The Influence of Natural and Anthropogenic Environmental Pressures on European Eel Abundances in French Estuaries
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Origin
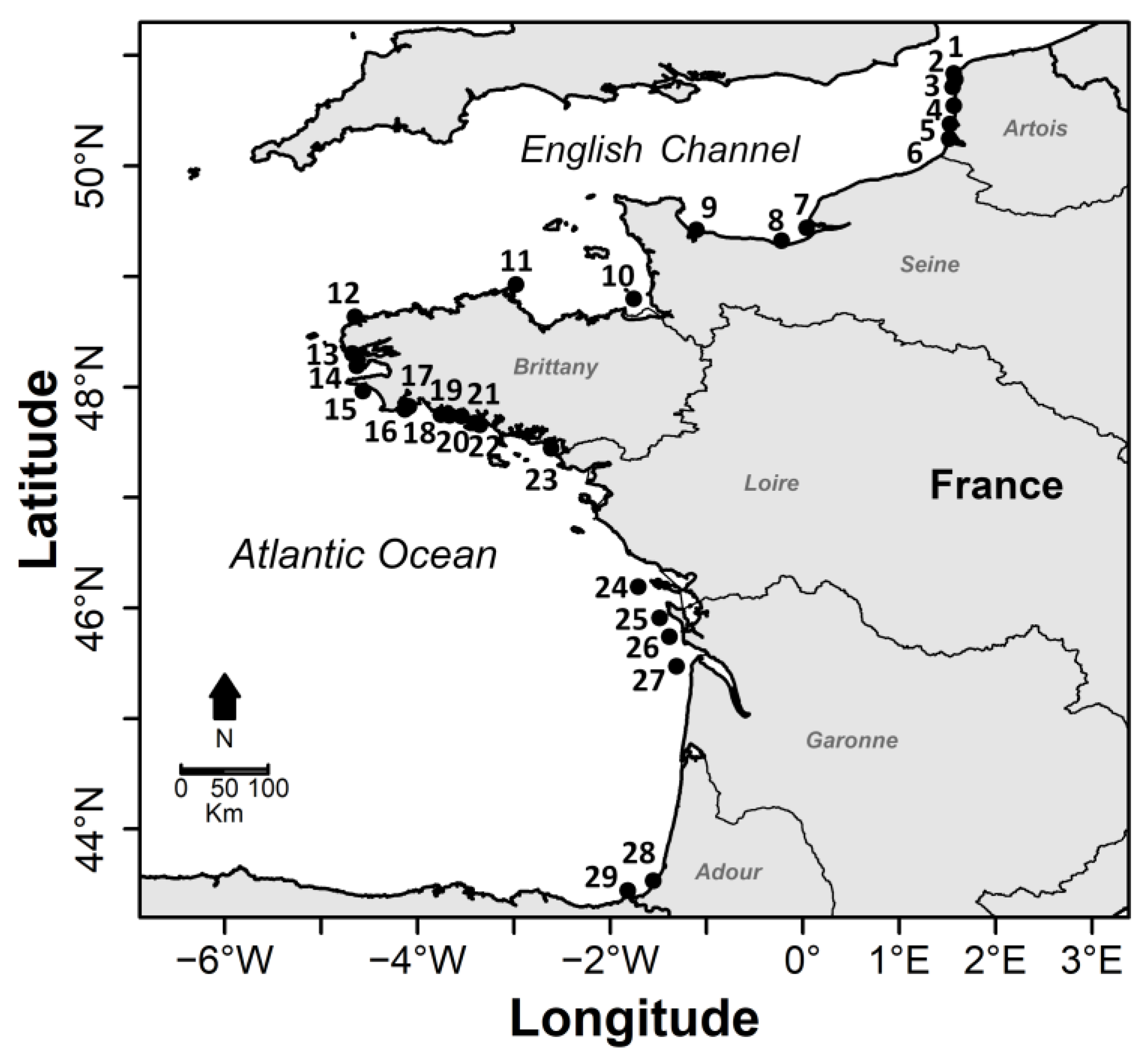
| Estuary Number | Estuary | Number of Sampling | Sampling Year | Sampling Season | Station Location |
|---|---|---|---|---|---|
| 1 | Slack | 72 | 2019–2020 | Spring–autumn | 1–2–3 |
| 2 | Wimereux | 72 | 2019–2020 | Spring–autumn | 1–2–3 |
| 3 | Liane | 56 | 2019–2020 | Spring–autumn | 1–2–3 |
| 4 | Canche | 72 | 2019–2020 | Spring–autumn | 1–2–3 |
| 5 | Authie | 72 | 2019–2020 | Spring–autumn | 1–2–3 |
| 6 | Somme | 96 | 2019–2020 | Spring–autumn | 1–2–3 |
| 7 | Seine | 80 | 2006 | Spring, autumn | 2–3 |
| 8 | Orne | 64 | 2006 | Spring, autumn | 3 |
| 9 | Veys bay | 128 | 2006 | Spring, autumn | 3 |
| 10 | Mont St Michel bay | 192 | 2006 | Spring, autumn | 1–2–3 |
| 11 | Trieux | 32 | 2007 | Spring, autumn | 2 |
| 12 | Aber Wrach | 32 | 2007 | Spring, autumn | 2 |
| 13 | Elorn | 32 | 2007 | Spring, autumn | 3 |
| 14 | Aulne | 32 | 2007 | Spring, autumn | 2 |
| 15 | Goyen | 32 | 2007 | Spring, autumn | 3 |
| 16 | Pont l’Abbe | 18 | 2007 | Summer-autumn | 1–2 |
| 17 | Odet | 24 | 2007 | Summer–autumn | 2 |
| 18 | Aven | 20 | 2007 | Summer–autumn | 2–3 |
| 19 | Belon | 20 | 2007 | Summer–autumn | 3 |
| 20 | Laita | 20 | 2007 | Summer–autumn | 2–3 |
| 21 | Scorff | 4 | 2007 | Spring | 2–3 |
| 22 | Blavet | 20 | 2007 | Spring, autumn | 3 |
| 23 | Vilaine | 32 | 2007 | Spring, autumn | 1–2–3 |
| 24 | Sevre Niortaise | 32 | 2007 | Spring, autumn | 2–3 |
| 25 | Charente | 32 | 2005 | Spring, autumn | 2–3 |
| 26 | Seudre | 38 | 2005 | Spring, autumn | 2–3 |
| 27 | Gironde | 32 | 2006–2008, 2010 | Summer–autumn | 3 |
| 28 | Adour | 34 | 2005 | Spring–summer | 1–2–3 |
| 29 | Bidassoa | 16 | 2005 | Spring, autumn | 3 |
2.2. Estuarine Environmental Factors and Anthropogenic Pressures
2.3. Eels Sampling
2.4. Eel Biological Characteristics
2.5. Statistical Analyses
3. Results
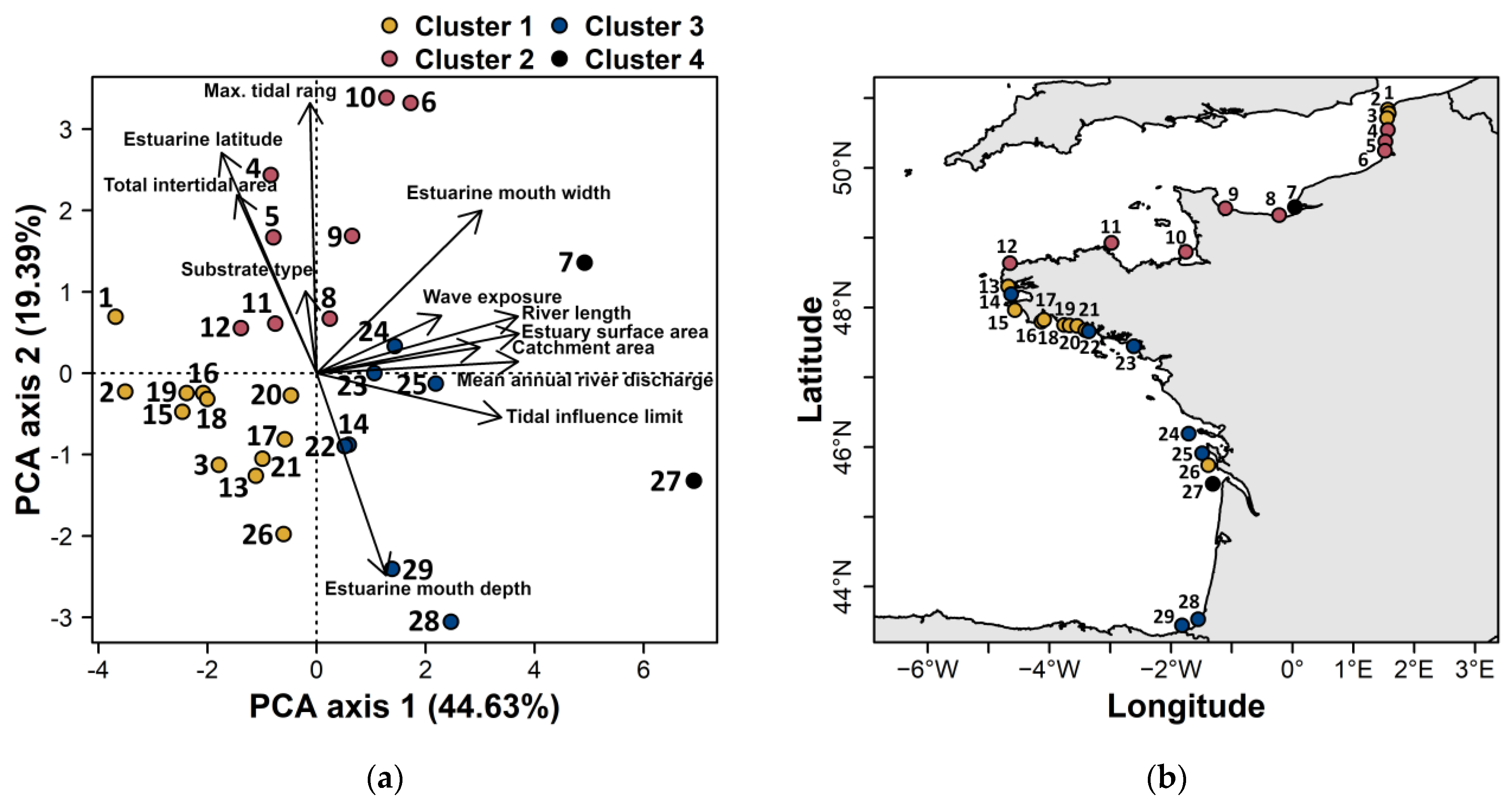
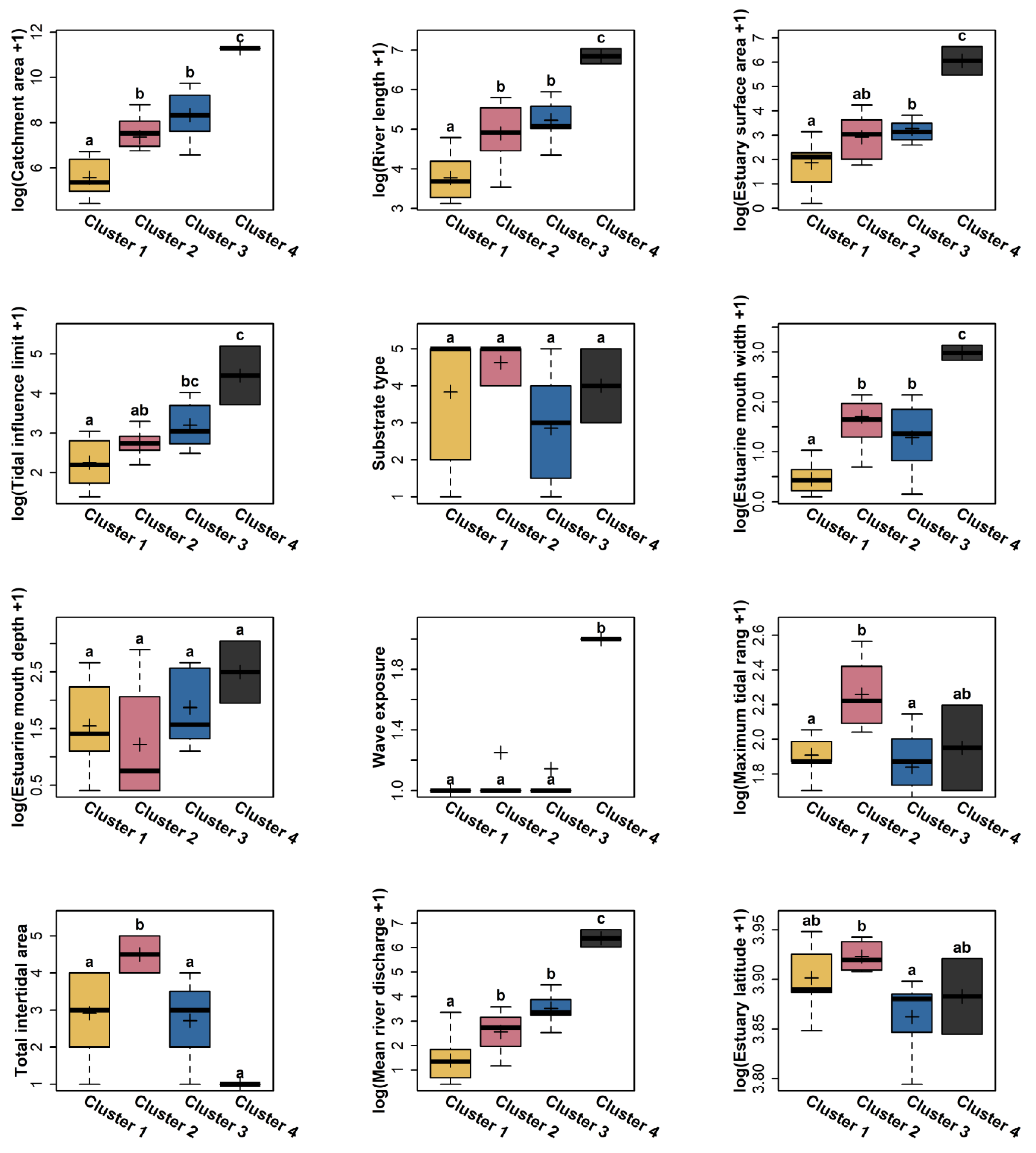
3.1. Estuarine Environmental Characteristics
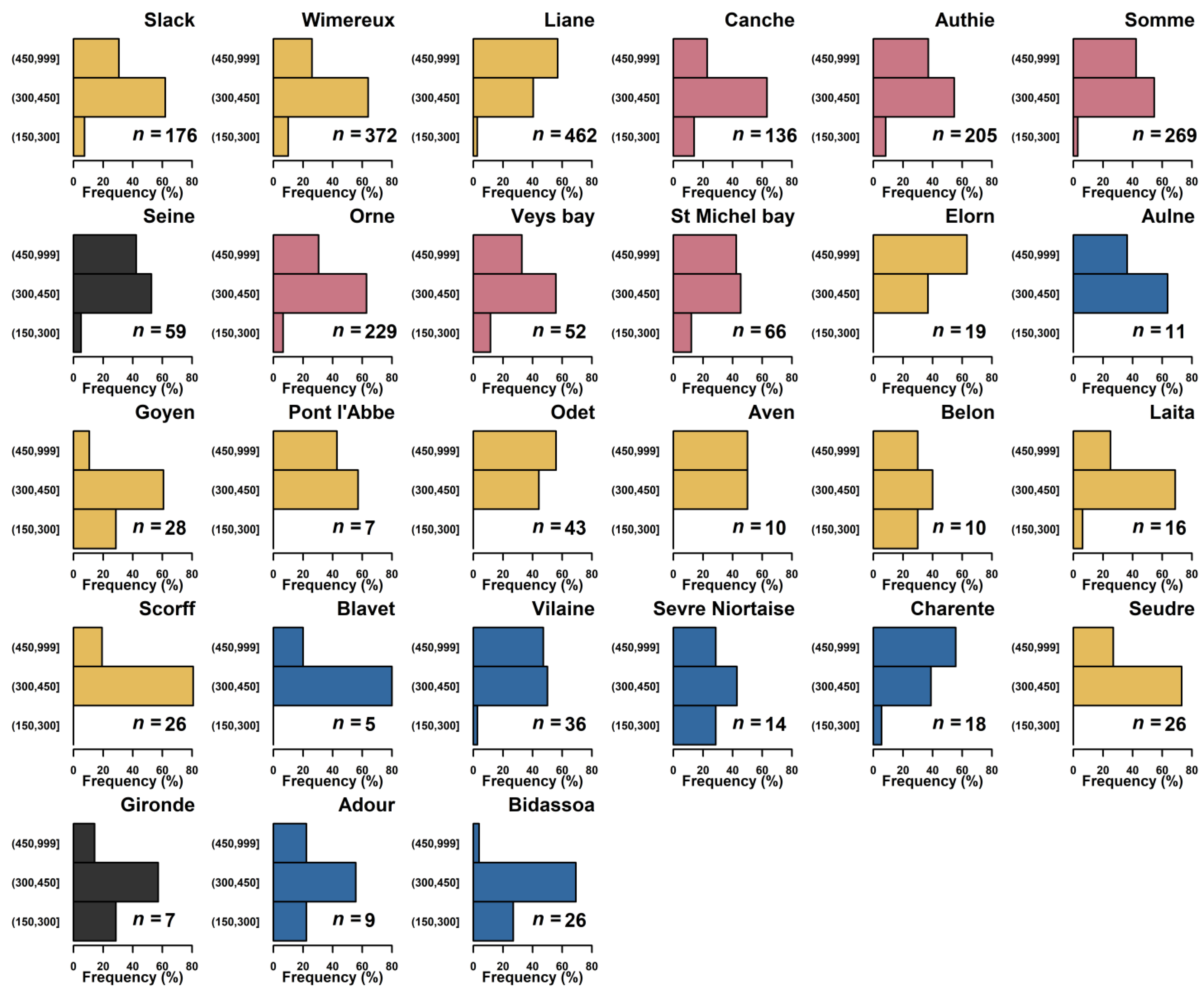
3.2. Eel Abundance and Total Length in Estuarine Habitats
3.3. Influence of Estuary Size on Eel Abundance
3.4. Relationship with Anthropogenic Pressures
4. Discussion
4.1. Spatial Variation in Eel Abundance in Estuarine Habitats
4.2. The Influence of Anthropogenic Pressures
4.3. Potential Sampling Biases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, J.; Regan, C.T. The Breeding Places of the Eel. Philos. Trans. R. Soc. Lond. B 1923, 211, 279–316. [Google Scholar]
- Tesch, F.W.; Thorpe, J.E. The Eel; Blackwell Science: Oxford, UK, 2003; Volume 15, ISBN 0-632-06389-0. [Google Scholar]
- Dekker, W. On the Distribution of the European Eel (Anguilla anguilla) and Its Fisheries. Can. J. Fish. Aquat. Sci. 2003, 60, 787–799. [Google Scholar] [CrossRef]
