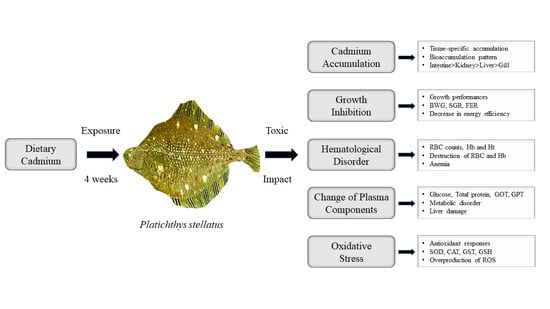
Toxic Impact of Dietary Cadmium on Bioaccumulation, Growth, Hematological Parameters, Plasma Components, and Antioxidant Responses in Starry Flounder (Platichthys stellatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Experimental Fish and Conditions
2.3. Bioaccumulation
2.4. Growth Performance
2.5. Hematological Parameters
2.6. Plasma Components
2.7. Antioxidant Responses
2.8. Statistical Analysis
3. Results
3.1. Bioaccumulation
3.2. Growth Performance
3.3. Hematological Parameters
3.4. Plasma Components
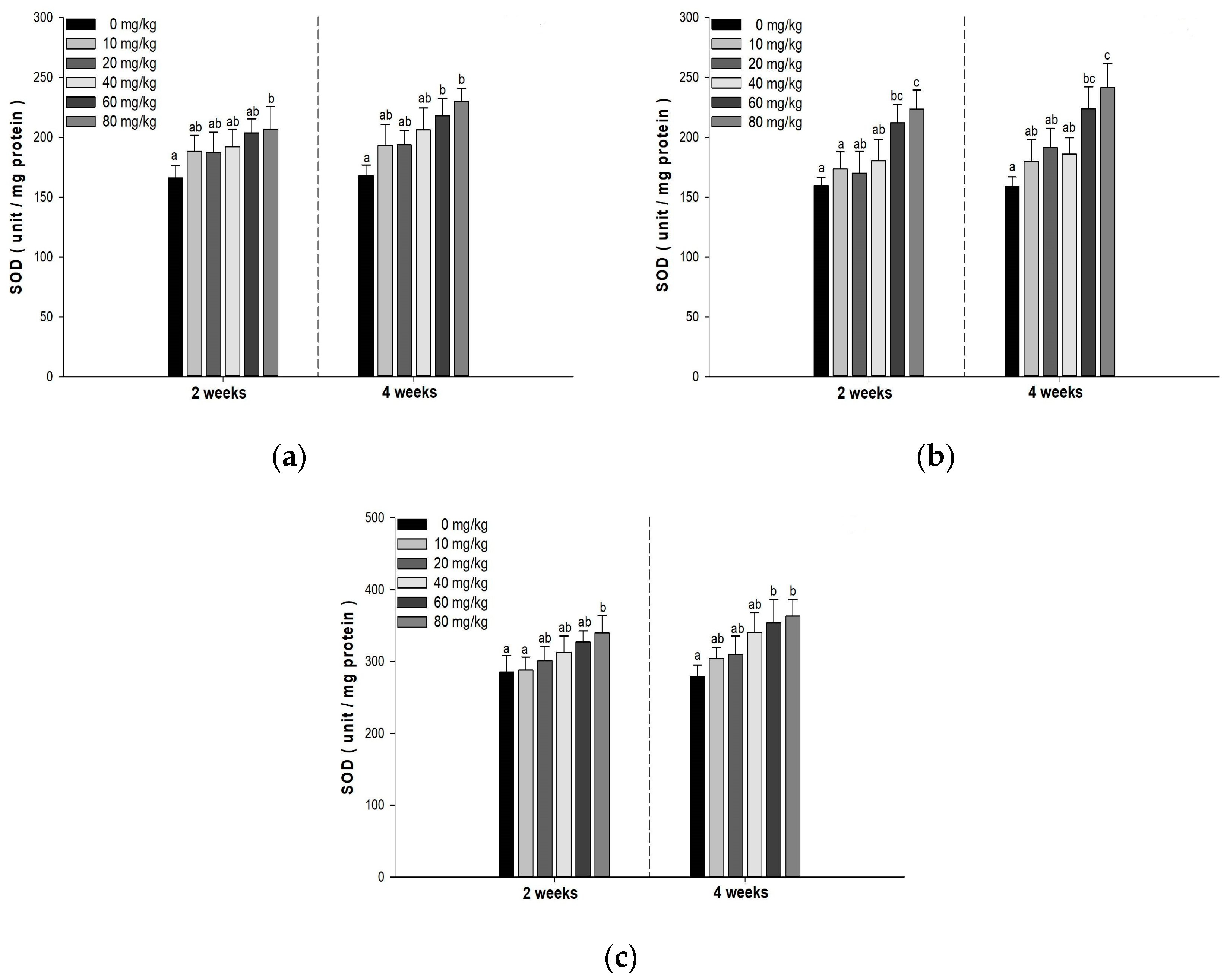
3.5. Antioxidant Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Singh, A. Cadmium toxicity in fish: An overview. GERF Bull. Biosci. 2010, 1, 41–47. [Google Scholar]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Tewari, S.; Bajpai, S.; Tripathi, M. Effect of cadmium on glycogen content in muscle, liver, gill and kidney tissues of freshwater fish Channa punctatus (Bloch). J. Appl. Nat. Sci. 2019, 11, 575–580. [Google Scholar] [CrossRef]
- Luo, W.; Wang, D.; Xu, Z.; Liao, G.; Chen, D.; Huang, X.; Wang, Y.; Yang, S.; Zhao, L.; Huang, H.; et al. Effects of cadmium pollution on the safety of rice and fish in a rice-fish coculture system. Environ. Int. 2020, 143, 105898. [Google Scholar] [CrossRef]
- Al-Asgah, N.A.; Abdel-Warith, A.W.; Younis el, S.M.; Allam, H.Y. Haematological and biochemical parameters and tissue accumulations of cadmium in Oreochromis niloticus exposed to various concentrations of cadmium chloride. Saudi J. Biol. Sci. 2015, 22, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Yeşilbudak, B.; Erdem, C. Cadmium accumulation in gill, liver, kidney and muscle tissues of common carp, Cyprinus carpio, and Nile tilapia, Oreochromis niloticus. Bull. Environ. Contam. Toxicol. 2014, 92, 546–550. [Google Scholar] [CrossRef]
- Demchenkov, E.L.; Nagdalian, A.A.; Budkevich, R.O.; Oboturova, N.P.; Okolelova, A.I. Usage of atomic force microscopy for detection of the damaging effect of CdCl(2) on red blood cells membrane. Ecotoxicol. Environ. Saf. 2021, 208, 111683. [Google Scholar] [CrossRef]
