Administration of Red Macroalgae (Galaxaura oblongata) in the Diet of the Rainbow Trout (Oncorhynchus mykiss) Improved Immunity and Hepatic Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Collection and Preparation
2.2. Experimental Diets
2.3. Experimental Design
2.4. Growth Performance
2.5. Sample Collection
2.6. Serum and Mucus Non-Specific Immune Parameters
2.7. Serum and Mucus Antioxidant Parameters
2.8. RNA Isolation, cDNA Synthesis, and Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Immunological Indices
3.3. Skin Mucus Immunological Indices
3.4. Serum Antioxidant Indices
3.5. Skin Mucus Antioxidant Indices
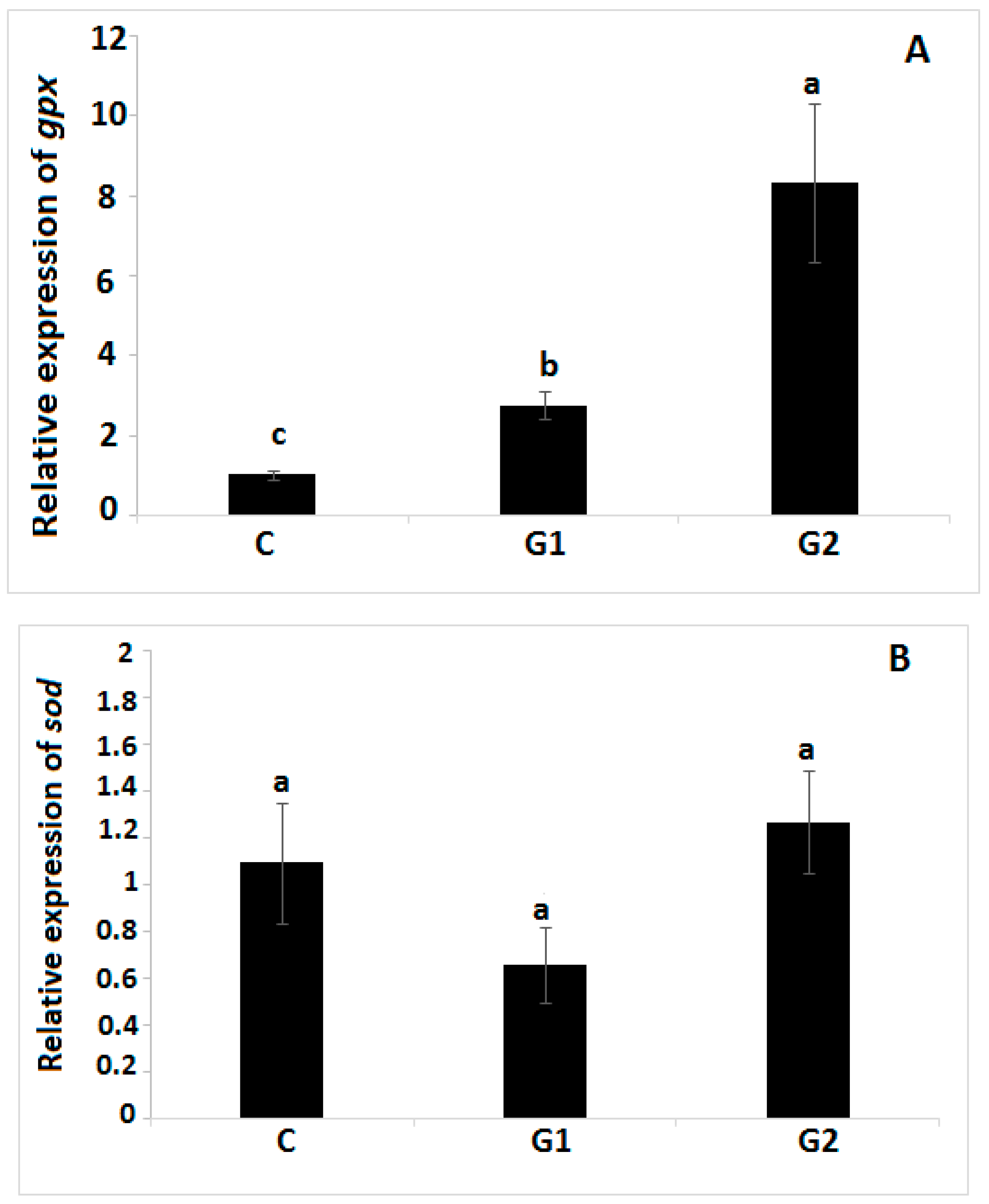
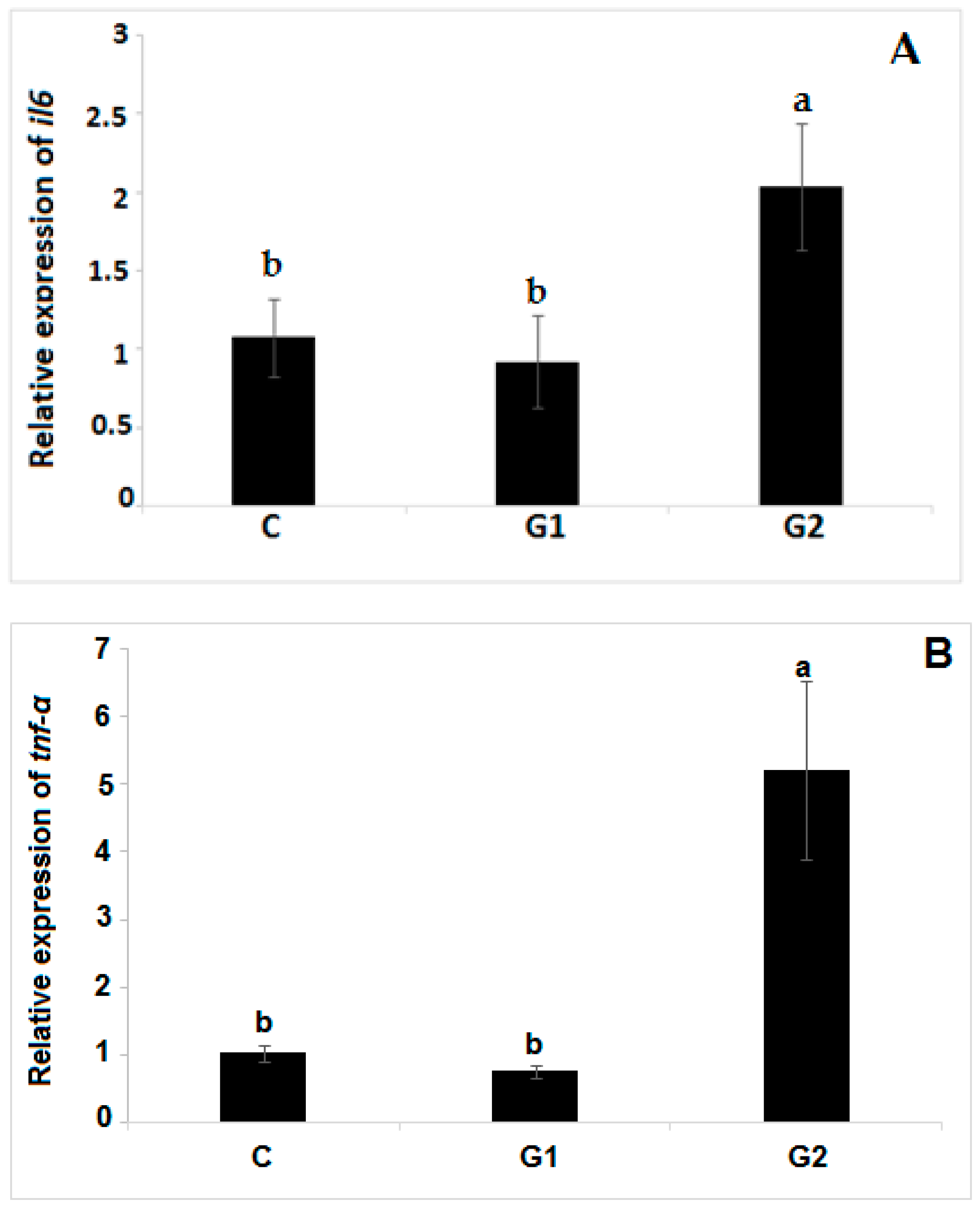
3.6. Antioxidant and Immune-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henriksson, P.J.; Rico, A.; Troell, M.; Klinger, D.H.; Buschmann, A.H.; Saksida, S.; Chadag, M.V.; Zhang, W. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: A review from a systems perspective. Sustain. Sci. 2018, 13, 1105–1120. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Sheikhzadeh, N.; Roshanaei, K.; Dargahi, N.; Faggio, C. Can dietary ginger (Zingiber officinale) alter biochemical and immunological parameters and gene expression related to growth, immunity and antioxidant system in zebrafish (Danio rerio)? Aquaculture 2019, 507, 341–348. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Manage, P.M. Heavy use of antibiotics in aquaculture; emerging human and animal health problems—A review. Sri Lanka J. Aquat. Sci. 2018, 23, 13–27. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; El-Ashram, A.M.; Tahoun, A.A.; Abdel-Razek, N.; Awad, S.M. Effects of dietary sweet basil (Ocimum basilicum) oil on the performance, antioxidants and immunity welfare, and resistance of indian shrimp (Penaeus indicus) against vibrio parahaemolyticus infection. Aquac. Nut. 2021, 27, 1244–1254. [Google Scholar] [CrossRef]
- Awad, E.; Awaad, A. Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol. 2017, 67, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Analysis of antioxidant capacity and bioactive compounds in marine macroalgal and lichenic extracts using different solvents and evaluation methods. Cienc. Mar. 2016, 42, 271–288. [Google Scholar] [CrossRef]
- Lailatussifa, R.; Husni, A.; Isnansetyo, A. Antioxidant activity and proximate analysis of dry powder from brown seaweed Sargassum hystrix. J. Perikan. Univ. Gad. Mada 2017, 19, 29–37. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Marhamati, A.; Chisti, Y. Seaweed-based diets lead to normal growth, improved fillet color but a down-regulated expression of somatotropic axis genes in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 554, 738183. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef]
