Another One Bites the Net: Assessing the Economic Impacts of Lagocephalus sceleratus on Small-Scale Fisheries in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Interviews with Local Fishers
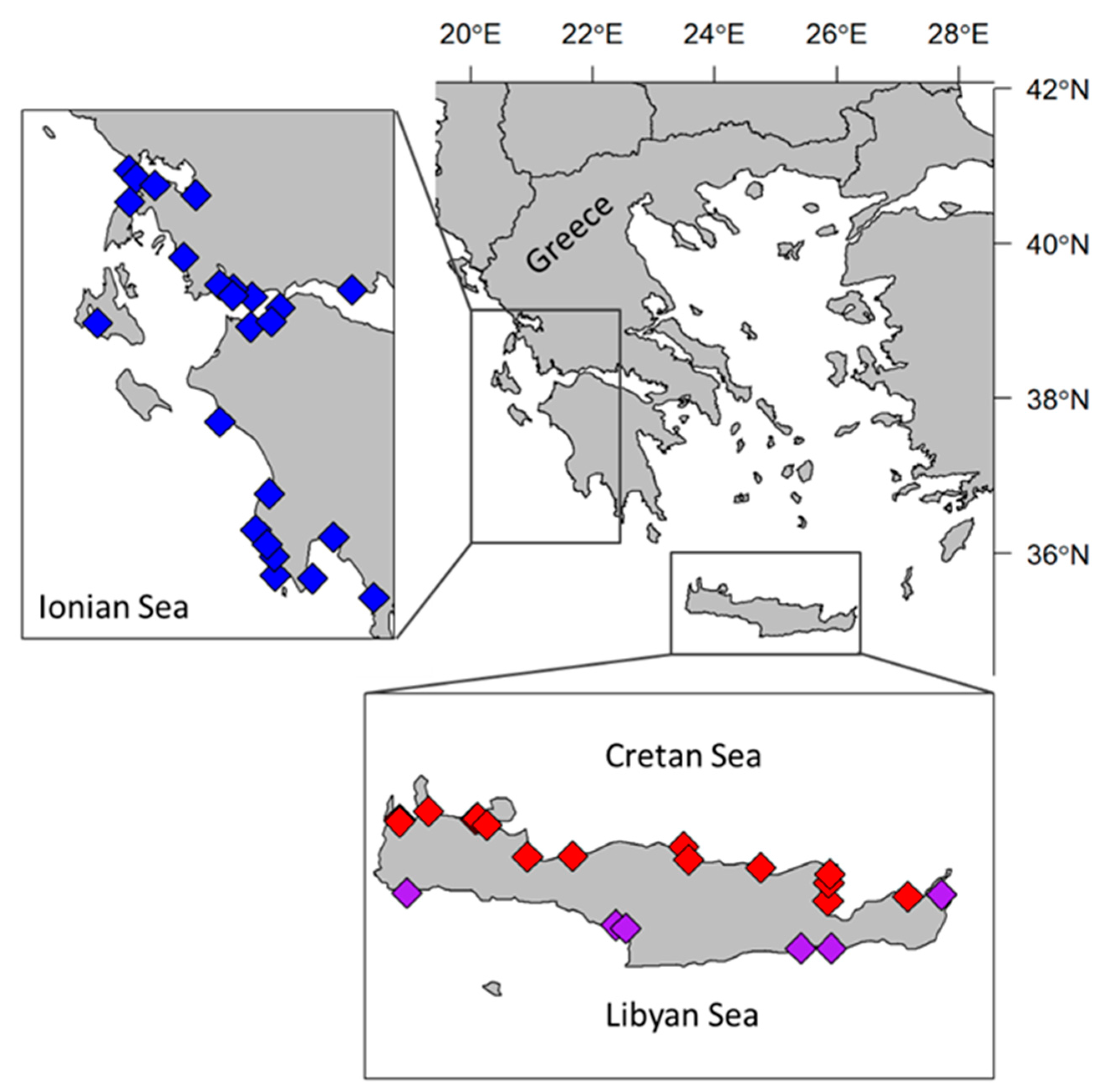
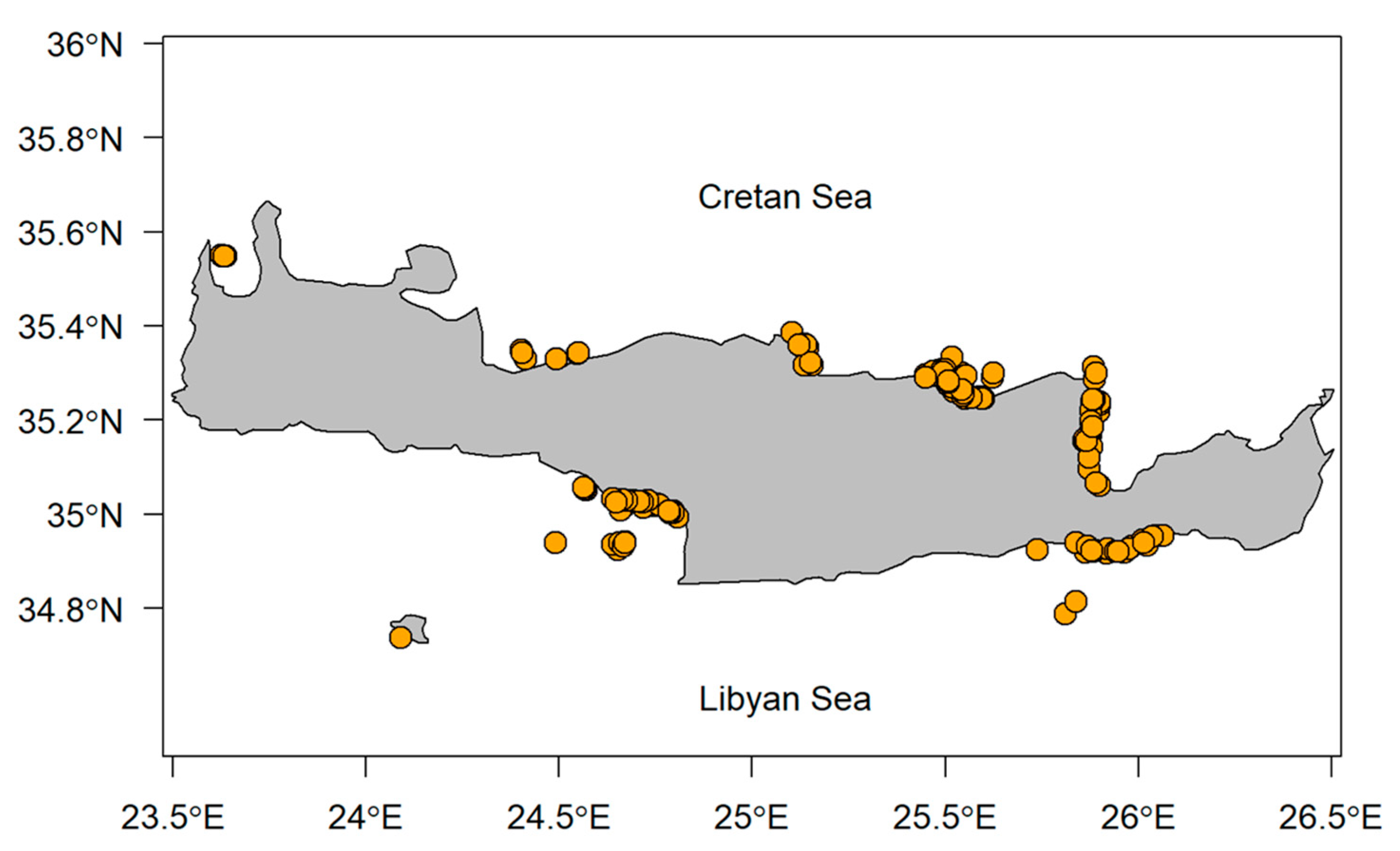
2.2. Onboard Sampling
2.3. Estimation of By-Catch and Impacts
2.4. Generalized Additive Models
3. Results
3.1. Interviews
3.2. Economic Impacts
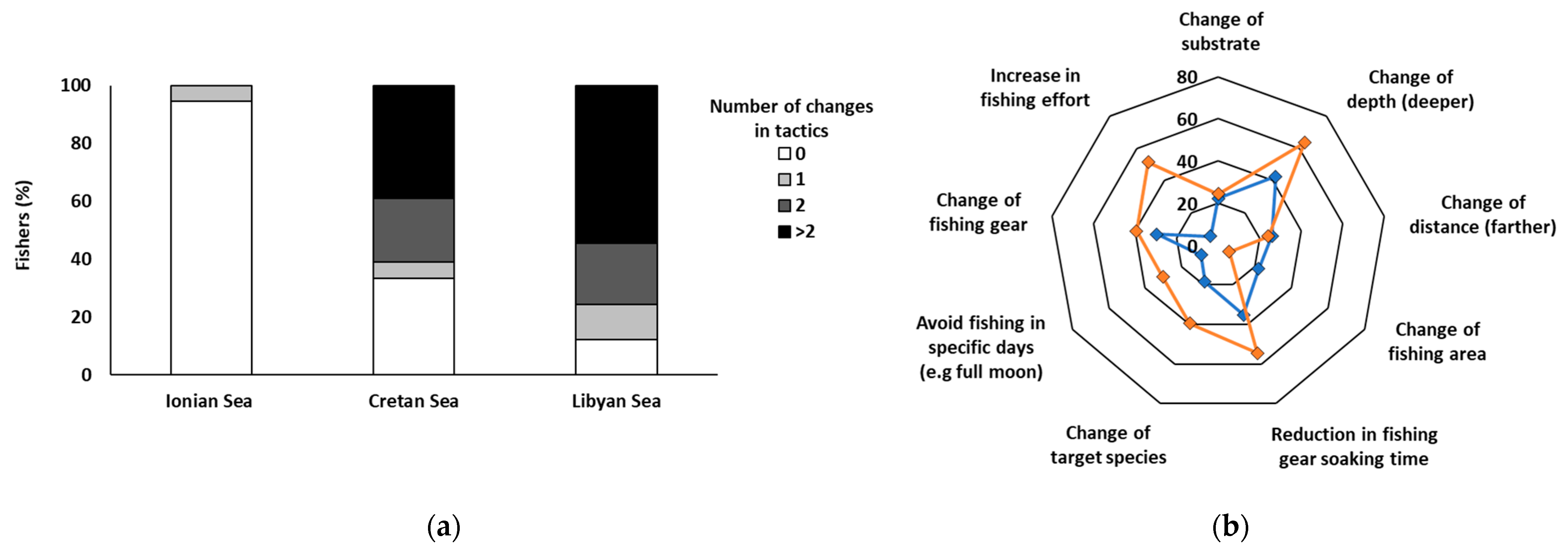
3.3. Adjustment in Fishing Tactics
3.4. Onboard Sampling
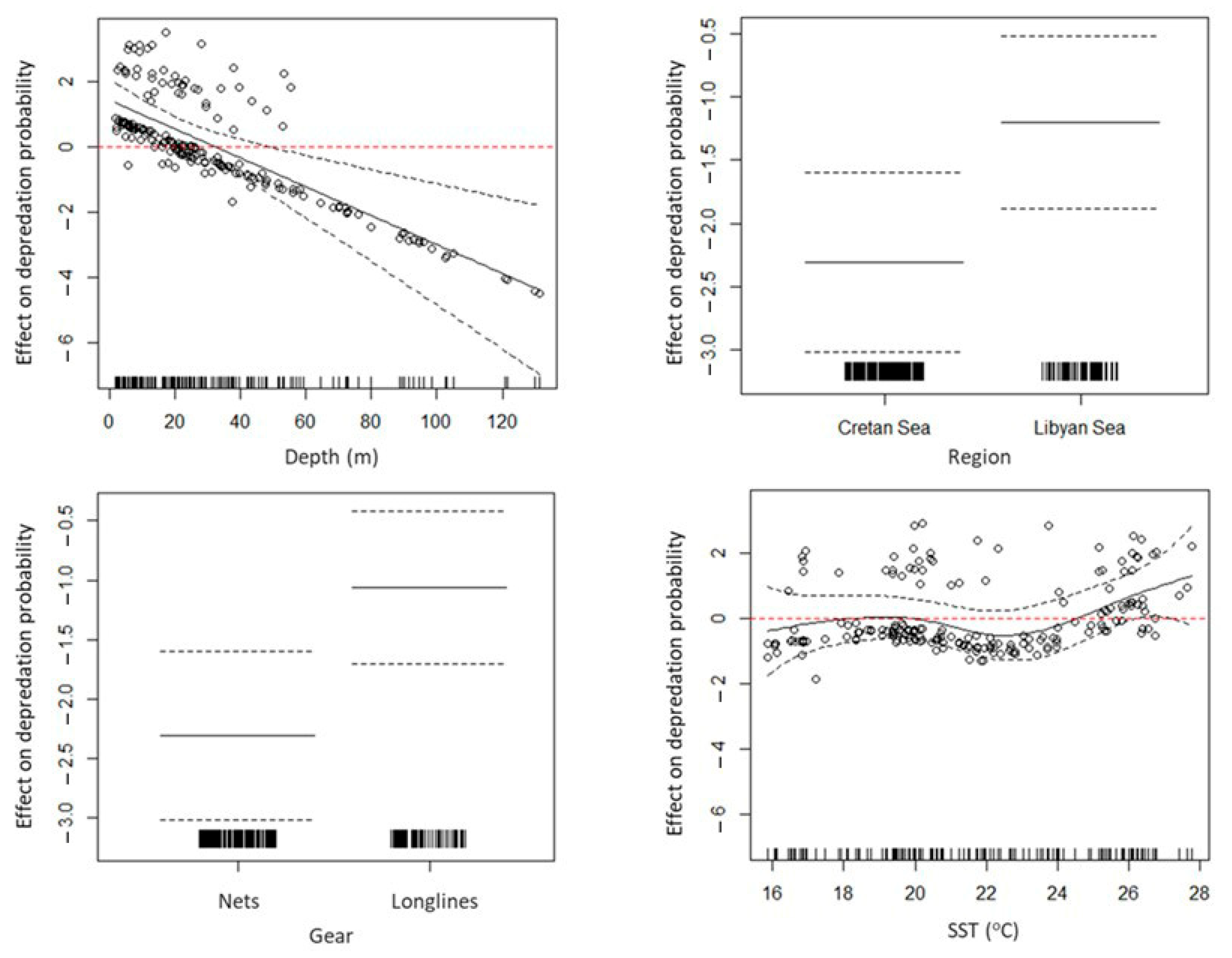
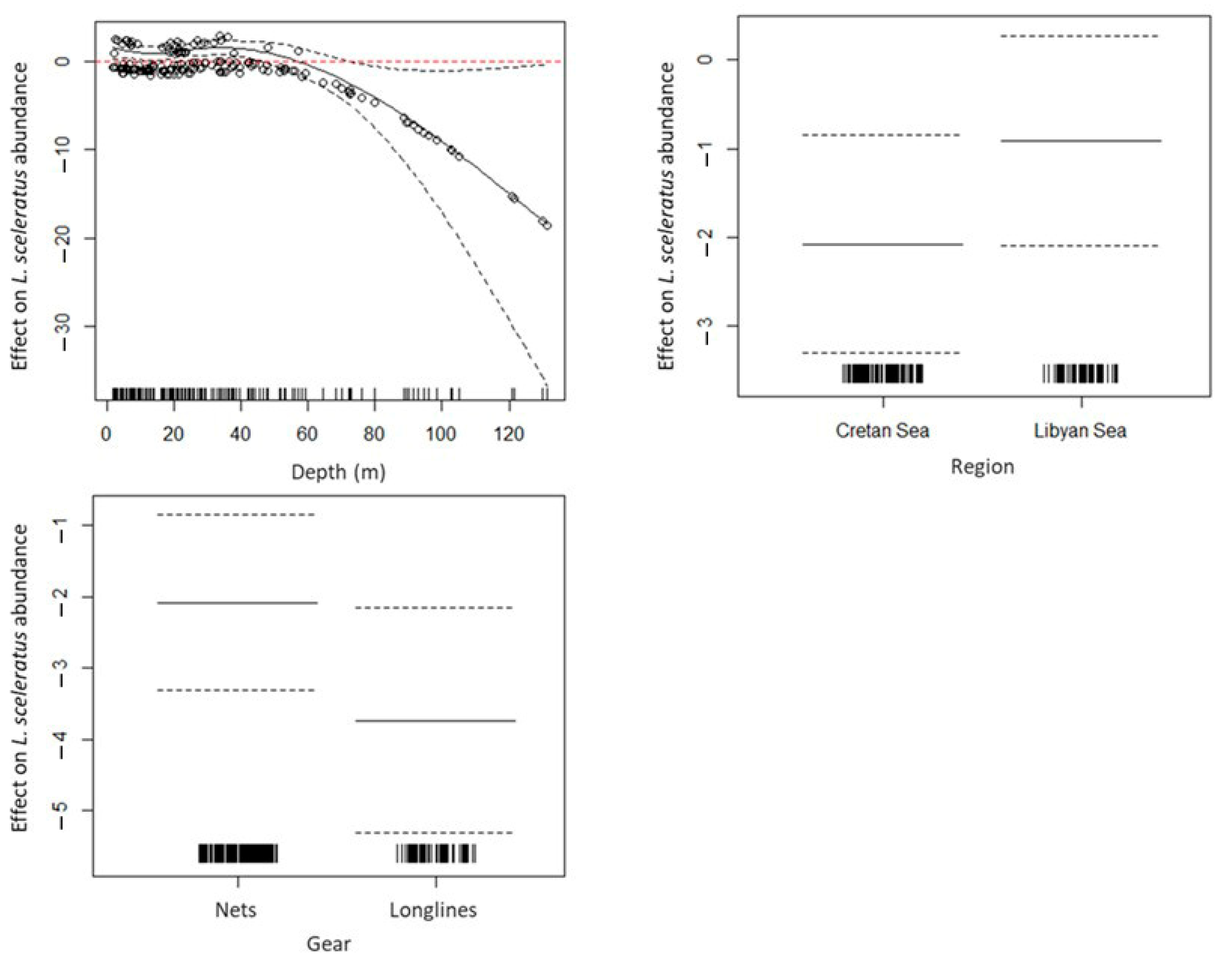
3.5. GAMs for Depredation Probability and By-Catch of L. sceleratus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-Bonaca, M. Non-indigenous marine species in the Mediterranean Sea—Myth and reality. Environ. Sci. Policy 2019, 96, 123–131. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Del Pasqua, M.; Gravili, C.; Gambi, M.C.; Gravina, M.F. The Mediterranean in check: Biological invasions in a changing sea. Mar. Ecol. 2020, 41, e12583. [Google Scholar] [CrossRef]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine invasive alien species: A threat to global biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Rilov, G.; Edelist, D. Impacts of marine invasive alien species on European fisheries and aquaculture–plague or boon? In Engaging Marine Scientists and Fishers to Share Knowledge and Perception—Early Lessons; Briand, F., Ed.; CIESM Monograph; CIESM: Villa Girasole, Monaco, 2018; Volume 50, pp. 125–132. [Google Scholar]