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL). ICES Sci. Rep. 2020, 2, 223. [Google Scholar] [CrossRef]
- Dekker, W.; Beaulaton, L. Faire Mieux Que La Nature? The History of Eel Restocking in Europe. Environ. Hist. 2016, 22, 255–300. [Google Scholar] [CrossRef]
- Morais, P.; Daverat, F. (Eds.) An Introduction to Fish Migration; CRC Press: Boca Raton, FL, USA, 2016; pp. 78–92. [Google Scholar]
- Jacoby, D.M.P.; Casselman, J.M.; Crook, V.; DeLucia, M.-B.; Ahn, H.; Kaifu, K.; Kurwie, T.; Sasal, P.; Silfvergrip, A.M.C.; Smith, K.G.; et al. Synergistic Patterns of Threat and the Challenges Facing Global Anguillid Eel Conservation. Glob. Ecol. Conserv. 2015, 4, 321–333. [Google Scholar] [CrossRef]
- Legrand, M.; Briand, C.; Buisson, L.; Artur, G.; Azam, D.; Baisez, A.; Barracou, D.; Bourré, N.; Carry, L.; Caudal, A.-L.; et al. Contrasting Trends between Species and Catchments in Diadromous Fish Counts over the Last 30 Years in France. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 7. [Google Scholar] [CrossRef]
- Waldman, J.; Wilson, K.A.; Mather, M.; Snyder, N.P. A Resilience Approach Can Improve Anadromous Fish Restoration. Fisheries 2016, 41, 116–126. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Brown, C.J.; Dwyer, R.G.; Harding, D.J.; Roberts, D.T.; Fuller, R.A.; Linke, S.; Possingham, H.P. Impacts of Fishing, River Flow and Connectivity Loss on the Conservation of a Migratory Fish Population. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 45–54. [Google Scholar] [CrossRef]
- Pike, C.; Crook, V.; Gollock, M. Anguilla anguilla. The IUCN Red List of Threatened Species 2020: E.T60344A152845178. Int. Union Conserv. Nat. 2020, 44. [Google Scholar] [CrossRef]
- Righton, D.; Piper, A.; Aarestrup, K.; Amilhat, E.; Belpaire, C.; Casselman, J.; Castonguay, M.; Díaz, E.; Dörner, H.; Faliex, E.; et al. Important Questions to Progress Science and Sustainable Management of Anguillid Eels. Fish Fish. 2021, 22, 762–788. [Google Scholar] [CrossRef]
- ICES. Report of the Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL). ICES Sci. Rep. 2023, 5, 175. [Google Scholar] [CrossRef]
- Daverat, F.; Limburg, K.; Thibault, I.; Shiao, J.; Dodson, J.; Caron, F.; Tzeng, W.; Iizuka, Y.; Wickström, H. Phenotypic Plasticity of Habitat Use by Three Temperate Eel Species, Anguilla anguilla, A. Japonica and A. Rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Daverat, F.; Tomás, J. Tactics and Demographic Attributes in the European Eel Anguilla anguilla in the Gironde Watershed, SW France. Mar. Ecol. Prog. Ser. 2006, 307, 247–257. [Google Scholar] [CrossRef]
- Moriarty, C. The Yellow Eel. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer Japan: Tokyo, Japan, 2003; pp. 89–105. ISBN 978-4-431-65907-5. [Google Scholar]
- Daverat, F.; Tomas, J.; Lahaye, M.; Palmer, M.; Elie, P. Tracking Continental Habitat Shifts of Eels Using Otolith Sr/Ca Ratios: Validation and Application to the Coastal, Estuarine and Riverine Eels of the Gironde—Garonne—Dordogne Watershed. Mar. Freshw. Res. 2005, 56, 619–627. [Google Scholar] [CrossRef]