- Nazima, B.; Manoharan, V.; Miltonprabu, S. Oxidative stress induced by cadmium in the plasma, erythrocytes and lymphocytes of rats: Attenuation by grape seed proanthocyanidins. Hum. Exp. Toxicol. 2016, 35, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M.; Kondera, E.; Szczygielska, K. The Effects of Cadmium on Common Carp Erythrocyte Morphology. Pol. J. Environ. Stud. 2011, 20, 783–788. [Google Scholar]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef] [PubMed]
- McRae, N.K.; Gaw, S.; Glover, C.N. Effects of waterborne cadmium on metabolic rate, oxidative stress, and ion regulation in the freshwater fish, inanga (Galaxias maculatus). Aquat. Toxicol. 2018, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sobha, K.; Poornima, A.; Harini, P.; Veeraiah, K. A study on biochemical changes in the fresh water fish, Catla catla (Hamilton) exposed to the heavy metal toxicant cadmium chloride. Kathmandu Univ. J. Sci. Eng. Technol. 2007, 1, 1–11. [Google Scholar] [CrossRef]
- Nair, A.R.; Degheselle, O.; Smeets, K.; Van Kerkhove, E.; Cuypers, A. Cadmium-Induced Pathologies: Where is the Oxidative Balance Lost (or Not)? Int. J. Mol. Sci. 2013, 14, 6116–6143. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.N.; MacDonald-Jay, N.; Kamunde, C. Effects of bioenergetics, temperature and cadmium on liver mitochondria reactive oxygen species production and consumption. Aquat. Toxicol. 2019, 214, 105264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Li, W.Y.; Zheng, J.L. Life-cycle exposure to cadmium induced compensatory responses towards oxidative stress in the liver of female zebrafish. Chemosphere 2018, 210, 949–957. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, J.C. Effects of sub-chronic exposure to lead (Pb) and ascorbic acid in juvenile rockfish: Antioxidant responses, MT gene expression, and neurotransmitters. Chemosphere 2017, 171, 520–527. [Google Scholar] [CrossRef]
- KOSTAT (Statistics Korea). Agriculture & Fishery Products, KOSTAT. 2019. Available online: https://kostat.go.kr/board.es?mid=a20102080000bid=11715 (accessed on 26 March 2020).