- Farrugio, H. Current situation of small-scale fisheries in the Mediterranean and Black Sea: Strategies and methodologies for an effective analysis of the sector. In Proceedings of the First Regional Symposium on Sustainable Small-Scale Fisheries in the Mediterranean and Black Sea, Saint Julian’s, Malta, 27–30 November 2013. [Google Scholar]
- Anonymous. National Strategic Plan for the Development of Fisheries, 2007–2013 (ESSAAL); Hellenic Republic Ministry of Rural Development and Foods, Directorate General of Fisheries: Athens, Greece, 2007; 77p. (In Greek) [Google Scholar]
- European Commission. Fleet Register 2024. Available online: https://webgate.ec.europa.eu/fleet-europa/search_en (accessed on 27 February 2024).
- Tzanatos, E.; Dimitriou, E.; Katselis, G.; Georgiadis, M.; Koutsikopoulos, C. Composition, temporal dynamics and regional characteristics of small-scale fisheries in Greece. Fish. Res. 2005, 73, 147–158. [Google Scholar] [CrossRef]
- Tzanatos, E.; Somarakis, S.; Tserpes, G.; Koutsikopoulos, C. Identifying and classifying small-scale fisheries métiers in the Mediterranean: A case study in the Patraikos Gulf, Greece. Fish. Res. 2006, 81, 158–168. [Google Scholar] [CrossRef]
- Skarvelis, K.; Tzanatos, E.; Lazarakis, G.; Peristeraki, P.; Tserpes, G. Typology of the activity of small-scale fisheries in Crete. In Proceedings of the 16th Hellenic Conference of Ichthyologists, Kavala, Greece, 6–9 October 2016. [Google Scholar]
