiring a morphologically reproductive-compatible (i.e., a wider) abdomen for carrying and incubating eggs [29,55]. The relative abdomen growth in crustacean females may be related to the maternal care of eggs [56] in the form of a shelter–incubator chamber [57]. However, it is important to note that, for N. norvegicus females, there is still doubt on whether physiological maturity (i.e., the ability to produce mature gonads) or functional maturity (i.e., the ability to brood eggs at a larger size even if they already have mature gonads) occurs first, as presented in previous studies comparing morphometry and visual gonadal maturity [28,58]. This issue implies that abdominal widening can continue even after females are physiologically mature [18,28]. These discrepancies remain an open question that requires further research.
5. Conclusions
Estimating size at maturity of unexploited M. binghami and overexploited N. norvegicus can help us to understand the potential effects of fishery artificial selection on the physiological and morphological traits of commercially targeted species. In the NW Mediterranean, N. norvegicus seems to achieve a physiologically adapted gonadal maturation at smaller sizes (and age) under overexploitation regimes, not reflected by changes in morphology. In contrast, M. binghami shows similarity in the two traits in the unexploited demersal habitat of the Colombian Caribbean Sea. The value of our data will be incorporated within the Multi-Annual Management Plan for Mediterranean N. norvegicus Stocks [59], with the aim of identifying suitable demographic indicators of recovery. Estimating size at maturity from morphometric data is as an alternative to physiological-based methods; it has the advantage that data are easier and cheaper to obtain, and are less prone to temporal and seasonal biases [18,60].
Author Contributions
J.P., conceptualization, methodology, sample collection, formal analysis, validation, visualization, writing—original draft, writing—review and editing. A.R., conceptualization, methodology, formal analysis, validation, visualization, writing—original draft, writing—review and editing. J.Q.Z., formal analysis, validation, visualization. J.B.C., conceptualization, methodology, sample collection, writing—review and editing. D.P., conceptualization, methodology, investigation. R.S.-B., validation. M.V., formal analysis, validation, visualization. J.A., conceptualization, methodology, formal analysis, visualization, writing—original draft, writing—review and editing. N.B., conceptualization, methodology, formal analysis, visualization, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The second author (AR) was sponsored by the Fondo de Ciencia, Tecnología e Innovación (FCTeI) del Sistema General de Regalías (SGR) and the Doctoral Excellence Scholarship Program Bicentenario del Ministerio de Ciencia, Tecnología e Innovación (Minciencias). The scientific fishery sampling was funded by Ministerio de Ciencia, Tecnología e Innovación (Minciencias) (grant number 1117-452-21288) and Autoridad Nacional de Acuicultura y Pesca (AUNAP) and Universidad del Magdalena through cooperation agreement number 153-2020 under the research project “Reproductive biology of deep-sea crustaceans of commercial importance in the Colombian Caribbean”.
Institutional Review Board Statement
The care and use of experimental animals complied with Autoridad Nacional de Licencias Ambientales de Colombia (ANLA), animal welfare laws, guidelines and policies as approved by Universidad del Magdalena reference number 1293-2013.
Data Availability Statement
Data will be made available on request.