- Wan, A.H.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed potential in the animal feed: A review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Xie, J.; Niu, J. Evaluation of four macro-algae on growth performance, antioxidant capacity and non-specific immunity in golden pompano (Trachinotus ovatus). Aquaculture 2022, 548, 737690. [Google Scholar] [CrossRef]
- Ashour, M.; Mabrouk, M.M.; Ayoub, H.F.; El-Feky, M.M.; Zaki, S.Z.; Hoseinifar, S.H.; Rossi, W.; Van Doan, H.; El-Haroun, E.; Goda, A. Effect of dietary seaweed extract supplementation on growth, feed utilization, hematological indices, and non-specific immunity of Nile tilapia, Oreochromis niloticus challenged with Aeromonas hydrophila. J. Appl. Phycol. 2020, 32, 3467–3479. [Google Scholar] [CrossRef]
- Niu, J.; Xie, S.W.; Fang, H.H.; Xie, J.J.; Guo, T.Y.; Zhang, Y.M.; Liu, Z.L.; Liao, S.Y.; He, J.Y.; Tian, L.X. Dietary values of macroalgae Porphyra haitanensis in Litopenaeus vannamei under normal rearing and wssv challenge conditions: Effect on growth, immune response and intestinal microbiota. Fish Shellfish Immunol. 2018, 81, 135–149. [Google Scholar] [CrossRef]
- Akbary, P.; Aminikhoei, Z. Effect of water-soluble polysaccharide extract from the green alga Ulva rigida on growth performance, antioxidant enzyme activity, and immune stimulation of grey mullet Mugil cephalus. J. Appl. Phycol. 2018, 30, 1345–1353. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Marhamati, A.; Rabiee, R.; Faggio, C. Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish Shellfish Immunol. 2020, 106, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Fazelan, Z.; Bayani, M.; Yousefi, M.; Van Doan, H.; Yazici, M. Dietary red macroalgae (Halopithys incurva) improved systemic and mucosal immune and antioxidant parameters and modulated related gene expression in zebrafish (Danio rerio). Fish Shellfish Immunol. 2022, 123, 164–171. [Google Scholar] [CrossRef]
- Khanzadeh, M.; Beikzadeh, B.; Hoseinifar, S.H. The Effects of Laurencia caspica Algae Extract on Hemato-Immunological Parameters, Antioxidant Defense, and Resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2023, 2023, 8882736. [Google Scholar] [CrossRef]
- Ortiz-Viedma, J.; Aguilera, J.M.; Flores, M.; Lemus-Mondaca, R.; Larrazabal, M.J.; Miranda, J.M.; Aubourg, S.P. Protective effect of red algae (Rhodophyta) extracts on essential dietary components of heat-treated salmon. Antioxidants 2021, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Archibald, J.M.; Simpson, A.G.; Slamovits, C.H.; Margulis, L.; Melkonian, M.; Chapman, D.J.; Corliss, J.O. Handbook of the Protists; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Tapilatu, Y. Biodiversity of tropical seaweeds. In Seaweed Biotechnology; Apple Academic Press: New York, NY, USA, 2023; pp. 1–13. [Google Scholar]