- Ahn, J.-J. The small octopus cadmium shock and risk communication. J. Environ. Health Sci. 2010, 36, 435–439. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Jeon, H.; Shin, H.-S. Risk Assessment and Determination of Arsenic and Heavy Metals in Fishery Products in Korea. Foods 2023, 12, 3750. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Y.; Shou, L. Concentration and potential health risk of heavy metals in seafoods collected from Sanmen Bay and its adjacent areas, China. Mar. Pollut. Bull. 2018, 131, 356–364. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Wafeek, M. Influence of water temperature and waterborne cadmium toxicity on growth performance and metallothionein-cadmium distribution in different organs of Nile tilapia, Oreochromis niloticus (L.). J. Therm. Biol. 2014, 45, 157–162. [Google Scholar] [CrossRef]
- Xie, D.; Li, Y.; Liu, Z.; Chen, Q. Inhibitory effect of cadmium exposure on digestive activity, antioxidant capacity and immune defense in the intestine of yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 222, 65–73. [Google Scholar] [CrossRef]
- Okorie, O.E.; Bae, J.Y.; Lee, J.H.; Lee, S.; Park, G.H.; Mohseni, M.; Bai, S.C. Effects of Different Dietary Cadmium Levels on Growth and Tissue Cadmium Content in Juvenile Parrotfish, Oplegnathus fasciatus. Asian-Australas. J. Anim. Sci. 2014, 27, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Eissa, B.L.; Salibián, A. Energy balance of juvenile Cyprinus carpio after a short-term exposure to sublethal water-borne cadmium. Fish. Physiol. Biochem. 2011, 37, 853–862. [Google Scholar] [CrossRef]
- Gandar, A.; Jean, S.; Canal, J.; Marty-Gasset, N.; Gilbert, F.; Laffaille, P. Multistress effects on goldfish (Carassius auratus) behavior and metabolism. Environ. Sci. Pollut. Res. Int. 2016, 23, 3184–3194. [Google Scholar] [CrossRef]
- Moiseenko, T.; Gashkina, N. Distribution and bioaccumulation of heavy metals (Hg, Cd and Pb) in fish: Influence of the aquatic environment and climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Le Croizier, G.; Lacroix, C.; Artigaud, S.; Le Floch, S.; Raffray, J.; Penicaud, V.; Coquillé, V.; Autier, J.; Rouget, M.L.; Le Bayon, N.; et al. Significance of metallothioneins in differential cadmium accumulation kinetics between two marine fish species. Environ. Pollut. 2018, 236, 462–476. [Google Scholar] [CrossRef]
- Kim, S.G.; Eom, K.H.; Kim, S.S.; Jin, H.G.; Kang, J.C. Kinetics of Cd accumulation and elimination in tissues of juvenile rockfish (Sebastes schlegeli) exposed to dietary Cd. Mar. Environ. Res. 2006, 62, 327–340. [Google Scholar] [CrossRef]
- Asagba, S.O.; Eriyamremu, G.E.; Igberaese, M.E. Bioaccumulation of cadmium and its biochemical effect on selected tissues of the catfish (Clarias gariepinus). Fish. Physiol. Biochem. 2008, 34, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Maunder, R.J.; Buckley, J.; Val, A.L.; Sloman, K.A. Accumulation of dietary and aqueous cadmium into the epidermal mucus of the discus fish Symphysodon sp. Aquat. Toxicol. 2011, 103, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Choi, Y.J.; Kim, J.H. Toxic effects of waterborne cadmium exposure on hematological parameters, oxidative stress, neurotoxicity, and heat shock protein 70 in juvenile olive flounder, Paralichthysolivaceus. Fish. Shellfish. Immunol. 2022, 122, 476–483. [Google Scholar] [CrossRef]