- Shiao, J.C.; Ložys, L.; Iizuka, Y.; Tzeng, W.N. Migratory Patterns and Contribution of Stocking to the Population of European Eel in Lithuanian Waters as Indicated by Otolith Sr:Ca Ratios. J. Fish Biol. 2006, 69, 749–769. [Google Scholar] [CrossRef]
- Tabouret, H.; Bareille, G.; Claverie, F.; Pécheyran, C.; Prouzet, P.; Donard, O.F.X. Simultaneous Use of Strontium:Calcium and Barium:Calcium Ratios in Otoliths as Markers of Habitat: Application to the European Eel (Anguilla anguilla) in the Adour Basin, South West France. Mar. Environ. Res. 2010, 70, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, W.N.; Severin, K.P.; Wickström, H. Use of Otolith Microchemistry to Investigate the Environmental History of European Eel Anguilla anguilla. Mar. Ecol. Prog. Ser. 1997, 149, 73–81. [Google Scholar] [CrossRef]
- Denis, J.; Mahé, K.; Amara, R. Abundance and Growth of the European Eels (Anguilla anguilla Linnaeus, 1758) in Small Estuarine Habitats from the Eastern English Channel. Fishes 2022, 7, 213. [Google Scholar] [CrossRef]
- Denis, J.; Mahé, K.; Tabouret, H.; Rabhi, K.; Boutin, K.; Diop, M.; Amara, R. Relationship between Habitat Use and Individual Condition of European Eel (Anguilla anguilla) in Six Estuaries of the Eastern English Channel (North-Eastern Atlantic Ocean). Estuar. Coast. Shelf Sci. 2023, 291, 108446. [Google Scholar] [CrossRef]
- Correia, M.J.; Domingos, I.; De Leo, G.A.; Costa, J.L. A Comparative Analysis of European Eel’s Somatic Growth in the Coastal Lagoon Santo André (Portugal) with Growth in Other Estuaries and Freshwater Habitats. Environ. Biol. Fishes 2021, 104, 837–850. [Google Scholar] [CrossRef]
- Gross, M.R. Evolution of Diadromy in Fishes. Am. Fish. Soc. Symp. 1987, 1, 14–25. [Google Scholar]
- Tsukamoto, K.; Arai, T. Facultative Catadromy of the Eel Anguilla japonica between Freshwater and Seawater Habitats. Mar. Ecol. Prog. Ser. 2001, 220, 265–276. [Google Scholar] [CrossRef]
- Copp, G.H.; Daverat, F.; Bašić, T. The Potential Contribution of Small Coastal Streams to the Conservation of Declining and Threatened Diadromous Fishes, Especially the European Eel. River Res. Appl. 2021, 37, 111–115. [Google Scholar] [CrossRef]
- Yokouchi, K.; Aoyama, J.; Miller, M.J.; McCarthy, T.K.; Tsukamoto, K. Depth Distribution and Biological Characteristics of the European Eel Anguilla anguilla in Lough Ennell, Ireland. J. Fish Biol. 2009, 74, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Denis, J.; Rabhi, K.; Le Loc’h, F.; Lasram, F.B.R.; Boutin, K.; Kazour, M.; Diop, M.; Gruselle, M.-C.; Amara, R. Role of Estuarine Habitats for the Feeding Ecology of the European Eel (Anguilla anguilla L.). PLoS ONE 2022, 17, e0270348. [Google Scholar] [CrossRef]
- Feunteun, E.; Acou, A.; Guillouët, J.; Laffaille, P.; Legault, A. Spatial Distribution of an Eel Population (Anguilla anguilla L.) in a Small Coastal Catchment of Northern Brittany (France). Consequences of Hydraulic Works. Bull. Fr. Pêche Piscic. 1998, 349, 129–139. [Google Scholar] [CrossRef]