Acknowledgments
This study is a contribution of the Tropical Fisheries Science and Technology Research Group (CITEPT) at the Universidad del Magdalena in Colombia. We thank the researchers of the CITEPT Research Group, who collected the data on board the vessel “Adriatic” and analyzed the crustacean samples in the laboratory. We greatly appreciate the assistance of the ICATMAR technical staff collecting samples in the Balearic (Catalan) Sea. This work acknowledges the “Severo Ochoa Centre of Excellence” accreditation (CEX2019-000928-S).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chang, Y.J.; Sun, C.L.; Chen, Y.; Yeh, S.Z. Modelling the growth of crustacean species. Rev. Fish Biol. Fish. 2012, 22, 157–187. [Google Scholar] [CrossRef]
- Boenish, R.; Kritzer, J.P.; Kleisner, K.; Steneck, R.S.; Werner, K.M.; Zhu, W.; Schram, F.; Rader, D.; Cheung, W.; Ingles, J.; et al. The global rise of crustacean fisheries. Front. Ecol. Environ. 2022, 20, 102–110. [Google Scholar] [CrossRef]
- Aguzzi, J.; Violino, S.; Costa, C.; Bahamon, N.; Navarro, J.; Chatzievangelou, D.; Robinson, J.; Doyle, J.; Martinelli, M.; Lordan, C.; et al. Established and emerging research trends in Norway lobster, Nephrops norvegicus. Biology 2022, 12, 225. [Google Scholar] [CrossRef]
- Scientific, Technical and Economic Committee for Fisheries (STECF). Stocks Assessments: Demersal Stocks in the Western Mediterranean Sea (EWG-22-09); Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Paramo, J.; Saint-Paul, U. Spatial structure of deep sea lobster (Metanephrops binghami) in the Colombian Caribbean Sea. Helg. Mar. Res. 2012, 66, 25–31. [Google Scholar] [CrossRef][Green Version]
- Vigo, M.; Navarro, J.; Rotllant, G.; Bahamon, N.; Carreton, M.; Quevedo, J.; Rojas, A.; Company, J.B. Before-after control-impact (BACI) assessment of the effects of a deep-water no-take fishery reserve to recover Norway lobster (Nephrops norvegicus) overfished populations and coexisting megafauna. ICES J. Mar. Sci. 2023, 80, 2008–2023. [Google Scholar] [CrossRef]
- Sardà, F.; Aguzzi, J. A review of burrow counting as an alternative to other typical methods of assessment of Norway lobster populations. Rev. Fish Biol. Fish. 2012, 22, 409–422. [Google Scholar] [CrossRef]
- Cadrin, S.X. Morphometric landmarks. In Stock Identification Methods: Applications in Fishery Science; Cadrin, S.X., Friedland, K.D., Waldman, J.R., Eds.; Academic Press: London, UK, 2005; pp. 153–172. [Google Scholar]
- King, M. Fisheries Biology, Assessment and Management; Wiley–Blackwell: London, UK, 2007; p. 396. [Google Scholar]
- Farmer, A.S. The development of the external sexual characters of Nephrops norvegicus (L.) (Decapoda: Nephropidae). J. Nat. Hist. 1974, 8, 241–255. [Google Scholar] [CrossRef]
- Bianchini, M.L.; Di Stefano, L.; Ragonese, S. Size and age at onset of sexual maturity of female Norway lobster Nephrops norvegicus L. (Crustacea: Nephropidae) in the Strait of Sicily (Central Mediterranean Sea). Sci. Mar. 1998, 62, 151–159. [Google Scholar] [CrossRef]
- Orsi-Relini, L.; Zamboni, A.; Fiorentino, F.; Massi, D. Reproductive patterns in Norway lobster (Nephrops norvegicus L., Crustacea Decapoda Nephropidae) of different Mediterranean areas. Sci. Mar. 1998, 62, 25–41. [Google Scholar] [CrossRef]
- Lizárraga-Cubedo, H.A.; Pierce, G.J.; Santos, M.B. Reproduction of crustaceans in relation to fisheries. In Reproductive Biology of Crustaceans; Mente, E., Ed.; Science Publishers: Enfield, NH, USA, 2008; pp. 169–222. [Google Scholar]
- Haynes, P.S.; Browne, P.; Fullbrook, L.; Graham, C.T.; Hancox, L.; Johnson, M.P.; Lauria, V.; Power, A.M. Growth in Nephrops norvegicus from a tag-recapture experiment. Sci. Rep. 2016, 6, 35143. [Google Scholar] [CrossRef] [PubMed]
- Roth, V.L.; Mercer, J.M. Morphometrics in development and evolution. Am. Zool. 2015, 40, 801–810. [Google Scholar] [CrossRef]
- Torrejon-Magallanes, J. sizeMat: Estimate Size at Sexual Maturity. R Package Version 1.1.2. 2020. Available online: https://CRAN.R-project.org/package=sizeMat (accessed on 1 June 2022).