- Ovie, K.-S.; Ikomi, U. Alterations in some haematological parameters of the African Snakehead: Parachanna africans exposed to cadmium. Not. Sci. Biol. 2011, 3, 29–34. [Google Scholar] [CrossRef]
- Ahmed, I.; Zakiya, A.; Fazio, F. Effects of aquatic heavy metal intoxication on the level of hematocrit and hemoglobin in fishes: A review. Front. Environ. Sci. 2022, 10, 919204. [Google Scholar] [CrossRef]
- Pereira, L.S.; Ribas, J.L.; Vicari, T.; Silva, S.B.; Stival, J.; Baldan, A.P.; Valdez Domingos, F.X.; Grassi, M.T.; Cestari, M.M.; Silva de Assis, H.C. Effects of ecologically relevant concentrations of cadmium in a freshwater fish. Ecotoxicol. Environ. Saf. 2016, 130, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Moniruzzaman, M.; Ghosal, I.; Pegu, T.; Das, D.N.; Chakraborty, S.B. Evaluating the role of dietary plant extracts to allow adaptation to thermal stress in a cold stream ornamental fish, Botia rostrata (Günther, 1868). J Therm Biol 2022, 105, 103224. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Prozialeck, W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009, 238, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.; Olsen, A.; Nguyen, J.; Wong, W.; El Muayed, M.; Edwards, J. Pancreatic Islets Accumulate Cadmium in a Rodent Model of Cadmium-Induced Hyperglycemia. Int. J. Mol. Sci. 2020, 22, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Wu, L.; Dong, B.; Jin, J.; Han, D.; Zhu, X.; Yang, Y.; Liu, H.; Xie, S. Differential regulation of endoplasmic reticulum stress-induced autophagy and apoptosis in two strains of gibel carp (Carassius gibelio) exposed to acute waterborne cadmium. Aquat. Toxicol. 2021, 231, 105721. [Google Scholar] [CrossRef]
- Vaboonian, A.; Movahedinia, A.; Safahieh, A.; Hedayati, A. Serum biochemical changes of yellowfin sea bream (Acanthopagrus latus) in response to sublethal cadmium toxicity. Comp. Clin. Pathol. 2015, 24, 35–39. [Google Scholar] [CrossRef]
- Santos, G.; Schrama, J.; Mamauag, R.; Rombout, J.; Verreth, J. Chronic stress impairs performance, energy metabolism and welfare indicators in European seabass (Dicentrarchus labrax): The combined effects of fish crowding and water quality deterioration. Aquaculture 2010, 299, 73–80. [Google Scholar] [CrossRef]
- El-Boshy, M.E.-S.; Gadalla, H.A.; El-Hamied, F. Immunological, hematological and biochemical changes induced by short term exposure to cadmium in catfish (Clarias gariepinus). J. Coast. Life Med. 2014, 2, 175–180. [Google Scholar] [CrossRef]
- Tabat, J.L.; Jehu, A.; Kogi, E.; Habila, J.D. Sublethal toxicity effects of cadmium (Cd2+) on serum biochemistry in fingerlings and juveniles of fresh water catfish, Clarias gariepinus (Burchell, 1822). Int. J. Fish. Aquat. Stud. 2021, 9, 1–6. [Google Scholar]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Messaoudi, I.; Barhoumi, S.; Saïd, K.; Kerken, A. Study on the sensitivity to cadmium of marine fish Salaria basilisca (Pisces: Blennidae). J. Environ. Sci. 2009, 21, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, Q.; Zhang, G.; Wu, F.; Zhang, Y.; Yuan, C.; Zhang, T.; Wang, Z. Single and combined exposures