Heritability Estimates for Growth Traits and Correlation Analysis between Weight and Metamorphosis Rate in the Bullfrog Rana (Aquarana) catesbeiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Family Construction
2.3. Tadpole Cultivation
2.4. Data Measurement
2.4.1. Weight of Tadpoles in Each Period
2.4.2. Metamorphosis Rate
2.4.3. Statistical Analysis
3. Results
4. Discussion
4.1. Metamorphosis of Tadpoles
4.2. Weight and Metamorphosis Rate of Tadpoles
4.3. Heritability and Correlation between Weight and Metamorphosis Rate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kikuyama, S.; Kawamura, K.; Tanaka, S.; Yamamoto, K. Aspects of amphibian metamorphosis: Hormonal control. Int. Rev. Cytol. 1993, 145, 105–148. [Google Scholar]
- Rose, C.S. Hormonal control in larval development and evolution—Amphibians. In The Origin and Evolution of Larval Forms; Elsevier: Amsterdam, The Netherlands, 1999; p. 167-VI. [Google Scholar]
- Werner, E.E. Amphibian metamorphosis: Growth rate, predation risk, and the optimal size at transformation. Am. Nat. 1986, 128, 319–341. [Google Scholar] [CrossRef]
- Altwegg, R.; Reyer, H.U. Patterns of natural selection on size at metamorphosis in water frogs. Evolution 2003, 57, 872–882. [Google Scholar]
- Wilbur, H.M. Competition, predation, and the structure of the Ambystoma-Rana sylvatica community. Ecology 1972, 53, 3–21. [Google Scholar] [CrossRef]
- Brockelman, W.Y. An analysis of density effects and predation in Bufo americanus tadpoles. Ecology 1969, 50, 632–644. [Google Scholar] [CrossRef]
- Collins, J.P. A Comparative Study of the Life History Strategies in a Community of Frogs. Ph.D. Dissertation, University of Michigan, Ann Arbor, MI, USA, 1975. [Google Scholar]
- Berven, K.A. The genetic basis of altitudinal variation in the wood frog Rana sylvatica II. An experimental analysis of larval development. Oecologia 1982, 52, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Smith-Gill, S.J.; Gill, D.E. Curvilinearities in the competition equations: An experiment with ranid tadpoles. Am. Nat. 1978, 112, 557–570. [Google Scholar] [CrossRef]