- MacDiarmid, A.B.; Sainte-Marie, B. Reproduction. In Lobsters: Biology, Management, Aquaculture and Fisheries; Phillips, B.F., Ed.; Blackwell Publishing: Oxford, UK, 2006; pp. 45–77. [Google Scholar]
- Queirós, A.M.; Weetman, A.; McLay, H.A.; Dobby, H. Geographical variation in size at the onset of maturity of male and female Norway lobster Nephrops norvegicus (L., Homarida: Decapoda) in Scottish waters. Fish. Res. 2013, 139, 132–144. [Google Scholar] [CrossRef]
- Severino-Rodrigues, E.; Gomes-Furquim, L.; da Graça-Lopes, R.; Ferreira-Alves, P.M. Crescimento relativo e tamanho na maturidade sexual do lagostim Metanephrops rubellus (Moreira, 1903) desembarcado no litoral do estado de São Paulo, Brasil. Bol. Inst. Pesca. 2016, 42, 431–442. [Google Scholar] [CrossRef]
- Vigo, M.; Galimany, E.; Poch, P.; Santos, R.; Sala-Coromina, J.; Bahamon, N.; Aguzzi, J.; Navarro, J.; Company, J. An update on the biological parameters of the Norway lobster (Nephrops norvegicus) in the northwestern Mediterranean Sea. ICES J. Mar. Sci. 2024; In Press. [Google Scholar] [CrossRef]
- Hall, N.G.; Smith, K.D.; de Lestang, S.; Potter, I.C. Does the largest chela of the males of three crab species undergo an allometric change that can be used to determine morphometric maturity? ICES J. Mar. Sci. 2006, 63, 140–150. [Google Scholar] [CrossRef]
- Claverie, T.; Smith, I.P. Morphological maturity and allometric growth in the squat lobster Munida rugosa. J. Mar. Biol. Assoc. U. K. 2009, 89, 1189–1194. [Google Scholar] [CrossRef]
- Josileen, J. Morphometrics and length-weight relationship in the blue swimming crab Portunus pelagicus (L. 1758) (Decapoda: Brachyura) from the Mandapam coast, India. Crustaceana 2011, 84, 1665–1681. [Google Scholar] [CrossRef]
- Bailey, N.; Chapman, C.J. A comparison of density, length composition and growth of two Nephrops populations off the west coast of Scotland. In ICES CM/K42; Thunen Institute: Braunschweig, Germany, 1983; pp. 1–18. [Google Scholar]
- Sigwart, J.D.; Lundy, M.; Dick, J.T.A.; Becker, C. Declining female size at onset of maturity in Nephrops norvegicus in long-term surveys (1997–2016). ICES J. Mar. Sci. 2020, 77, 3031–3038. [Google Scholar] [CrossRef]
- Pérez, D.; Paramo, J.; Vargas, Y.; Rodriguez, A.; Atencia, M.; Hurtado, M.; Marín, D.; Jiménez, O.; Arango, N.; Bustos-Montes, D. Aspectos Reproductivos de los Crustáceos de Aguas Profundas de Importancia Comercial en el Caribe Norte Colombiano; Aunap-Unimagdalena-Citept: Santa Marta, Colombia, 2022; p. 35. ISBN 978-958-746-511-2. [Google Scholar]
- ICATMAR. Institut Català de Recerca per a la Governança del Mar. 2023. Available online: www.icatmar.cat (accessed on 10 December 2023).
- McQuaid, N.; Briggs, R.P.; Roberts, D. Estimation of the size of onset of sexual maturity in Nephrops norvegicus (L.). Fish. Res. 2006, 81, 26–36. [Google Scholar] [CrossRef]
- Cusba, J.; Paramo, J. Morphometric relation. ships and size at sexual maturity of the deep-sea Caribbean lobster Metanephrops binghami (Decapoda: Nephropidae) in the Colombian Caribbean. Univ. Sci. 2017, 22, 145–160. [Google Scholar] [CrossRef]
- Tzeng, T.D.; Chiu, C.S.; Yeh, S.Y. Morphometric variation in red-spot prawn (Metapenaeopsis barbata) in different geographic waters off Taiwan. Fish. Res. 2001, 53, 211–217. [Google Scholar] [CrossRef]
- Tzeng, T.D.; Yeh, S.Y. Multivariate allometric comparisons for kuruma shrimp (Penaeus japonicus) off Taiwan. Fish. Res. 2002, 59, 279–288. [Google Scholar] [CrossRef]
- Corgos, A.; Freire, J. Morphometric and gonad maturity in the spider crab Maja brachydactyla: A comparison of methods for estimating size at maturity in species with determinate growth. ICES J. Mar. Sci. 2006, 63, 851–859. [Google Scholar] [CrossRef]
- Somerton, D.A. A computer technique for estimating the size of sexual maturity in crabs. Can. J. Fish. Aquat. Sci. 1980, 37, 1488–1494. [Google Scholar] [CrossRef]
- Roa, R.; Ernst, B.; Tapia, F. Estimation of size at sexual maturity: An evaluation of analytical and resampling procedures. Fish. Bull. 1999, 97, 570–580. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Bergen, NJ, USA, 2010; p. 944. [Google Scholar]
- McQuaid, N.; Briggs, R. Sexual Maturity of Male Nephrops norvegicus (L.) in the Irish Sea, 2004. Available online: https://www.ices.dk/sites/pub/CM%20Doccuments/2005/Q/Q3005ABS.pdf (accessed on 1 June 2023).