of waterborne Cu and Cd induced oxidative stress responses and tissue injury in female rare minnow (Gobiocypris rarus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 222, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Hellou, J.; Ross, N.W.; Moon, T.W. Glutathione, glutathione S-transferase, and glutathione conjugates, complementary markers of oxidative stress in aquatic biota. Environ. Sci. Pollut. Res. Int. 2012, 19, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Pereira, E.; Duarte, A.C.; Ahmad, I. Glutathione and its dependent enzymes’ modulatory responses to toxic metals and metalloids in fish—A review. Environ. Sci. Pollut. Res. Int. 2013, 20, 2133–2149. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, A.M.; Ashry, K.E.; El-Sayed, Y.S. Physiological and Oxidative Stress Biomarkers in the Freshwater Nile Tilapia, Oreochromis niloticus L., exposed to sublethal doses of cadmium. Alex. J. Vet. Sci. 2014, 40, 29–43. [Google Scholar] [CrossRef]
- Dabas, A.; Nagpure, N.S.; Kumar, R.; Kushwaha, B.; Kumar, P.; Lakra, W.S. Assessment of tissue-specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrel, Channa punctatus. Fish. Physiol. Biochem. 2012, 38, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, H.; Liu, X. Low levels of cadmium exposure induce DNA damage and oxidative stress in the liver of Oujiang colored common carp Cyprinus carpio var. color. Fish. Physiol. Biochem. 2011, 37, 97–103. [Google Scholar] [CrossRef] [PubMed]




| Ingredients (%) | Cadmium Concentration (mg/kg) | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | 60 | 80 | |
| White fish meal 1 | 62 | 62 | 62 | 62 | 62 | 62 |
| Casein 2 | 10 | 10 | 10 | 10 | 10 | 10 |
| Dextrin 3 | 20 | 20 | 20 | 20 | 20 | 20 |
| Fish oil 4 | 2 | 2 | 2 | 2 | 2 | 2 |
| Squid liver oil 5 | 2 | 2 | 2 | 2 | 2 | 2 |
| Carboxymethylcellulose 6 | 1 | 1 | 1 | 1 | 1 | 1 |
| α-Cellulose 6 | 0.2 | 0.18 | 0.16 | 0.12 | 0.08 | 0.04 |
| Vitamin premix 7 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Mineral premix 8 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cadmium premix 9 | 0 | 0.02 | 0.04 | 0.08 | 0.12 | 0.16 |
| Colin salt 10 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Actual Cadmium levels (mg/kg) | 0.11 | 7.65 | 16.51 | 36.87 | 47.32 | 64.14 |
| Item | Value |
|---|---|
| Temperature (°C) | 20.8 ± 1.2 |
| pH | 8.0 ± 0.6 |
| Salinity (‰) | 32.9 ± 1.1 |
| Dissolved Oxygen (mg/L) | 7.2 ± 0.4 |
| Chemical Oxygen Demand (mg/L) | 1.0 ± 0.4 |
| Ammonia (μg/L) | 12.1 ± 0.8 |
| Nitrite (μg/L) | 1.3 ± 0.3 |
| Nitrate (μg/L) | 11.5 ± 0.9 |
| Growth Performances | Exposure Period | Cadmium Concentration (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | 60 | 80 | ||
| BWG (%) | 2 weeks | 19.93 ± 1.02 a | 18.51 ± 1.49 ab | 18.64 ± 1.66 ab | 18.31 ± 1.49 ab | 17.20 ± 1.14 ab | 16.30 ± 0.93 b |
| 4 weeks | 29.95 ± 2.75 a | 24.50 ± 2.52 ab | 25.18 ± 1.96 ab | 24.88 ± 2.17 ab | 23.14 ± 1.11 b | 22.12 ± 1.60 b | |
| SGR (%) | 2 weeks | 1.29 ± 0.08 a | 1.18 ± 0.08 ab | 1.20 ± 0.09 ab | 1.23 ± 0.09 ab | 1.16 ± 0.05 ab | 1.06 ± 0.07 b |
| 4 weeks | 0.92 ± 0.08 a | 0.76 ± 0.08 ab | 0.77 ± 0.05 ab | 0.78 ± 0.06 ab | 0.73 ± 0.04 b | 0.67 ± 0.03 b | |