- Berven, K.A. Mate choice in the wood frog, Rana sylvatica. Evolution 1981, 35, 707–722. [Google Scholar] [CrossRef]
- Wilbur, H.M. Interactions of food level and population density in Rana sylvatica. Ecology 1977, 58, 206–209. [Google Scholar] [CrossRef]
- McDiarmid, R.W.; Altig, R. Tadpoles: The Biology of Anuran Larvae; University of Chicago Press: Chicago, IL, USA, 1999. [Google Scholar]
- Brooks, R.; Endler, J.A. Direct and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata). Evolution 2001, 55, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, H.M.; Collins, J.P. Ecological aspects of amphibian metamorphosis: Nonnormal distributions of competitive ability reflect selection for facultative metamorphosis. Science 1973, 182, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.A.; McCoy, M.W.; Amick, A.; Guillette, L.J., Jr.; St. Mary, C.M. Tradeoffs between somatic and gonadal investments during development in the African clawed frog (Xenopus laevis). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2007, 307, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.P. Intrapopulation variation in the body size at metamorphosis and timing of metamorphosis in the bullfrog, Rana catesbeiana. Ecology 1979, 60, 738–749. [Google Scholar] [CrossRef]
- Gosner, K.L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Gilmour, A.R.; Gogel, B.J.; Cullis, B.R.; Welham, S.; Thompson, R. ASReml User Guide Release 1.0; University of Hamburg: Hamburg, Germany, 2002. [Google Scholar]
- Watkins, T.B. A quantitative genetic test of adaptive decoupling across metamorphosis for locomotor and life-history traits in the Pacific tree frog, Hyla regilla. Evolution 2001, 55, 1668–1677. [Google Scholar]
- Liu, X.L.; Chang, Y.Q.; Xiang, J.H.; Cao, X.B. Estimates of genetic parameters for growth traits of the sea urchin, Strongylocentrotus intermedius. Aquaculture 2005, 243, 27–32. [Google Scholar] [CrossRef]
- Rice, W.R. Analyzing tables of statistical tests. Evolution 1989, 43, 223–225. [Google Scholar] [CrossRef]
- Traijitt, T.; Kitana, N.; Khonsue, W.; Kitana, J. Chronological changes in the somatic development of Hoplobatrachus rugulosus (Wiegmann, 1834) (Anura: Dicroglossidae). Trop. Nat. Hist. 2021, 21, 184–199. [Google Scholar]