- Bearzi, G. Interactions between cetaceans and fisheries in the Mediterranean Sea. In Cetaceans of the Mediterranean and Black Seas: State of Knowledge and Conservation Strategies; A Report to the ACCOBAMS Secretariat; di Sciara, N., Ed.; ACCOBAMS: Monaco City, Monaco, 2002; p. 20. [Google Scholar]
- Güçlüsoy, H. Damage by monk seals to gear of the artisanal fishery in the Foça Monk Seal Pilot Conservation Area, Turkey. Fish. Res. 2008, 90, 70–77. [Google Scholar] [CrossRef]
- Öztürk, B.; Dede, A. Present Status of the Mediterranean Monk Seal, Monachus monachus, (Hermann, 1779) on the Coasts of Foça in the Bay of Izmir. Turkish J. Mar. Sci. 1995, 1, 95–107. [Google Scholar]
- Panagopoulou, A.; Meletis, Z.A.; Margaritoulis, D.; Spotila, J.R. Caught in the same net? Small-scale fishermen’s perceptions of fisheries interactions with sea turtles and other protected species. Front. Mar. Sci. 2017, 4, 180. [Google Scholar] [CrossRef]
- Turan, C. Status and Trend of Lessepsian Species in Marine Waters of Turkey. In EastMed, 2010. Report of the Sub-Regional Technical Meeting on the Lessepsian Migration and Its Impact on Eastern Mediterranean Fishery; GCP/INT/041/EC-GRE-ITA/TD-04; FAO: Athens, Greece, 2010; pp. 109–118. [Google Scholar]
- Marchessaux, G.; Mangano, M.C.; Bizzarri, S.; M’Rabet, C.; Principato, E.; Lago, N.; Veyssiere, D.; Garrido, M.; Scyphers, S.B.; Sarà, G. Invasive blue crabs and small-scale fisheries in the Mediterranean Sea: Local ecological knowledge, impacts and future management. Mar. Policy 2023, 148, 105461. [Google Scholar] [CrossRef]