- Rotllant, G.; Ribes, E.; Company, J.B.; Durfort, M. The ovarian maturation cycle of the Norway lobster Nephrops norvegicus (Linnaeus, 1758) (Crustacea, Decapoda) from the western Mediterranean Sea. Invertebr. Reprod. Dev. 2005, 48, 161–169. [Google Scholar] [CrossRef]
- Kuparinen, A.; O’Hara, R.B.; Merilä, J. The role of growth history in determining age and size at maturation in exploited fish populations. Fish Fish 2008, 9, 201–207. [Google Scholar] [CrossRef]
- de Roos, A.M.; Boukal, D.S.; Persson, L. Evolutionary regime shifts in age and size at maturation of exploited fish stocks. Proc. R. Soc. B. 2006, 273, 1873–1880. [Google Scholar] [CrossRef]
- Conover, D.O.; Clarke, L.M.; Munch, S.B.; Wagner, G.N. Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. J. Fish Biol. 2006, 69, 21–47. [Google Scholar] [CrossRef]
- Jones, J.B. Environmental impact of trawling on the seabed: A review. N. Z. J. Mar. Freshwater Res. 1992, 26, 59–67. [Google Scholar] [CrossRef]
- Pusceddu, A.; Bianchelli, S.; Martín, J.; Puig, P.; Palanques, A.; Masqué, P.; Danovaro, R. Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. USA 2014, 111, 8861–8866. [Google Scholar] [CrossRef]
- Romano, C.; Fanelli, E.; D’Anna, G.; Pipitone, C.; Vizzini, S.; Mazzola, A.; Badalamenti, F. Spatial variability of soft-bottom macrobenthic communities in northern Sicily (Western Mediterranean): Contrasting trawled vs. untrawled areas. Mar. Environ. Res. 2016, 122, 113–125. [Google Scholar] [CrossRef]
- Aguzzi, J.; Costa, C.; Antonucci, F.; Company, J.B.; Menesatti, P.; Sardá, F. Influence of diel behaviour in the morphology of decapod Natantia. Biol. J. Linn. Soc. 2008, 96, 517–532. [Google Scholar] [CrossRef]
- Yang, C.P.; Li, H.X.; Li, L.; Xu, J.; Yan, Y. Population Structure, Morphometric Analysis and Reproductive Biology of Portunus sanguinolentus (Herbst, 1783) (Decapoda: Brachyura: Portunidae) in Honghai Bay, South China Sea. J. Crust. Biol. 2014, 34, 722–730. [Google Scholar] [CrossRef]
- Ribeiro, F.B.; Cascon, H.M.; Bezerra, L.E.A. Morphometric sexual maturity and allometric growth of the crab Sesarma rectum Randall, 1840 (Crustacea: Sesarmidae) in an impacted tropical mangrove in northeast Brazil. Lat. Am. J. Aquat. Res. 2013, 41, 361–368. [Google Scholar] [CrossRef]
- Marković, O.; Ikica, Z.; Đurović, M.; Mandić, M.; Pešić, A.; Petović, S.; Joksimović, A. Some preliminary data about reproductive activity of female of Nephrops norvegicus (Linnaeus, 1758), in the South Adriatic Sea (Montenegro). Turkish J. Fish. Aquat. Sci. 2016, 16, 743–748. [Google Scholar] [CrossRef]
- Di Salvatore, P.; Sacristán, H.J.; Florentín, O.; Varisco, M.; Lovrich, G.A. Female reproductive output and potential recruitment of three fished southern king crab stocks from the Southern Atlantic Ocean. ICES J. Mar. Sci. 2021, 78, 2628–2642. [Google Scholar] [CrossRef]
- Galimany, E.; Baeta, M.; Durfort, M.; Lleonart, J.; Ramón, M. Reproduction and size at first maturity in a Mediterranean exploited Callista chione bivalve bed. Sci. Mar. 2015, 79, 233–242. [Google Scholar] [CrossRef]