- Dodd, M.; Dodd, J. The biology of metamorphosis. Physiol. Amphib. 1976, 3, 467–599. [Google Scholar]
- Loman, J. Food, feeding rates and prey size selection in juvenile and adult frogs, Rana arvalis Nilss. and R. temporaria L. Ekol. Pol. 1979, 27, 581–601. [Google Scholar]
- Tollrian, R. Predator-induced morphological defenses: Costs, life history shifts, and maternal effects in Daphnia pulex. Ecology 1995, 76, 1691–1705. [Google Scholar] [CrossRef]
- Segers, F.H.; Taborsky, B. Juvenile exposure to predator cues induces a larger egg size in fish. Proc. R. Soc. B Biol. Sci. 2012, 279, 1241–1248. [Google Scholar] [CrossRef]
- Michimae, H.; Nishimura, K.; Tamori, Y.; Wakahara, M. Maternal effects on phenotypic plasticity in larvae of the salamander Hynobius retardatus. Oecologia 2009, 160, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, M.; Chang, L.; Zhu, W.; Li, C.; Xie, F.; Zhang, H.; Zhao, T.; Jiang, J. Characterizing the composition, metabolism and physiological functions of the fatty liver in Rana omeimontis tadpoles. Front. Zool. 2019, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T. Growth and survival rate of the larvae of Hynobius nebulosus tokyoensis Tago (Amphibia, Hynobiidae). Popul. Ecol. 1981, 23, 360–378. [Google Scholar] [CrossRef]
- Pakkasmaa, S.; Merilä, J.; O’Hara, R. Genetic and maternal effect influences on viability of common frog tadpoles under different environmental conditions. Heredity 2003, 91, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gjedrem, T.; Robinson, N.; Rye, M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture 2012, 350, 117–129. [Google Scholar] [CrossRef]
- Vandeputte, M.; Quillet, E.; Chevassus, B. Early development and survival in brown trout (Salmo trutta fario L.): Indirect effects of selection for growth rate and estimation of genetic parameters. Aquaculture 2002, 204, 435–445. [Google Scholar] [CrossRef]
- Berven, K.A. The heritable basis of variation in larval developmental patterns within populations of the wood frog (Rana sylvatica). Evolution 1987, 41, 1088–1097. [Google Scholar]