- Nabil-Adam, A.; Shreadah, M.A. Red algae natural products for prevention of lipopolysaccharides (lps)-induced liver and kidney inflammation and injuries. Biosci. Rep. 2021, 41, BSR20202022. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.I.; Daley, D.; Watson, C.; Powell, S.A.; Ayeah, K.N.; Toyang, N.J.; Bryant, J.; Lamm, A.S. Anticancer activity of three jamaican macroalgae against prostate, pancreatic and skin cancers. Eur. J. Med. Plants 2016, 13, 1–5. [Google Scholar] [CrossRef]
- Huang, H.L.; Wu, S.L.; Liao, H.F.; Jiang, C.M.; Huang, R.L.; Chen, Y.Y.; Yang, Y.C.; Chen, Y.J. Induction of apoptosis by three marine algae through generation of reactive oxygen species in human leukemic cell lines. Agric. Food Chem. 2005, 53, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Sayin, S.; Kohlhaas, T.; Veziroglu, S.; Okudan, E.Ş.; Naz, M.; Schröder, S.; Saygili, E.; Açil, Y.; Faupel, F.; Wiltfang, J. Marine algae-pla composites as de novo alternative to porcine derived collagen membranes. Mater. Today Chem. 2020, 17, 100276. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Majidiyan, N.; Mirghaed, A.T.; Hoseinifar, S.H.; Van Doan, H. Dietary glycine supplementation alleviates transportation-induced stress in common carp, Cyprinus carpio. Aquaculture 2022, 551, 737959. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Hoseinifar, S.H.; Tongsiri, S.; Chitmanat, C.; Musthafa, M.S.; El-Haroun, E.; Ringo, E. Modulation of growth, innate immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) culture under biofloc system by supplementing pineapple peel powder and Lactobacillus plantarum. Fish Shellfish Immunol. 2021, 115, 212–220. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Adorian, T.J.; Ferrigolo, F.R.G.; Raissy, M.; Van Doan, H. The effects of combined inclusion of malvae sylvestris, Origanum vulgare, and Allium hirtifolium boiss for common carp (Cyprinus carpio) diet: Growth performance, antioxidant defense, and immunological parameters. Fish Shellfish Immunol. 2021, 119, 670–677. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Siwicki, A.K.; Anderson, D.P.; Rumsey, G.L. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet. Immunol. Immuno Pathol. 1994, 41, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Nedaei, S.; Noori, A.; Valipour, A.; Khanipour, A.A.; Hoseinifar, S.H. Effects of dietary galactooligosaccharide enriched commercial prebiotic on growth performance, innate immune response, stress resistance, intestinal microbiota and digestive enzyme activity in narrow clawed crayfish (Astacus leptodactylus eschscholtz, 1823). Aquaculture 2019, 499, 80–89. [Google Scholar]