- Laffaille, P.; Feunteun, E.; Baisez, A.; Robinet, T.; Acou, A.; Legault, A.; Lek, S. Spatial Organisation of European Eel (Anguilla anguilla L.) in a Small Catchment. Ecol. Freshw. Fish 2003, 12, 254–264. [Google Scholar] [CrossRef]
- Domingos, I.; Costa, J.L.; Costa, M.J. Factors Determining Length Distribution and Abundance of the European Eel, Anguilla anguilla, in the River Mondego (Portugal). Freshw. Biol. 2006, 51, 2265–2281. [Google Scholar] [CrossRef]
- Degerman, E.; Tamario, C.; Watz, J.; Nilsson, P.A.; Calles, O. Occurrence and Habitat Use of European Eel (Anguilla anguilla) in Running Waters: Lessons for Improved Monitoring, Habitat Restoration and Stocking. Aquat. Ecol. 2019, 53, 639–650. [Google Scholar] [CrossRef]
- Henriques, S.; Guilhaumon, F.; Villéger, S.; Amoroso, S.; França, S.; Pasquaud, S.; Cabral, H.N.; Vasconcelos, R.P. Biogeographical Region and Environmental Conditions Drive Functional Traits of Estuarine Fish Assemblages Worldwide. Fish Fish. 2017, 18, 752–771. [Google Scholar] [CrossRef]
- Teichert, N.; Lepage, M.; Lobry, J. Beyond Classic Ecological Assessment: The Use of Functional Indices to Indicate Fish Assemblages Sensitivity to Human Disturbance in Estuaries. Sci. Total Environ. 2018, 639, 465–475. [Google Scholar] [CrossRef]
- Nicolas, D.; Lobry, J.; Lepage, M.; Sautour, B.; Le Pape, O.; Cabral, H.; Uriarte, A.; Boët, P. Fish under Influence: A Macroecological Analysis of Relations between Fish Species Richness and Environmental Gradients among European Tidal Estuaries. Estuar. Coast. Shelf Sci. 2010, 86, 137–147. [Google Scholar] [CrossRef]
- Teichert, N.; Lepage, M.; Sagouis, A.; Borja, A.; Chust, G.; Ferreira, M.T.; Pasquaud, S.; Schinegger, R.; Segurado, P.; Argillier, C. Functional Redundancy and Sensitivity of Fish Assemblages in European Rivers, Lakes and Estuarine Ecosystems. Sci. Rep. 2017, 7, 17611. [Google Scholar] [CrossRef]
- Teichert, N.; Pasquaud, S.; Borja, A.; Chust, G.; Uriarte, A.; Lepage, M. Living under Stressful Conditions: Fish Life History Strategies across Environmental Gradients in Estuaries. Estuar. Coast. Shelf Sci. 2017, 188, 18–26. [Google Scholar] [CrossRef]
- Selleslagh, J.; Amara, R.; Laffargue, P.; Lesourd, S.; Lepage, M.; Girardin, M. Fish Composition and Assemblage Structure in Three Eastern English Channel Macrotidal Estuaries: A Comparison with Other French Estuaries. Estuar. Coast. Shelf Sci. 2009, 81, 149–159. [Google Scholar] [CrossRef]
- Courrat, A.; Lobry, J.; Nicolas, D.; Laffargue, P.; Amara, R.; Lepage, M.; Girardin, M.; Le Pape, O. Anthropogenic Disturbance on Nursery Function of Estuarine Areas for Marine Species. Estuar. Coast. Shelf Sci. 2009, 81, 179–190. [Google Scholar] [CrossRef]
- Rochette, S.; Rivot, E.; Morin, J.; Mackinson, S.; Riou, P.; Le Pape, O. Effect of Nursery Habitat Degradation on Flatfish Population: Application to solea solea in the Eastern Channel (Western Europe). J. Sea Res. 2010, 64, 34–44. [Google Scholar] [CrossRef]