- Öndes, F.; Ünal, V.; Özbilgin, Y.; Deval, C.; Turan, C. By-catch and monetary loss of pufferfish in Turkey, the Eastern Mediterranean. Ege J. Fish. Aquat. Sci. 2018, 35, 361–372. [Google Scholar] [CrossRef]
- Ulman, A.; Kalogirou, S.; Pauly, D. The dynamics of maximum lengths for the invasive silver-cheeked toadfish (Lagocephalus sceleratus) in the Eastern Mediterranean Sea. J. Mar. Sci. Eng. 2022, 10, 387. [Google Scholar] [CrossRef]
- Akyol, O.; Ünal, V.; Ceyhan, T.; Bilecenoglou, M. First confirmed record of Lagocephalus sceleratus (Gmelin, 1789) in the Mediterranean Sea. J. Fish Biol. 2005, 66, 1183–1186. [Google Scholar] [CrossRef]
- Michailidis, N. Study on the lessepsian migrant Lagocephalus sceleratus in Cyprus. In EastMed, 2010. Report of the Sub-Regional Technical Meeting on the Lessepsian Migration and Its Impact on Eastern Mediterranean Fishery; GCP/INT/041/EC-GRE-ITA/TD-04; FAO: Athens, Greece, 2010; pp. 74–87. [Google Scholar]
- Khalaf, G.; Saad, A.; Jemaa, S.; Sabour, W.; Lteif, M.; Lelli, S. Population structure and sexual maturity of the pufferfish Lagocephalus sceleratus (Osteichthyes, Tetraodontidae) in the Lebanese and Syrian marine waters (Eastern Mediterranean). J. Earth Sci. Eng. 2014, 4, 236–244. [Google Scholar]
- Çinar, M.; Bilecenoglu, M.; Öztürk, B.; Katagan, T.; Yokes, M.; Aysel, V.; Dagli, E.; Acik, S.; Ozcan, T.; Erdogan, H. An updated review of alien species on the coasts of Turkey. Mediterr. Mar. Sci. 2011, 12, 257–315. [Google Scholar] [CrossRef]