- Hoseinifar, S.H.; Shakouri, M.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Raeisi, M.; Yousefi, S.; Harikrishnan, R.; Reverter, M. Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss). Fish Shellfish Immunol. 2020, 99, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Mahboub, H.H.; Rashidian, G.; Hoseinifar, S.H.; Kamel, S.; Zare, M.; Ghafarifarsani, H.; Algharib, S.A.; Moonmanee, T.; Van Doan, H. Protective effects of Allium hirtifolium extract against foodborne toxicity of zinc oxide nanoparticles in common carp (Cyprinus carpio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 257, 109345. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Hoseinifar, S.H.; Dadar, M. Enrichment of common carp (Cyprinus carpio) diet with malic acid: Effects on skin mucosal immunity, antioxidant defecne and growth performance. Ann. Anim. Sci. 2021, 21, 561–573. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Seaweed as a source of novel nutraceuticals: Sulfated polysaccharides and peptides. Adv. Food Nutr. Res. 2011, 64, 325–337. [Google Scholar] [PubMed]
- Choi, Y.H.; Kim, K.W.; Han, H.S.; Nam, T.J.; Lee, B.J. Dietary Hizikia fusiformis glycoprotein-induced igf-i and igfbp-3 associated to somatic growth, polyunsaturated fatty acid metabolism, and immunity in juvenile olive flounder Paralichthys olivaceus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 167, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Abdelrhman, A.M.; Ashour, M.; Al-Zahaby, M.A.; Sharawy, Z.Z.; Nazmi, H.; Zaki, M.A.; Ahmed, N.H.; Ahmed, S.R.; El-Haroun, E.; Van Doan, H. Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquac. Rep. 2022, 25, 101212. [Google Scholar] [CrossRef]
- Shi, Q.; Rong, H.; Hao, M.; Zhu, D.; Aweya, J.J.; Li, S.; Wen, X. Effects of dietary Sargassum horneri on growth performance, serum biochemical parameters, hepatic antioxidant status, and immune responses of juvenile black sea bream Acanthopagrus schlegelii. Appl. Phycol. 2019, 31, 2103–2113. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y. The growth performance and non-specific immunity of juvenile grass carp (Ctenopharyngodon idella) affected by dietary Porphyra yezoensis polysaccharide supplementation. Fish Shellfish Immunol. 2019, 87, 615–619. [Google Scholar] [CrossRef]
- Passos, R.; Correia, A.P.; Ferreira, I.; Pires, P.; Pires, D.; Gomes, E.; do Carmo, B.; Santos, P.; Simões, M.; Afonso, C. Effect on health status and pathogen resistance of gilthead seabream (Sparus aurata) fed with diets supplemented with Gracilaria gracilis. Aquaculture 2021, 531, 735888. [Google Scholar] [CrossRef]
- Mazlum, Y.; Yazici, M.; Sayin, S.; Habiboğlu, O.U.; Sinem, U. Effects of two different macroalgae (Ulva lactuca and Jania rubens) species on growth and survival of red swamp crayfish (Procambarus clarkii) as feed additive. Mar. Sci. Technol. Bull. 2020, 10, 154–162. [Google Scholar] [CrossRef]
- Nahavandi, R.; Sadeghi, A.; Pormozaffar, S.; Jahromi, S.T.; Khajehrahimi, A.E. Investigation of the effect of diet containing red algae (Laurencia caspica) on blood parameters and activity of digestive enzymes of goldfish (Carassius auratus). J. Surv. Fish. Sci. 2023, 10, 48–59. [Google Scholar]