- Durif, C.M.F.; Arts, M.; Bertolini, F.; Cresci, A.; Daverat, F.; Karlsbakk, E.; Koprivnikar, J.; Moland, E.; Olsen, E.M.; Parzanini, C.; et al. The Evolving Story of Catadromy in the European Eel (Anguilla anguilla). ICES J. Mar. Sci. 2023, 80, 2253–2265. [Google Scholar] [CrossRef]
- Hyndes, G.A.; Potter, I.C.; Lenanton, R.C.J. Habitat Partitioning by Whiting Species (Sillaginidae) in Coastal Waters. Environ. Biol. Fishes 1996, 45, 21–40. [Google Scholar] [CrossRef]
- Lowry, M.; Suthers, I. Population Structure of Aggregations, and Response to Spear Fishing, of a Large Temperate Reef Fish Cheilodactylus Fuscus. Mar. Ecol. Prog. Ser. 2004, 273, 199–210. [Google Scholar] [CrossRef]
- Van der Veer, H.W.; Dapper, R.; Witte, J.I. The Nursery Function of the Intertidal Areas in the Western Wadden Sea for 0-Group Sole solea solea (L.). J. Sea Res. 2001, 45, 271–279. [Google Scholar] [CrossRef]
- Gibson, R.N.; Robb, L.; Wennhage, H.; Burrows, M. Ontogenetic Changes in Depth Distribution of Juvenile Flatfishes in Relation to Predation Risk and Temperature on a Shallow Water Nursery Ground. Mar. Ecol. Prog. Ser. 2002, 229, 233–244. [Google Scholar] [CrossRef]
- McLusky, D.S.; Elliott, M. The Estuarine Ecosystem: Ecology, Threats and Management; OUP: Oxford, UK, 2004; ISBN 978-0-19-154623-5. [Google Scholar]
- Aubry, A.; Elliott, M. The Use of Environmental Integrative Indicators to Assess Seabed Disturbance in Estuaries and Coasts: Application to the Humber Estuary, UK. Mar. Pollut. Bull. 2006, 53, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Lepage, M.; Harrison, T.; Breine, J.; Cabral, H.; Coates, S.; Galván, C.; García, P.; Jager, Z.; Kelly, F.; Mosch, E.C.; et al. An Approach to Intercalibrate Ecological Classification Tools Using Fish in Transitional Water of the North East Atlantic. Ecol. Indic. 2016, 67, 318–327. [Google Scholar] [CrossRef]
- Durif, C.; Dufour, S.; Elie, P. The Silvering Process of Anguilla anguilla: A New Classification from the Yellow Resident to the Silver Migrating Stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Frontier, S. Étude de La Décroissance Des Valeurs Propres Dans Une Analyse En Composantes Principales: Comparaison Avec Le Modéle Du Bâton Brisé. J. Exp. Mar. Biol. Ecol. 1976, 25, 67–75. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R.; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7975-9. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.5-7; ResearchGate: Berlin, Germany, 2020. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria; Sage Publications: Thousand Oaks, CA, USA, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Dinno, A. Dunn.Test: Dunn’s Test of Multiple Comparisons Using Rank Sums; R Package Version 1.3.5. 2017. Available online: https://cran.r-project.org/web/packages/dunn.test/index.html (accessed on 1 January 2024).