- Kalogirou, S. Ecological characteristics of the invasive pufferfish Lagocephalus sceleratus (Gmelin, 1789) in the eastern Mediterranean Sea—A case study from Rhodes. Mediterr. Mar. Sci. 2013, 14, 251–260. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien marine species in the Mediterranean-the 100 ‘Worst Invasives’ and their impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Halim, Y.; Rizkalla, S. Aliens in Egyptian Mediterranean waters. A check-list of Erythrean fish with new records. Mediterr. Mar. Sci. 2011, 12, 479–490. [Google Scholar] [CrossRef]
- Nader, M.; Indray, S.; Boustany, L. The Puffer Fish Lagocephalus sceleratus (Gmelin, 1789) in the Eastern Mediterranean. In East Med Technical Documents 2012; GCP/INT/041/EC–GRE–ITA; FAO: Rome, Italy, 2012; p. 39. [Google Scholar]
- Galanidi, M.; Zenetos, A.; Bacher, S. Assessing the socio-economic impacts of priority marine invasive fishes in the Mediterranean with the newly proposed SEICAT methodology. Mediterr. Mar. Sci. 2018, 19, 107–123. [Google Scholar] [CrossRef]
- Shakman, E.; Eteayb, K.; Taboni, I.; Abdalha, A.B. Status of marine alien species along the Libyan coast. J. Black Sea/Medit. Environ. 2019, 25, 188–209. [Google Scholar]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campàs, M.; Ozogul, F. An updated review of tetrodotoxin and its peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef]
- Regulation 853/2004/EC; Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004. Laying Down Specific Hygiene Rules for Food of Animal Origin. L226. Regulation (EC). Publications Office of the European Union: Brussels, Belgium, 25 June 2004; pp. 22–82.