- Abbas, E.M.; Al-Souti, A.S.; Sharawy, Z.Z.; El-Haroun, E.; Ashour, M. Impact of dietary administration of seaweed polysaccharide on growth, microbial abundance, and growth and immune-related genes expression of the pacific whiteleg shrimp (Litopenaeus vannamei). Life 2023, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Yousefi, S.; Capillo, G.; Paknejad, H.; Khalili, M.; Tabarraei, A.; Van Doan, H.; Spanò, N.; Faggio, C. Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol. 2018, 83, 232–237. [Google Scholar] [CrossRef]
- Adel, M.; Omidi, A.H.; Dawood, M.A.; Karimi, B.; Shekarabi, S.P.H. Dietary Gracilaria persica mediated the growth performance, fillet colouration, and immune response of persian sturgeon (Acipenser persicus). Aquaculture 2021, 530, 735950. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.A. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Sutili, F.J.; Gatlin III, D.M.; Heinzmann, B.M.; Baldisserotto, B. Plant essential oils as fish diet additives: Benefits on fish health and stability in feed. Rev. Aquac. 2018, 10, 716–726. [Google Scholar] [CrossRef]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Sringarm, K.; Hoseinifar, S.H.; Dawood, M.A.; El-Haroun, E.; Harikrishnan, R.; Jaturasitha, S.; Paolucci, M. Impacts of amla (Phyllanthus emblica) fruit extract on growth, skin mucosal and serum immunities, and disease resistance of Nile tilapia (Oreochromis niloticus) raised under biofloc system. Aquac. Rep. 2022, 22, 100953. [Google Scholar] [CrossRef]
- Ángeles Esteban, M. An overview of the immunological defenses in fish skin. Int. Sch. Res. Not. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Aftabgard, M.; Van Doan, H. The improving role of savory (Satureja hortensis) essential oil for caspian roach (Rutilus caspicus) fry: Growth, haematological, immunological, and antioxidant parameters and resistance to salinity stress. Aquaculture 2022, 548, 737653. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Jiang, J.; Zhang, Y.; Zhou, X. Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2014, 41, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, V.; Nafisi Bahabadi, M.; Sotoudeh, E.; Azodi, M.; Hafezieh, M. Nutritional evaluation of Gracilaria pulvinata as partial substitute with fish meal in practical diets of barramundi (Lates calcarifer). Appl. Phycol. 2018, 30, 619–628. [Google Scholar] [CrossRef]
- Rudtanatip, T.; Lynch, S.A.; Wongprasert, K.; Culloty, S.C. Assessment of the effects of sulfated polysaccharides extracted from the red seaweed irish moss Chondrus crispus on the immune-stimulant activity in mussels Mytilus spp. Fish Shellfish Immunol. 2018, 75, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wen, G.; Lin, H.; Yang, Y.; Huang, X.; Zhou, C.; Zhang, Z.; Duan, Y.; Huang, Z.; Li, T. Effects of dietary Spirulina platensis on growth performance, hematological and serum biochemical parameters, hepatic antioxidant status, immune responses and disease resistance of coral trout Plectropomus leopardus (lacepede, 1802). Fish Shellfish Immunol. 2018, 74, 649–655. [Google Scholar] [CrossRef]