- Ebbert, D. Chisq.Posthoc.Test: A Post Hoc Analysis for Pearson’s Chi-Squared Test for Count Data; R Package Version 0.1.2; ResearchGate: Berlin, Germany, 2019; Available online: https://cran.r-project.org/web/packages/chisq.posthoc.test/index.html (accessed on 1 January 2024).
- Acou, A.; Robinet, T.; Lance, E.; Gerard, C.; Mounaix, B.; Brient, L.; Le Rouzic, B.; Feunteun, E. Evidence of Silver Eels Contamination by Microcystin-LR at the Onset of Their Seaward Migration: What Consequences for Breeding Potential? J. Fish Biol. 2008, 72, 753–762. [Google Scholar] [CrossRef]
- Belpaire, C.; Hodson, P.; Pierron, F.; Freese, M. Impact of Chemical Pollution on Atlantic Eels: Facts, Research Needs, and Implications for Management. Curr. Opin. Environ. Sci. Health 2019, 11, 26–36. [Google Scholar] [CrossRef]
- Robinet, T.; Feunteun, E. Sublethal Effects of Exposure to Chemical Compounds: A Cause for the Decline in Atlantic Eels? Ecotoxicology 2002, 11, 265–277. [Google Scholar] [CrossRef]
- Dauvin, J.-C.; Bachelet, G.; Barillé, A.-L.; Blanchet, H.; De Montaudouin, X.; Lavesque, N.; Ruellet, T. Benthic Indicators and Index Approaches in the Three Main Estuaries along the French Atlantic Coast (Seine, Loire and Gironde). Mar. Ecol. 2009, 30, 228–240. [Google Scholar] [CrossRef]
- Piscart, C.; Moreteau, J.-C.; Beisel, J.-N. Biodiversity and Structure of Macroinvertebrate Communities Along a Small Permanent Salinity Gradient (Meurthe River, France). Hydrobiologia 2005, 551, 227–236. [Google Scholar] [CrossRef]
- Blanchet, H.; Gouillieux, B.; Alizier, S.; Amouroux, J.-M.; Bachelet, G.; Barillé, A.-L.; Dauvin, J.-C.; De Montaudouin, X.; Derolez, V.; Desroy, N.; et al. Multiscale Patterns in the Diversity and Organization of Benthic Intertidal Fauna among French Atlantic Estuaries. J. Sea Res. 2014, 90, 95–110. [Google Scholar] [CrossRef]
- Foulquier, C.; Baills, J.; Arraud, A.; D’Amico, F.; Blanchet, H.; Rihouey, D.; Bru, N. Hydrodynamic Conditions Effects on Soft-Bottom Subtidal Nearshore Benthic Community Structure and Distribution. J. Mar. Sci. 2020, 2020, e4674580. [Google Scholar] [CrossRef]
- Bouchereau, J.-L.; Marques, C.; Pereira, P.; Guélorget, O.; Vergne, Y. Food of the European Eel Anguilla anguilla in the Mauguio Lagoon (Mediterranean, France). Acta Adriat. 2009, 50, 159–170. [Google Scholar]
- Vasconi, M.; Lopez, A.; Galimberti, C.; Rojas, J.M.M.; Redondo, J.M.M.; Bellagamba, F.; Moretti, V.M. Authentication of Farmed and Wild European Eel (Anguilla anguilla) by Fatty Acid Profile and Carbon and Nitrogen Isotopic Analyses. Food Control. 2019, 102, 112–121. [Google Scholar] [CrossRef]
- Arai, T. Biology and Ecology of Anguillid Eels. CRC Press: Boca Raton, FL, USA, 2016; pp. 181–187. [Google Scholar]
- Dörner, H.; Skov, C.; Berg, S.; Schulze, T.; Beare, D.J.; Van der Velde, G. Piscivory and Trophic Position of Anguilla anguilla in Two Lakes: Importance of Macrozoobenthos Density. J. Fish Biol. 2009, 74, 2115–2131. [Google Scholar] [CrossRef]
- Bilkovic, D.M. Response of Tidal Creek Fish Communities to Dredging and Coastal Development Pressures in a Shallow-Water Estuary. Estuaries Coasts 2011, 34, 129–147. [Google Scholar] [CrossRef]
- Teichert, N.; Borja, A.; Chust, G.; Uriarte, A.; Lepage, M. Restoring Fish Ecological Quality in Estuaries: Implication of Interactive and Cumulative Effects among Anthropogenic Stressors. Sci. Total Environ. 2016, 542, 383–393. [Google Scholar] [CrossRef]
- Bruslé, J. L’anguille européenne Anguilla anguilla, un poisson sensible aux stress environnementaux et vulnérable à diverses atteintes pathogènes. Bull. Fr. Pêche Piscic. 1994, 335, 237–260. [Google Scholar] [CrossRef]
- Feunteun, E. Management and Restoration of European Eel Population (Anguilla anguilla): An Impossible Bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Acou, A.; Rivot, E.; Van Gils, J.A.; Legault, A.; Ysnel, F.; Feunteun, E. Habitat Carrying Capacity Is Reached for the European Eel in a Small Coastal Catchment: Evidence and Implications for Managing Eel Stocks: Carrying Capacity Limitation for an Eel Population. Freshw. Biol. 2011, 56, 952–968. [Google Scholar] [CrossRef]
- Teichert, N.; Carassou, L.; Sahraoui, Y.; Lobry, J.; Lepage, M. Influence of Intertidal Seascape on the Functional Structure of Fish Assemblages: Implications for Habitat Conservation in Estuarine Ecosystems. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 798–809. [Google Scholar] [CrossRef]
- Baudoin, J.M.; Burgun, V.; Chanseau, M.; Larinier, M.; Ovidio, M.; Sremski, W. Evaluer Le Franchissement Des Obstacles Par Les Poissons; Principes et Méthodes: Informations Sur La Continuité Écologique, ICE; ONEMA: Vincennes, France, 2014; pp. 12–32. [Google Scholar]
- Verreault, G.; Dumont, P.; Mailhot, Y. Habitat Losses and Anthropogenic Barriers as a Cause of Population Decline for American Eel (anguilla rostrata) in the St. Lawrence Watershed, Canada; CM Document; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2004. [Google Scholar]
- Tamario, C.; Calles, O.; Watz, J.; Nilsson, P.A.; Degerman, E. Coastal River Connectivity and the Distribution of Ascending Juvenile European Eel (Anguilla anguilla L.): Implications for Conservation Strategies Regarding Fish-Passage Solutions. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 612–622. [Google Scholar] [CrossRef]