- Newman, R.A. Genetic variation for larval anuran (Scaphiopus couchii) development time in an uncertain environment. Evolution 1988, 42, 763–773. [Google Scholar]
- Laurila, A.; Karttunen, S.; Merilä, J. Adaptive phenotypic plasticity and genetics of larval life histories in two Rana temporaria populations. Evolution 2002, 56, 617–627. [Google Scholar] [PubMed]
- Sommer, S.; Pearman, P.B. Quantitative genetic analysis of larval life history traits in two alpine populations of Rana temporaria. Genetica 2003, 118, 1–10. [Google Scholar] [CrossRef]
- Travis, J.; Emerson, S.B.; Blouin, M. A quantitative-genetic analysis of larval life-history traits in Hyla crucifer. Evolution 1987, 41, 145–156. [Google Scholar]
- Emerson, S.B.; Travis, J.; Blouin, M. Evaluating a hypothesis about heterochrony: Larval life-history traits and juvenile hind-limb morphology in Hyla crucifer. Evolution 1988, 42, 68–78. [Google Scholar] [PubMed]
- Kaplan, R.H. The implications of ovum size variability for offspring fitness and clutch size within several populations of salamanders (Ambystoma). Evolution 1980, 34, 51–64. [Google Scholar]
- Atkinson, B.G.; Warkman, A.S.; Chen, Y. Thyroid hormone induces a reprogramming of gene expression in the liver of premetamorphic Rana catesbeiana tadpoles. Wound Repair Regen. 1998, 6, S323–S336. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. Metamorphosis and the amphibian immune system. Immunol. Rev. 1998, 166, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Berven, K.A.; Gill, D.E.; Smith-Gill, S.J. Countergradient selection in the green frog, Rana clamitans. Evolution 1979, 33, 609–623. [Google Scholar] [CrossRef]
- Murray, D.L. The effects of food and density on growth and metamorphosis in larval wood frogs (Rana sylvatica) from central Labrador. Can. J. Zool. 1990, 68, 1221–1226. [Google Scholar] [CrossRef]
- Berven, K.A.; Chadra, B.G. The relationship among egg size, density and food level on larval development in the wood frog (Rana sylvatica). Oecologia 1988, 75, 67–72. [Google Scholar] [CrossRef]