- Rufchaei, R.; Nedaei, S.; Hoseinifar, S.H.; Hassanpour, S.; Golshan, M.; Sayad Bourani, M. Improved growth performance, serum and mucosal immunity, haematology and antioxidant capacity in pikeperch (Sander lucioperca) using dietary water hyacinth (Eichhornia crassipes) leaf powder. Aquac. Res. 2021, 52, 2194–2204. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Thamizharasan, S.; Devi, G.; Van Doan, H.; Kumar, T.T.A.; Hoseinifar, S.H.; Balasundaram, C. Dried lemon peel enriched diet improves antioxidant activity, immune response and modulates immuno-antioxidant genes in Labeo rohita against Aeromonas sorbia. Fish Shellfish Immunol. 2020, 106, 675–684. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Hoseinifar, S.H.; Van Doan, H. Growth performance and hematological and antioxidant characteristics of rainbow trout, Oncorhynchus mykiss, fed diets supplemented with roselle, Hibiscus sabdariffa. Aquaculture 2021, 530, 735827. [Google Scholar] [CrossRef]
- Yousefi, S.; Shokri, M.M.; Noveirian, H.A.; Hoseinifar, S.H. Effects of dietary yeast cell wall on biochemical indices, serum and skin mucus immune responses, oxidative status and resistance against Aeromonas hydrophila in juvenile persian sturgeon (Acipenser persicus). Fish Shellfish Immunol. 2020, 106, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Kiadaliri, M.; Firouzbakhsh, F.; Deldar, H. Effects of feeding with red algae (Laurencia caspica) hydroalcoholic extract on antioxidant defense, immune responses, and immune gene expression of kidney in rainbow trout (Oncorhynchus mykiss) infected with Aeromonas hydrophila. Aquaculture 2020, 526, 735361. [Google Scholar] [CrossRef]
- Latif, M.; Faheem, M.; Asmatullah; Hoseinifar, S.H.; Van Doan, H. Dietary black seed effects on growth performance, proximate composition, antioxidant and histo-biochemical parameters of a culturable fish, rohu (Labeo rohita). Animals 2020, 11, 48. [Google Scholar] [CrossRef]
- Mirghaed, A.T.; Hoseini, S.M.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary thyme (Zataria multiflora) extract on antioxidant and immunological responses and immune-related gene expression of rainbow trout (Oncorhynchus mykiss) juveniles. Fish Shellfish Immunol. 2020, 106, 502–509. [Google Scholar] [CrossRef]
- Teimouri, M.; Yeganeh, S.; Mianji, G.R.; Najafi, M.; Mahjoub, S. The effect of Spirulina platensis meal on antioxidant gene expression, total antioxidant capacity, and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2019, 45, 977–986. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Arockiaraj, J.; Jagruthi, C. Efficacy of ulvan on immune response and immuno-antioxidant gene modulation in Labeo rohita against columnaris disease. Fish Shellfish Immunol. 2021, 117, 262–273. [Google Scholar] [CrossRef]