- Drouineau, H.; Durif, C.; Castonguay, M.; Mateo, M.; Rochard, E.; Verreault, G.; Yokouchi, K.; Lambert, P. Freshwater Eels: A Symbol of the Effects of Global Change. Fish Fish. 2018, 19, 903–930. [Google Scholar] [CrossRef]
- ICES. Report of the Workshop of a Planning Group on the Monitoring of Eel Quality under the Subject “Development of Standardized and Harmonized Protocols for the Estimation of Eel Quality” (WKPGMEQ); ICES: Brussels, Belgium, 2015; pp. 6–56. [Google Scholar]
- Bourillon, B.; Feunteun, E.; Acou, A.; Trancart, T.; Teichert, N.; Belpaire, C.; Dufour, S.; Bustamante, P.; Aarestrup, K.; Walker, A.; et al. Anthropogenic Contaminants Shape the Fitness of the Endangered European Eel: A Machine Learning Approach. Fishes 2022, 7, 274. [Google Scholar] [CrossRef]
- Pannetier, P.; Caron, A.; Campbell, P.G.C.; Pierron, F.; Baudrimont, M.; Couture, P. A Comparison of Metal Concentrations in the Tissues of Yellow American Eel (anguilla rostrata) and European Eel (Anguilla anguilla). Sci. Total Environ. 2016, 569–570, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Pierron, F.; Baudrimont, M.; Dufour, S.; Elie, P.; Bossy, A.; Baloche, S.; Mesmer-Dudons, N.; Gonzalez, P.; Bourdineaud, J.-P.; Massabuau, J.-C. How Cadmium Could Compromise the Completion of the European Eel’s Reproductive Migration. Environ. Sci. Technol. 2008, 42, 4607–4612. [Google Scholar] [CrossRef]
- Pierron, F.; Baudrimont, M.; Lucia, M.; Durrieu, G.; Massabuau, J.-C.; Elie, P. Cadmium Uptake by the European Eel: Trophic Transfer in Field and Experimental Investigations. Ecotoxicol. Environ. Saf. 2008, 70, 10–19. [Google Scholar] [CrossRef]
- Bilau, M.; Sioen, I.; Matthys, C.; De Vocht, A.; Goemans, G.; Belpaire, C.; Willems, J.L.; De Henauw, S. Probabilistic Approach to Polychlorinated Biphenyl (PCB) Exposure through Eel Consumption in Recreational Fishermen vs. the General Population. Food Addit. Contam. 2007, 24, 1386–1393. [Google Scholar] [CrossRef]
- Blanchet-Letrouvé, I.; Zalouk-Vergnoux, A.; Vénisseau, A.; Couderc, M.; Le Bizec, B.; Elie, P.; Herrenknecht, C.; Mouneyrac, C.; Poirier, L. Dioxin-like, Non-Dioxin like PCB and PCDD/F Contamination in European Eel (Anguilla anguilla) from the Loire Estuarine Continuum: Spatial and Biological Variabilities. Sci. Total Environ. 2014, 472, 562–571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byer, J.D.; Lebeuf, M.; Alaee, M.; Stephen, B.R.; Trottier, S.; Backus, S.; Keir, M.; Couillard, C.M.; Casselman, J.; Hodson, P.V. Spatial Trends of Organochlorinated Pesticides, Polychlorinated Biphenyls, and Polybrominated Diphenyl Ethers in Atlantic Anguillid Eels. Chemosphere 2013, 90, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Privitera, L.; Aarestrup, K.; Moore, A. Impact of a Short-Term Exposure to Tributyl Phosphate on Morphology, Physiology and Migratory Behaviour of European Eels during the Transition from Freshwater to the Marine Environment. Ecol. Freshw. Fish 2014, 23, 171–180. [Google Scholar] [CrossRef]
- Teles, M.; Pacheco, M.; Santos, M.A. Physiological and Genetic Responses of European Eel (Anguilla anguilla L.) to Short-Term Chromium or Copper Exposure—Influence of Preexposure to a PAH-like Compound. Environ. Toxicol. 2005, 20, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Capela, R.C.; Sérgio, T.; Caldeira, C.; Gonçalves, F.; Correia, A.T. Effects of Chronic Exposure to Lead, Copper, Zinc, and Cadmium on Biomarkers of the European Eel, Anguilla anguilla. Environ. Sci. Pollut. Res. 2014, 21, 5689–5700. [Google Scholar] [CrossRef] [PubMed]
- Quadroni, S.; Galassi, S.; Capoccioni, F.; Ciccotti, E.; Grandi, G.; De Leo, G.A.; Bettinetti, R. Contamination, Parasitism and Condition of Anguilla anguilla in Three Italian Stocks. Ecotoxicology 2013, 22, 94–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gollock, M.J.; Kennedy, C.R.; Brown, J.A. European Eels, Anguilla anguilla (L.), Infected with Anguillicola Crassus Exhibit a More Pronounced Stress Response to Severe Hypoxia than Uninfected Eels. J. Fish Dis. 2005, 28, 429–436. [Google Scholar] [CrossRef]
- Lefebvre, F.; Contournet, P.; Crivelli, A. Interaction between the Severity of the Infection by the Nematode Anguillicola Crassus and the Tolerance to Hypoxia in the European Eel Anguilla anguilla. Acta Parasitol. 2007, 52, 171–175. [Google Scholar] [CrossRef]
- Diop, M.; Couteau, J.; Bado-Nilles, A.; Tavernier, E.; Ouddane, B.; Denis, J.; Duong, G.; Gevaert, F.; Monchy, S.; Laroche, J.; et al. Bioaccumulation of Trace Metal Elements and Biomarker Responses in Caged Juvenile Flounder at a Polluted Site: Effects of Fish Density and Time Exposure. Mar. Pollut. Bull. 2022, 185, 114289. [Google Scholar] [CrossRef]
- Acolas, M.-L.; Davail, B.; Gonzalez, P.; Jean, S.; Clérandeau, C.; Morin, B.; Gourves, P.-Y.; Daffe, G.; Labadie, P.; Perrault, A.; et al. Health Indicators and Contaminant Levels of a Critically Endangered Species in the Gironde Estuary, the European Sturgeon. Environ. Sci. Pollut. Res. 2020, 27, 3726–3745. [Google Scholar] [CrossRef]