- Travis, J. Variation in development patterns of larval anurans in temporary ponds. I. Persistent variation within a Hyla gratiosa population. Evolution 1983, 37, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.S. Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends Ecol. Evol. 2005, 20, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Travis, J. Anuran size at metamorphosis: Experimental test of a model based on intraspecific competition. Ecology 1984, 65, 1155–1160. [Google Scholar] [CrossRef]
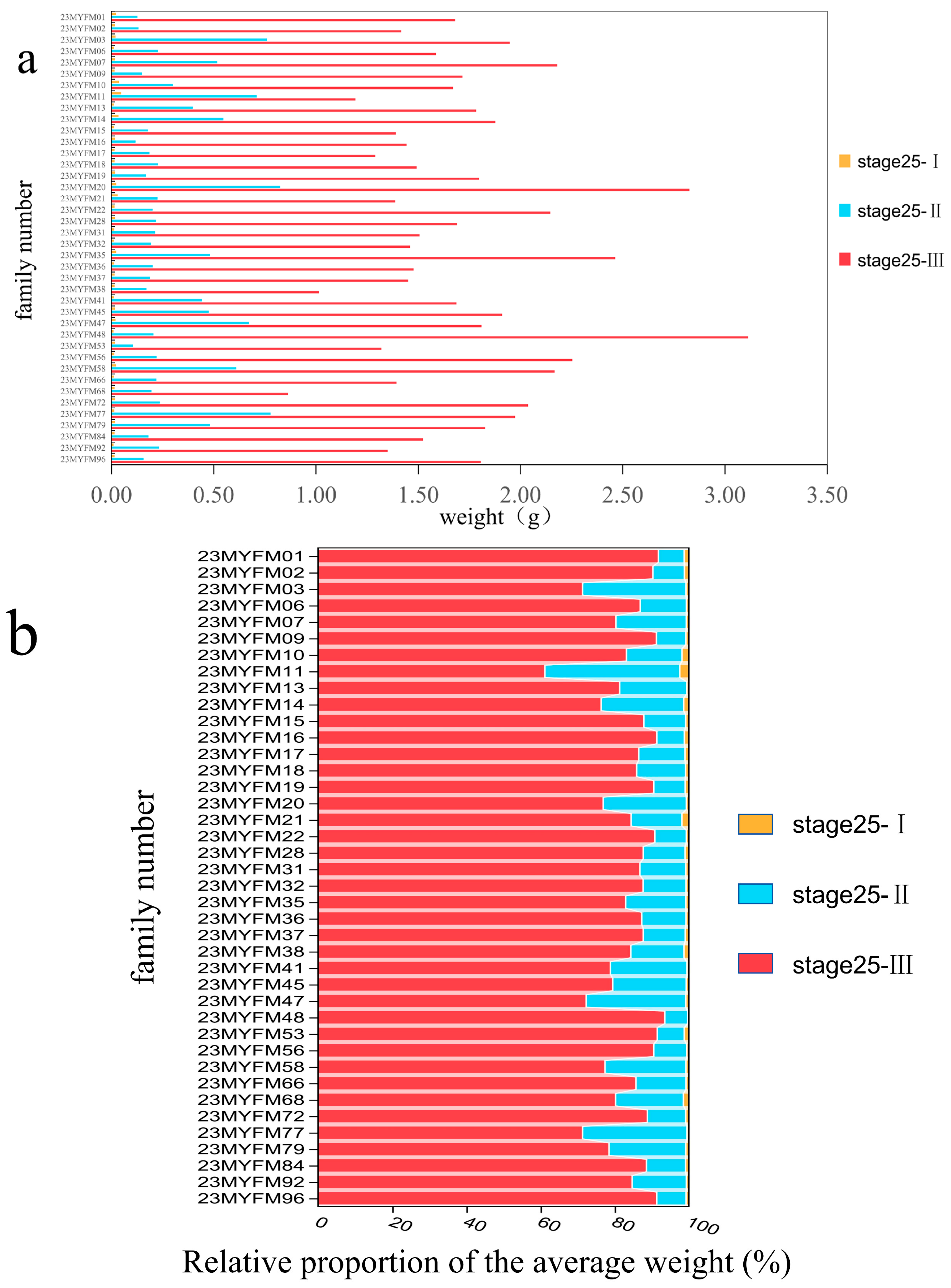


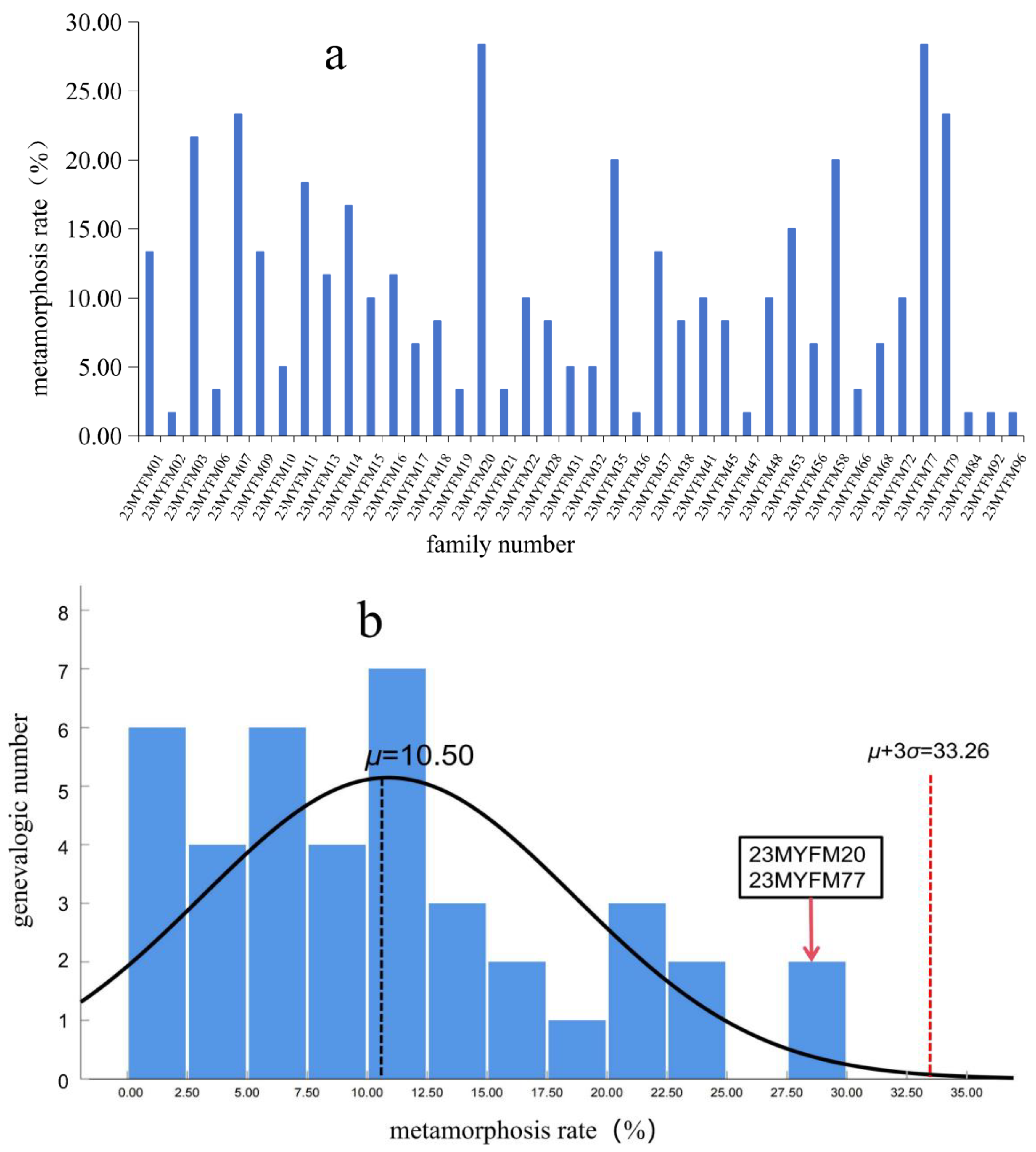
| h2 | Growth | Stage 25-I Weight | Stage 25-II Weight | Stage 25-III Weight | Metamorphosis Rate | |
|---|---|---|---|---|---|---|
| growth | 0.13 ± 0.06 | - | - | - | - | |
| stage 25-I weight | 0.99 ± 0.00 | 0.20 | 0.40 * | −0.03 | 0.21 | |
| stage 25-II weight | 0.46 ± 0.12 | 0.60 * | 0.22 | 0.42 * | 0.57 ** | |
| stage 25-III weight | 0.44 ± 0.20 | 0.66 * | −0.03 | −0.09 | 0.38 * | |
| metamorphosis rate | 0.18 ± 0.20 | 0.26 | 0.21 | −0.03 | 0.38 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Wang, Y.; Wang, G.; Zhang, L.; Zhang, G.; Huo, Z.; Ge, H. Heritability Estimates for Growth Traits and Correlation Analysis between Weight and Metamorphosis Rate in the Bullfrog Rana (Aquarana) catesbeiana. Fishes 2024, 9, 105. https://doi.org/10.3390/fishes9030105
Xu W, Wang Y, Wang G, Zhang L, Zhang G, Huo Z, Ge H. Heritability Estimates for Growth Traits and Correlation Analysis between Weight and Metamorphosis Rate in the Bullfrog Rana (Aquarana) catesbeiana. Fishes. 2024; 9(3):105. https://doi.org/10.3390/fishes9030105
Chicago/Turabian StyleXu, Wencheng, Yanzhe Wang, Guodong Wang, Lili Zhang, Guiling Zhang, Zhipeng Huo, and Hui Ge. 2024. "Heritability Estimates for Growth Traits and Correlation Analysis between Weight and Metamorphosis Rate in the Bullfrog Rana (Aquarana) catesbeiana" Fishes 9, no. 3: 105. https://doi.org/10.3390/fishes9030105
APA StyleXu, W., Wang, Y., Wang, G., Zhang, L., Zhang, G., Huo, Z., & Ge, H. (2024). Heritability Estimates for Growth Traits and Correlation Analysis between Weight and Metamorphosis Rate in the Bullfrog Rana (Aquarana) catesbeiana. Fishes, 9(3), 105. https://doi.org/10.3390/fishes9030105