- Bilecenoglu, M. Alien marine fishes of Turkey-an updated review. In Fish Invasions in the Mediterranean Sea: Change and Renewal; Golani, D., Appelbaum-Golani, B., Eds.; Pensoft: Sofia, Bulgaria; Moscow, Russia, 2010; pp. 189–217. [Google Scholar]
- Farrag, M.M.S. Fisheries and Biological Studies on Lessepsian Pufferfish, Lagocephalus sceleratus (Gmelin, 1789) (Family: Tetraodontidae) in the Egyptian Mediterranean Waters. Ph.D. Thesis, Faculty of Science, Al-Azhar University, Assuit, Egypt, 2014. [Google Scholar]
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught in the Northeastern Mediterranean Sea. Food Chem. 2016, 210, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Christidis, G.; Mandalakis, M.; Anastasiou, T.I.; Tserpes, G.; Peristeraki, P.; Somarakis, S. Keeping Lagocephalus sceleratus off the table: Sources of variation in the quantity of TTX, TTX analogues, and risk of tetrodotoxication. Toxins 2021, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Alkassar, M.; Sanchez-Henao, A.; Reverté, J.; Barreiro, L.; Rambla-Alegre, M.; Leonardo, S.; Mandalakis, M.; Peristeraki, P.; Diogène, J.; Campàs, M. Evaluation of Toxicity Equivalency Factors of Tetrodotoxin Analogues with a Neuro-2a Cell-Based Assay and Application to Puffer Fish from Greece. Mar. Drugs 2023, 21, 432. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, T.I.; Kagiampaki, E.; Kondylatos, G.; Tselepides, A.; Peristeraki, P.; Mandalakis, M. Assessing the Toxicity of Lagocephalus sceleratus Pufferfish from the Southeastern Aegean Sea and the Relationship of Tetrodotoxin with Gonadal Hormones. Mar. Drugs 2023, 21, 520. [Google Scholar] [CrossRef] [PubMed]
- Kosker, A.R.; Karakus, M.; Katikou, P.; Dal, İ.; Durmus, M.; Ucar, Y.; Ayas, D.; Özogul, F. Monthly Variation of Tetrodotoxin Levels in Pufferfish (Lagocephalus sceleratus) Caught from Antalya Bay, Mediterranean Sea. Mar. Drugs 2023, 21, 527. [Google Scholar] [CrossRef]
- Ünal, V.; Göncüoğlu, H.; Durgun, D.; Tosunoğlu, Z.; Deval, C.; Turan, C. Silver-cheeked toadfish, Lagocephalus sceleratus (Actinopterygii: Tetraodontiformes: Tetraodontidae), causes a substantial economic losses in the Turkish Mediterranean coast: A call for decision makers. Acta Ichthyol. Pisc. 2015, 45, 231–237. [Google Scholar] [CrossRef]
- Ünal, V.; Bodur, H.G. The socio-economic impacts of the silver-cheeked toadfish on small-scale fishers: A comparative study from the Turkish coast. J. Fish. Aquat. Sci. 2017, 34, 119–127. [Google Scholar] [CrossRef]
- Geraci, M.L.; Falsone, F.; Scannella, D.; Sardo, G.; Vitale, S. Dolphin-fisheries interactions: An increasing problem for Mediterranean small-scale fisheries. Examines Mar. Biol. Oceanogr. 2019, 3, 271–272. [Google Scholar] [CrossRef]
- Cochran, W.G. Sampling Techniques, 3rd ed.; Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- May, J.L.; Maxwell, J.G.H. Trawl Fish from Temperate Waters of Australia; CSIRO Division of Fisheries Research: Hobart, Tasmania, 1986; p. 492. [Google Scholar]
- Yaglioglu, D.; Turan, C.; Erguden, D.; Mevlut, G. Range Expansion of silverstripe blaasop, Lagocephalus sceleratus (Gmelin, 1789), to the northeastern Mediterranean Sea. Biharean Biol. 2011, 5, 159–161. [Google Scholar]
- Ramsay, T.O.; Burnett, R.T.; Krewski, D. The effect of concurvity in generalized additive models linking mortality to ambient particulate matter. Epidemiology 2003, 14, 18–23. [Google Scholar] [CrossRef]
- Otero, M.; Cebrian, E.; Francour, P.; Galil, B.; Savini, D. Monitoring Marine Invasive Species in Mediterranean Marine Protected Areas (MPAS)—A Strategy and Practical Guide for Managers; IUCN Centre for Mediterranean Cooperation: Gland, Switzerland, 2013. [Google Scholar]
- Cánovas-Molina, A.; García-Frapolli, E. A review of vulnerabilities in worldwide small-scale fisheries. Fish. Manag. Ecol. 2022, 29, 491–501. [Google Scholar] [CrossRef]
- Anonymous. Greek Fishing Fleet 2020 Annual Report; Hellenic Republic Ministry of Rural Development and Food, Directorate General of Fisheries: Athens, Greece, 2021. [Google Scholar]
- Gonzalvo, J.; Giovos, I.; Moutopoulos, D.K. Fishermen’s perception on the sustainability of small-scale fisheries and dolphin–fisheries interactions in two increasingly fragile coastal ecosystems in western Greece. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 91–106. [Google Scholar] [CrossRef]
- Bonizzoni, S.; Bearzi, G.; Santostasi, N.L.; Furey, N.B.; Valavanis, V.D.; Würsig, B. Dolphin depredation of bottom-set fishing nets in the Gulf of Corinth, Mediterranean Sea. In Proceedings of the 30th Annual Conference of the European Cetacean Society, Madeira, Portugal, 14–16 March 2016. [Google Scholar]