- Fazelan, Z.; Hoseini, S.M.; Yousefi, M.; Khalili, M.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary eucalyptol administration on antioxidant and inflammatory genes in common carp (Cyprinus carpio) exposed to ambient copper. Aquaculture 2020, 520, 734988. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, F.; Gong, P.; Qiu, Y.; Zhou, W.; Cui, Y.; Li, J.; Chen, H. Subneurotoxic copper (II)-induced nf-κb-dependent microglial activation is associated with mitochondrial ros. Toxicol. Appl. Pharmacol. 2014, 276, 95–103. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M.; Mirghaed, A.T.; Paray, B.A.; Hoseinifar, S.H.; Van Doan, H. Effects of rearing density and dietary tryptophan supplementation on intestinal immune and antioxidant responses in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 528, 735537. [Google Scholar] [CrossRef]
- Zheng, J.L.; Yuan, S.S.; Wu, C.W.; Lv, Z.M. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio). Aquat. Toxicol. 2016, 180, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Vijay, S.; Balasundaram, C.; Ringø, E.; Hoseinifar, S.H.; Jaturasithaf, S. Dietary plant pigment on blood-digestive physiology, antioxidant-immune response, and inflammatory gene transcriptional regulation in spotted snakehead (Channa punctata) infected with Pseudomonas aeruginosa. Fish Shellfish Immunol. 2022, 120, 716–736. [Google Scholar] [CrossRef]
- Hong, S.; Li, R.; Xu, Q.; Secombes, C.J.; Wang, T. Two types of tnf-α exist in teleost fish: Phylogeny, expression, and bioactivity analysis of type-ii tnf-α3 in rainbow trout Oncorhynchus mykiss. J. Immunol. 2013, 191, 5959–5972. [Google Scholar] [CrossRef] [PubMed]
- Eggestøl, H.Ø.; Lunde, H.S.; Haugland, G.T. The pro-inflammatory cytokines tnf-α and il-6 in lumpfish (Cyclopterus lumpus L.) identification, molecular characterization, phylogeny and gene expression analyses. Dev. Comp. Immunol. 2020, 105, 103608. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Lee, B.J.; Nam, T.J. Effect of dietary inclusion of Pyropia yezoensis extract on biochemical and immune responses of olive flounder Paralichthys olivaceus. Aquaculture 2015, 435, 347–353. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Firouzbakhsh, F.; Rahimi-Mianji, G.; Paknejad, H. Immunostimulatory effect of aloe vera (Aloe barbadensis) on non-specific immune response, immune gene expression, and experimental challenge with Saprolegnia parasitica in rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 503, 330–338. [Google Scholar] [CrossRef]
| Gene Name | Sequences of Primers | Accession No | Efficiency |
|---|---|---|---|
| ß-actin | Forward: GACATCAGGGTGTCATGGTTGGT | M24113.1 | 97% |
| Reverse: CTCAAACATGATCTGTGTCAT | |||