| FER (%) | 2 weeks | 71.17 ± 2.22 a | 66.09 ± 3.04 ab | 66.56 ± 4.06 ab | 68.07 ± 2.31 ab | 65.40 ± 3.60 ab | 61.44 ± 2.65 b |
| 4 weeks | 53.47 ± 4.48 a | 45.57 ± 4.08 ab | 44.42 ± 3.44 ab | 43.74 ± 4.07 ab | 41.31 ± 2.34 b | 39.51 ± 3.57 b | |
| Hematological Parameters | Exposure Period | Cadmium Concentration (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | 60 | 80 | ||
| RBC count (×104/mm3) | 2 weeks | 220.0 ± 15.8 a | 205.4 ± 13.7 ab | 192.6 ± 11.3 ab | 197.1 ± 13.1 ab | 185.1 ± 13.0 ab | 179.4 ± 9.8 b |
| 4 weeks | 215.1 ± 19.4 a | 204.0 ± 18.1 ab | 190.3 ± 12.4 ab | 184.6 ± 14.7 ab | 178.6 ± 11.8 ab | 164.0 ± 12.3 b | |
| Ht (%) | 2 weeks | 21.43 ± 0.90 a | 19.71 ± 1.36 ab | 18.86 ± 0.88 ab | 19.14 ± 1.65 ab | 18.29 ± 0.89 ab | 17.14 ± 0.99 b |
| 4 weeks | 20.43 ± 1.69 a | 18.43 ± 1.44 ab | 17.57 ± 1.10 ab | 17.00 ± 1.36 ab | 17.07 ± 1.14 ab | 16.43 ± 1.00 b | |
| Hb (mg/dL) | 2 weeks | 5.73 ± 0.51 a | 5.53 ± 0.38 ab | 5.23 ± 0.41 ab | 5.25 ± 0.25 ab | 4.80 ± 0.32 ab | 4.53 ± 0.36 b |
| 4 weeks | 5.69 ± 0.52 a | 5.15 ± 0.48 ab | 4.69 ± 0.37 ab | 4.73 ± 0.42 ab | 4.53 ± 0.42 ab | 4.13 ± 0.39 b | |
| Glucose (mg/dL) | 2 weeks | 48.39 ± 1.84 a | 53.89 ± 2.87 ab | 54.80 ± 2.25 ab | 53.59 ± 2.04 ab | 55.20 ± 3.05 b | 55.13 ± 2.34 b |
| 4 weeks | 47.98 ± 2.07 a | 54.25 ± 3.06 ab | 53.85 ± 1.74 ab | 55.35 ± 2.47 ab | 57.78 ± 4.10 b | 61.59 ± 3.42 b | |
| Total protein (g/dL) | 2 weeks | 4.56 ± 0.20 a | 4.45 ± 0.13 a | 4.34 ± 0.18 ab | 4.39 ± 0.21 ab | 4.10 ± 0.21 ab | 3.94 ± 0.15 b |
| 4 weeks | 4.58 ± 0.14 a | 4.46 ± 0.15 a | 4.29 ± 0.22 ab | 4.16 ± 0.19 ab | 3.83 ± 0.16 b | 3.86 ± 0.17 b | |
| GOT (Karmen/mL) | 2 weeks | 52.43 ± 2.61 a | 53.29 ± 2.15 ab | 53.46 ± 2.87 ab | 55.87 ± 3.22 ab | 56.83 ± 3.26 ab | 60.52 ± 2.67 b |
| 4 weeks | 51.43 ± 2.24 a | 55.20 ± 2.45 ab | 57.34 ± 2.73 ab | 57.15 ± 3.35 ab | 59.00 ± 3.58 ab | 62.66 ± 3.37 b | |
| GPT (Karmen/mL) | 2 weeks | 34.33 ± 2.12 a | 35.03 ± 1.80 a | 36.44 ± 1.46 ab | 36.14 ± 1.17 ab | 36.63 ± 1.83 b | 39.95 ± 1.66 b |
| 4 weeks | 34.54 ± 1.50 a | 34.97 ± 1.79 a | 35.30 ± 1.47 a | 36.95 ± 1.99 ab | 37.71 ± 1.98 ab | 40.24 ± 1.52 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.-C.; Kim, J.-H.; Kang, J.-C. Toxic Impact of Dietary Cadmium on Bioaccumulation, Growth, Hematological Parameters, Plasma Components, and Antioxidant Responses in Starry Flounder (Platichthys stellatus). Fishes 2024, 9, 59. https://doi.org/10.3390/fishes9020059
Jung H-C, Kim J-H, Kang J-C. Toxic Impact of Dietary Cadmium on Bioaccumulation, Growth, Hematological Parameters, Plasma Components, and Antioxidant Responses in Starry Flounder (Platichthys stellatus). Fishes. 2024; 9(2):59. https://doi.org/10.3390/fishes9020059
Chicago/Turabian StyleJung, Hyeok-Chan, Jun-Hwan Kim, and Ju-Chan Kang. 2024. "Toxic Impact of Dietary Cadmium on Bioaccumulation, Growth, Hematological Parameters, Plasma Components, and Antioxidant Responses in Starry Flounder (Platichthys stellatus)" Fishes 9, no. 2: 59. https://doi.org/10.3390/fishes9020059
APA StyleJung, H.-C., Kim, J.-H., & Kang, J.-C. (2024). Toxic Impact of Dietary Cadmium on Bioaccumulation, Growth, Hematological Parameters, Plasma Components, and Antioxidant Responses in Starry Flounder (Platichthys stellatus). Fishes, 9(2), 59. https://doi.org/10.3390/fishes9020059