- Garagouni, M.; Avgerinou, G.; Mouchlianitis, F.A.; Minos, G.; Ganias, K. Questionnaire and experimental surveys show that dolphins cause substantial losses to a gillnet fishery in the eastern Mediterranean Sea. ICES J. Mar. Sci. 2022, 79, 2552–2561. [Google Scholar] [CrossRef]
- Ríos, N.; Drakulic, M.; Paradinas, I.; Milliou, A.; Cox, R. Occurrence and impact of interactions between small-scale fisheries and predators, with focus on Mediterranean monk seals (Monachus monachus Hermann 1779), around Lipsi Island complex, Aegean Sea, Greece. Fish. Res. 2017, 187, 1–10. [Google Scholar] [CrossRef]
- Coro, G.; Vilas, L.G.; Magliozzi, C.; Ellenbroek, A.; Scarponi, P.; Pagano, P. Forecasting the ongoing invasion of Lagocephalus sceleratus in the Mediterranean Sea. Ecol. Modell. 2018, 371, 37–49. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsiamis, K.; Ioannou, G.; Michailidis, N.; Zenetos, A. Inventory of alien marine species of Cyprus. Mediterr. Mar. Sci. 2009, 10, 109–134. [Google Scholar] [CrossRef]
- Gücü, A.C. Impact of depth and season on the demersal trawl discard. Turk. J. Fish. Aquat. Sci. 2012, 12, 817–830. [Google Scholar]
- Özbek, E.; Çardak, M.; Kebapçioğlu, T. Spatio-temporal patterns of abundance, biomass and length of the silver-cheeked toadfish Lagocephalus sceleratus in the Gulf of Antalya, Turkey (Eastern Mediterranean Sea). Turk. J. Fish. Aquat. Sci. 2017, 17, 725–733. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Peristeraki, P.; Lazarakis, G.; Tserpes, G. First results on the maturity of the lessepsian migrant Lagocephalus sceleratus (Gmelin 1789) in the eastern Mediterranean Sea. Rapp. Comm. Int. Mer Médit. 2010, 39, 628. [Google Scholar]
- Kawase, H.; Okata, Y.; Ito, K.; Ida, A. Spawning behavior and paternal egg care in a circular structure constructed by pufferfish, Torquigener albomaculosus (Pisces: Tetraodontidae). Bull. Mar. Sci. 2014, 91, 33–43. [Google Scholar] [CrossRef]
- Ulman, A.; Çiçek, B.A.; Salihoglu, I.; Petrou, A.; Patsalidou, M.; Pauly, D.; Zeller, D. Unifying the catch data of a divided island: Cyprus’s marine fisheries catches, 1950–2010. Environ. Dev. Sustain. 2015, 17, 801–821. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A. Risk assessment & annex on measures for L. sceleratus. In Study on Invasive Alien Species–Development of Risk Assessments to Tackle Priority Species and Enhance Prevention–Final Report; European Commission, Directorate-General for Environment, Publications Office: Ispra, Italy, 2018; Available online: https://data.europa.eu/doi/10.2779/84029 (accessed on 29 February 2024).
- Ulman, A.; Yildiz, T.; Demirel, N.; Canak, O.; Yemişken, E.; Pauly, D. The biology and ecology of the invasive silver-cheeked toadfish (Lagocephalus sceleratus), with emphasis on the Eastern Mediterranean. NeoBiota 2021, 68, 145–175. [Google Scholar] [CrossRef]
- Ulman, A.; Ali, F.Z.; Harris, H.E.; Adel, M.; Al Mabruk, S.A.; Bariche, M.; Candelmo, A.C.; Chapman, J.K.; Çiçek, B.A.; Clements, K.R.; et al. Lessons from the Western Atlantic lionfish invasion to inform management in the Mediterranean. Front. Mar. Sci. 2022, 9, 865162. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef]
- Papadaki, S.; Pappou, S.; Dimou, P.; Krokida, M. Isolation and Utilization of Toxins from Marine Invasive Species towards the management of their population. Eur. J. Sustain. Dev. 2022, 11, 61–71. [Google Scholar] [CrossRef]
- Papadaki, S.; Pappou, S.; Krokida, M.; Batjakas, I.; Metai, S.; Frakolaki, G. Extraction and Characterization of Collagen and Fatty Acids from Marine Invasive Species Lagocephalus Sceleratus. In Pterois Miles and Fistularia Commersonii; SSRN: Rochester, NY, USA, 2023; preprint. [Google Scholar] [CrossRef]
- Bucciarelli, G.M.; Lechner, M.; Fontes, A.; Kats, L.B.; Eisthen, H.L.; Shaffer, H.B. From poison to promise: The evolution of tetrodotoxin and its potential as a therapeutic. Toxins 2021, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Olta Azul-Crafting Luxury, Restoring Oceans, One Invasive Fish at a Time. Available online: https://pufferfishleather.com (accessed on 28 February 2024).
- Kasapidis, P.; Peristeraki, P.; Tserpes, G.; Magoulas, A. First record of the Lessepsian migrant Lagocephalus sceleratus (Gmelin 1789) (Osteichthyes: Tetraodontidae) in the Cretan Sea (Aegean, Greece). Aquat. Invasions 2007, 2, 71–73. [Google Scholar] [CrossRef]
- Kaykaç, M.; Tosunoğlu, Z.; Aydın, C.; Ünal, V. Characteristics of fishing gears and methods proposed for combating silver-cheeked toadfish Lagocephalus sceleratus (Gmelin, 1789). In Proceedings of the International Symposium on Pufferfish, Bodrum, Turkey, 13–14 October 2017. [Google Scholar]