| il-6 | Forward: ACTCCCCTCTGTCACACACC | DQ866150 | 98% |
| Reverse: GGCAGACAGGTCCTCCACTA | |||
| tnf-α | Forward: GGTGATGGTGTCGAGGAGGAA | AJ311800.1 | 97% |
| Reverse: TGGAAAGACACCTGGCTGTA | |||
| sod | Forward: GTAGTCGTGGCTCAATGGTAAG | AJ311800.1 | 97% |
| Reverse: GCTTTATATTCTGCGGGTCATT | |||
| gpx | Forward: AAATTGCCATTCCCCTCCGA | AF281338 | 97% |
| Reverse: TCCATCAGGACTGACCAGGA |
| C | G1 | G2 | |
|---|---|---|---|
| IW (g) | 4.50 ± 0.50 a | 4.26 ± 0.20 a | 4.30 ± 0.26 a |
| FW (g) | 22.60 ± 2.25 a | 23.40 ± 1.40 a | 23.66 ± 3.88 a |
| WG (g) | 18.10 ± 2.68 a | 19.13 ± 1.18 a | 19.36 ± 3.64 a |
| SGR (%/d) | 2.88 ± 0.36 a | 3.03 ± 0.10 a | 3.13 ± 0.19 a |
| FCR | 1.16 ± 0.14 a | 1.08 ± 0.10 a | 1.09 ± 0.19 a |
| C | G1 | G2 | |
|---|---|---|---|
| Total Ig (g/dL) | 1.04 ± 0.01 c | 1.38 ± 0.04 a | 1.21 ± 0.04 b |
| Lysozyme (U/mL) | 45.69 ± 2.80 c | 56.47 ± 3.31 a | 49.84 ± 3.47 b |
| C | G1 | G2 | |
|---|---|---|---|
| Total Ig (g/dL) | 0.61 ± 0.03 b | 0.96 ± 0.08 a | 0.85 ± 0.09 a |
| Lysozyme (U/mL) | 20.33 ± 1.43 a | 21.56 ± 2.32 a | 22.49 ± 1.91 a |
| C | G1 | G2 | |
|---|---|---|---|
| Catalase (mL/min/n) | 0.22 ± 0.04 b | 0.73 ± 0.10 a | 0.63 ± 0.06 a |
| SOD (U/mL) | 656.26 ± 7.13 b | 780.52 ± 24.28 a | 719.61 ± 18.56 a |
| GPx (nmol/min/mL) | 131.50 ± 0.52 b | 170.90 ± 1.84 a | 166.87 ± 1.18 a |
| C | G1 | G2 | |
|---|---|---|---|
| Catalase (mL/min/n) | 0.25 ±0.01 a | 0.26 ± 0.01 a | 0.25 ± 0.01 a |
| SOD (U/mL) | 502.83 ± 1.29 b | 499.61 ± 0.98 b | 509.12 ± 5.33 a |
| GPx (nmol/min/mL) | 136.78 ± 0.96 b | 141.78 ± 0.92 a | 142.02 ± 1.11 a |
| MDA (nmol/mL) | 417.13 ± 3.32 a | 416.97 ± 2.34 a | 414.93 ± 1.25 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazici, M.; Zavvar, F.; Hoseinifar, S.H.; Nedaei, S.; Doan, H.V. Administration of Red Macroalgae (Galaxaura oblongata) in the Diet of the Rainbow Trout (Oncorhynchus mykiss) Improved Immunity and Hepatic Gene Expression. Fishes 2024, 9, 48. https://doi.org/10.3390/fishes9020048
Yazici M, Zavvar F, Hoseinifar SH, Nedaei S, Doan HV. Administration of Red Macroalgae (Galaxaura oblongata) in the Diet of the Rainbow Trout (Oncorhynchus mykiss) Improved Immunity and Hepatic Gene Expression. Fishes. 2024; 9(2):48. https://doi.org/10.3390/fishes9020048
Chicago/Turabian StyleYazici, Metin, Fatemeh Zavvar, Seyed Hossein Hoseinifar, Shiva Nedaei, and Hien Van Doan. 2024. "Administration of Red Macroalgae (Galaxaura oblongata) in the Diet of the Rainbow Trout (Oncorhynchus mykiss) Improved Immunity and Hepatic Gene Expression" Fishes 9, no. 2: 48. https://doi.org/10.3390/fishes9020048
APA StyleYazici, M., Zavvar, F., Hoseinifar, S. H., Nedaei, S., & Doan, H. V. (2024). Administration of Red Macroalgae (Galaxaura oblongata) in the Diet of the Rainbow Trout (Oncorhynchus mykiss) Improved Immunity and Hepatic Gene Expression. Fishes, 9(2), 48. https://doi.org/10.3390/fishes9020048