- Molinet, C.; Olguín, A.; Gebauer, P.; Díaz, P.A.; Díaz, M.; Matamala, T.; Mora, P.; Paschke, K. Upswing and expansion of the southern king crab (Lithodes santolla) fishery in Northwest Patagonia: Drivers, trends and opportunities for management. Reg. Stud. Mar. Sci. 2020, 34, 101073. [Google Scholar] [CrossRef]
- Le Bris, A.; Pershing, A.; Gaudette, J.; Pugh, T.L.; Reardon, K.M. Multi-scale quantification of the effects of temperature on size at maturity in the American lobster (Homarus americanus). Fish. Res. 2017, 186, 397–406. [Google Scholar] [CrossRef]
- Sbrana, M.; Zupa, W.; Ligas, A.; Capezzuto, F.; Chatzispyrou, M.; Follesa, C.; Gancitano, V.; Guijarro, B.; Isajlovic, I.; Jadaud, A.; et al. Spatiotemporal abundance pattern of deep-water rose shrimp, Parapenaus longirostris, and Norway lobster, Nephrops norvegicus, in European Mediterranean waters. Sci. Mar. 2019, 83, 71–80. [Google Scholar] [CrossRef]
- Bahamon, N.; Aguzzi, J.; Ahumada-Sempoal, M.A.; Bernardello, R.; Reuschel, C.; Company, J.B.; Peters, F.; Gordoa, A.; Navarro, J.; Velásquez, Z.; et al. Stepped Coastal Water Warming Revealed by Multiparametric Monitoring at NW Mediterranean Fixed Stations. Sensors 2020, 20, 2658. [Google Scholar] [CrossRef] [PubMed]
- Muggeo, V.M.R. Segmented: An R package to fit regression models with broken-line relationships. R News 2008, 8, 20–25. [Google Scholar]
- Rudolph, E. New records of intersexuality in the freshwater crayfish Samastacus spinifrons (Philippi 1882) (Decapoda, Parastacidae). J. Crust. Biol. 2002, 22, 377–389. [Google Scholar] [CrossRef]
- Williner, V.; Torres, M.V.; Carvalho, D.A.; König, N. Relative growth and morphological sexual maturity size of the freshwater crab Trichodactylus borellianus (Crustacea, Decapoda, Trichodactylidae) in the Middle Paraná River, Argentina. ZooKeys 2014, 457, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Viau, V.; López, G.L.; Bond-Buckup, G.; Rodríguez, E. Size at the onset of sexual maturity in the anomuran crab, Aegla uruguayana (Aeglidae). Acta Zool. 2006, 87, 253–264. [Google Scholar] [CrossRef]
- Tuck, I.D.; Atkinson, R.J.A.; Chapman, C.J. Population biology of the Norway lobster, Nephrops norvegicus (L.) in the Firth of Clyde, Scotland II: Fecundity and size at onset of sexual maturity. ICES J. Mar. Sci. 2000, 57, 1227–1239. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EU) 2019/1022 of the European Parliament and of the Council of 20 June 2019 establishing a multiannual plan for the fisheries exploiting demersal stocks in the western Mediterranean Sea and amending Regulation (EU) No 508/2014. Off. J. Eur. Union 2019.
- Da Silva-Santana, C.A.; Lordan, C.; Power, A.M. Theoretical size at the onset of maturity and its density-dependent variability as an option in crustacean fisheries management. ICES J. Mar. Sci. 2021, 78, 1421–1433. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).