| Data Type | Data | |
|---|---|---|
| Presence/absence | Small/large individuals per depth zone (m) | |
| Qualitative | Fishing tactic shifts | |
| Quantitative | Gear-related loss | Extra cost for gear repair and/or replacement (EUR/year) |
| Labor-related loss | Extra fisher’s time (person-hours/month) | |
| Hiring of extra workers (person-months/year) | ||
| Lost fishing days/month | ||
| Catch loss | Loss of commercial species (kg/month) | |
| L. sceleratus by-catch | kg/year |
| Region | Size Class | Depth Zone (m) | ||||
|---|---|---|---|---|---|---|
| 0–10 | 10–25 | 25–45 | 45–65 | 65–100 | ||
| Cretan Sea | Large | 42 | 77 | 90 | 42 | 13 |
| Small | 52 | 67 | 52 | 3 | 0 | |
| Libyan Sea | Large | 36 | 85 | 91 | 82 | 18 |
| Small | 63 | 88 | 47 | 6 | 0 | |
| Ionian Sea | Large | 13 | 67 | 67 | 13 | 13 |
| Small | 14 | 64 | 71 | 7 | 7 | |
| Region | Gear | N of Fishers | Economic Losses | L. sceleratus By-Catch | ||
|---|---|---|---|---|---|---|
| Gear | Labor | Catch | ||||
| Libyan Sea | Longlines | 2 | 650 ± 650 | 1960 ± 1960 | 720 ± 720 | 145 ± 118 |
| Nets | 7 | 1364 ± 209 | 1662 ± 1003 | 2263 ± 288 | 477 ± 276 | |
| Nets/Longlines | 24 | 2495 ± 266 | 5348 ± 688 | 2649 ± 169 | 865 ± 165 | |
| Cretan Sea | Longlines | 6 | 628 ± 201 | 1255 ± 965 | 444 ± 115 | 33 ± 24 |
| Nets | 19 | 1550 ± 304 | 1906 ± 590 | 1360 ± 333 | 209 ± 76 | |
| Nets/Longlines | 29 | 1459 ± 182 | 1904 ± 444 | 1756 ± 282 | 270 ± 94 | |
| Ionian Sea | Longlines | 5 | 40 ± 25 | 672 ± 412 | - | 50 ± 50 |
| Nets | 38 | 8 ± 8 | - | - | 18 ± 13 | |
| Nets/Longlines | 11 | 364 ± 364 | 554 ± 415 | - | 27 ± 14 | |
| Gear | N of Fishers | Economic Losses | L. sceleratus By-Catch | ||
|---|---|---|---|---|---|
| Gear | Labor | Catch | |||
| Longlines | 8 | 634 ± 6 | 1431 ± 262 | 513 ± 73 | 61 ± 30 |
| Nets | 26 | 1500 ± 52 | 1840 ± 68 | 1603 ± 251 | 281 ± 74 |
| Nets/Longlines | 53 | 1928 ± 363 | 3464 ± 944 | 2160 ± 313 | 540 ± 208 |
| Region | Gear | FO% | Mean | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L. sceleratus By-Catch | Gear Damages | Catch Damages | L. sceleratus BPUE (kg) | L. sceleratus NPUE (n) | DPUE (n) | EPUE (kg) | TL (mm) | ||
| Cretan Sea | Nets | 17 | 11 | 12 | 0.9 ± 3.0 | 0.4 ± 1.4 | 2.1 ± 14.6 | 0.04 ± 0.22 | 544 ± 114 |
| Longlines | 6 | 24 | 6 | 0.1 ± 0.3 | 0.03 ± 0.14 | 2.1 ± 5.9 | 0.01 ± 0.08 | 557 ± 74 | |
| Libyan Sea | Nets | 49 | 19 | 26 | 0.5 ± 0.8 | 0.6 ± 1.0 | 4.5 ± 15.1 | 0.4 ± 2.1 | 385 ± 139 |
| Longlines | 0 | 19 | 13 | - | - | 2.9 ± 9.0 | 0.004 ± 0.01 | - | |
| Explanatory Variable | Residual d.f | Residual Deviance | Cumulative Variance Explained (%) | p-Value |
|---|---|---|---|---|
| Depredation probability (binomial model) | ||||
| Mean | 202.00 | 214.78 | ||
| Region | 201.00 | 213.39 | 0.65 | 0.0074 |
| Gear | 200.00 | 211.63 | 1.47 | 0.0042 |
| Depth | 199.01 | 191.20 | 11.0 | 0.0001 |
| SST | 195.26 | 180.90 | 15.8 | 0.0305 |
| L. sceleratus biomass (Tweedie model) | ||||
| Mean | 202.00 | 735.87 | ||
| Region | 201.00 | 732.06 | 0.52 | 0.2692 |
| Gear | 200.00 | 673.85 | 8.43 | 0.0383 |
| Depth | 197.29 | 597.13 | 18.8 | 0.0097 |
| SST | 194.76 | 578.33 | 21.4 | 0.2600 |
| L. sceleratus abundance (Tweedie model) | ||||
| Mean | 202.00 | 760.22 | ||
| Region | 201.00 | 711.18 | 6.45 | 0.0261 |
| Gear | 200.00 | 657.02 | 13.57 | 0.0098 |
| Depth | 196.25 | 564.95 | 25.7 | 0.0196 |
| SST | 196.25 | 564.95 | 25.7 | 0.7863 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christidis, G.; Batziakas, S.; Peristeraki, P.; Tzanatos, E.; Somarakis, S.; Tserpes, G. Another One Bites the Net: Assessing the Economic Impacts of Lagocephalus sceleratus on Small-Scale Fisheries in Greece. Fishes 2024, 9, 104. https://doi.org/10.3390/fishes9030104
Christidis G, Batziakas S, Peristeraki P, Tzanatos E, Somarakis S, Tserpes G. Another One Bites the Net: Assessing the Economic Impacts of Lagocephalus sceleratus on Small-Scale Fisheries in Greece. Fishes. 2024; 9(3):104. https://doi.org/10.3390/fishes9030104
Chicago/Turabian StyleChristidis, Georgios, Stratos Batziakas, Panagiota Peristeraki, Evangelos Tzanatos, Stylianos Somarakis, and George Tserpes. 2024. "Another One Bites the Net: Assessing the Economic Impacts of Lagocephalus sceleratus on Small-Scale Fisheries in Greece" Fishes 9, no. 3: 104. https://doi.org/10.3390/fishes9030104
APA StyleChristidis, G., Batziakas, S., Peristeraki, P., Tzanatos, E., Somarakis, S., & Tserpes, G. (2024). Another One Bites the Net: Assessing the Economic Impacts of Lagocephalus sceleratus on Small-Scale Fisheries in Greece. Fishes, 9(3), 104. https://doi.org/10.3390/fishes9030104