- Knights, B. A Review of the Possible Impacts of Long-Term Oceanic and Climate Changes and Fishing Mortality on Recruitment of Anguillid Eels of the Northern Hemisphere. Sci. Total Environ. 2003, 310, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Dekker, W. Status of the European Eel Stock and Fisheries. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer Japan: Tokyo, Japan, 2003; pp. 237–254. ISBN 978-4-431-65909-9. [Google Scholar]
- Prigge, E.; Marohn, L.; Hanel, R. Tracking the Migratory Success of Stocked European Eels Anguilla anguilla in the Baltic Sea: Tracking Stocked Anguilla anguilla in the Baltic Sea. J. Fish Biol. 2013, 82, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Josset, Q.; Trancart, T.; Mazel, V.; Charrier, F.; Frotté, L.; Acou, A.; Feunteun, E. Pre-Release Processes Influencing Short-Term Mortality of Glass Eels in the French Eel (Anguilla anguilla, Linnaeus 1758) Stocking Programme. ICES J. Mar. Sci. 2016, 73, 150–157. [Google Scholar] [CrossRef]
- Feunteun, E.; Serranito, B.; Le Peru, Y. Etude ADRAF: Analyse Des Données de 10 Années de Repeuplement Anguille En France—2011/2021; Ara France: Paris, France, 2022; pp. 15–48. [Google Scholar]
- Arai, T.; Kotake, A.; Harrod, C.; Morrissey, M.; McCarthy, T.K. Ecological Plasticity of the European Eel Anguilla anguilla in a Tidal Atlantic Lake System in Ireland. J. Mar. Biol. Assoc. UK 2019, 99, 1189–1195. [Google Scholar] [CrossRef]
- Capoccioni, F.; Lin, D.-Y.; Iizuka, Y.; Tzeng, W.-N.; Ciccotti, E. Phenotypic Plasticity in Habitat Use and Growth of the European Eel (Anguilla anguilla) in Transitional Waters in the Mediterranean Area. Ecol. Freshw. Fish 2014, 23, 65–76. [Google Scholar] [CrossRef]
- Harrod, C.; Grey, J.; McCarthy, T.K.; Morrissey, M. Stable Isotope Analyses Provide New Insights into Ecological Plasticity in a Mixohaline Population of European Eel. Oecologia 2005, 144, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Teichert, N.; Lizé, A.; Tabouret, H.; Gérard, C.; Bareille, G.; Acou, A.; Carpentier, A.; Trancart, T.; Virag, L.-S.; Robin, E.; et al. A Multi-Approach Study to Reveal Eel Life-History Traits in an Obstructed Catchment before Dam Removal. Hydrobiologia 2022, 849, 1885–1903. [Google Scholar] [CrossRef]
- Fablet, R.; Daverat, F.; Pontual, H.D. Unsupervised Bayesian Reconstruction of Individual Life Histories from Otolith Signatures: Case Study of Sr:Ca Transects of European Eel (Anguilla anguilla) Otoliths. Can. J. Fish. Aquat. Sci. 2007, 64, 152–165. [Google Scholar] [CrossRef]
- Caraguel, J.-M.; Charrier, F.; Mazel, V.; Feunteun, E. Mass Marking of Stocked European Glass Eels (Anguilla anguilla) with Alizarin Red S. Ecol. Freshw. Fish 2015, 24, 435–442. [Google Scholar] [CrossRef]
- Desprez, M.; Crivelli, A.J.; Lebel, I.; Massez, G.; Gimenez, O. Demographic Assessment of a Stocking Experiment in European Eels. Ecol. Freshw. Fish 2013, 22, 412–420. [Google Scholar] [CrossRef]
- Westin, L. Migration Failure in Stocked Eels Anguilla anguilla. Mar. Ecol. Prog. Ser. 2003, 254, 307–311. [Google Scholar] [CrossRef]
- Rohtla, M.; Silm, M.; Tulonen, J.; Paiste, P.; Wickström, H.; Kielman-Schmitt, M.; Kooijman, E.; Vaino, V.; Eschbaum, R.; Saks, L.; et al. Conservation Restocking of the Imperilled European Eel Does Not Necessarily Equal Conservation. ICES J. Mar. Sci. 2021, 78, 101–111. [Google Scholar] [CrossRef]
- ICES. Report of the Workshop on Eel Stocking (WKSTOCKEEL); ICES: Copenhagen, Denmark, 2016. [Google Scholar] [CrossRef]
- Verhelst, P.; Reubens, J.; Pauwels, I.; Buysse, D.; Aelterman, B.; Van Hoey, S.; Goethals, P.; Moens, T.; Coeck, J.; Mouton, A. Movement Behaviour of Large Female Yellow European Eel (Anguilla anguilla L.) in a Freshwater Polder Area. Ecol. Freshw. Fish 2018, 27, 471–480. [Google Scholar] [CrossRef]
- De Meyer, J.; Verhelst, P.; Adriaens, D. Saving the European Eel: How Morphological Research Can Help in Effective Conservation Management. Integr. Comp. Biol. 2020, 60, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.J.; Beach, M.H. Fish Pass Design for Eel and Elver (Anguilla anguilla); R&D Technical Report W2-070/TR; Environment Agency: London, UK, 2004; pp. 4–23. [Google Scholar]
- Selleslagh, J. Fonctionnement des Nourriceries Intertidales et Estuariennes: Influence de L’environnement Sur La Dynamique et les Performances Physiologiques de L’ichtyofaune. Ph.D. Thesis, Université du Littoral Côte d’Opale, Wimereux, France, 2008. [Google Scholar]
- Weldon, L.; O’Leary, C.; Steer, M.; Newton, L.; Macdonald, H.; Sargeant, S.L. A Comparison of European Eel Anguilla anguilla eDNA Concentrations to Fyke Net Catches in Five Irish Lakes. Environ. DNA 2020, 2, 587–600. [Google Scholar] [CrossRef]
- Collins, R.A.; Wangensteen, O.S.; O’Gorman, E.J.; Mariani, S.; Sims, D.W.; Genner, M.J. Persistence of Environmental DNA in Marine Systems. Commun. Biol. 2018, 1, 185. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Chen, J.; Ruan, H.; Li, Z.; Guo, W.; Li, M.; Liu, L. eDNA Metabarcoding as a Promising Conservation Tool for Monitoring Fish Diversity in a Coastal Wetland of the Pearl River Estuary Compared to Bottom Trawling. Sci. Total Environ. 2020, 702, 134704. [Google Scholar] [CrossRef]
- Nagarajan, R.P.; Bedwell, M.; Holmes, A.E.; Sanches, T.; Acuña, S.; Baerwald, M.; Barnes, M.A.; Blankenship, S.; Connon, R.E.; Deiner, K.; et al. Environmental DNA Methods for Ecological Monitoring and Biodiversity Assessment in Estuaries. Estuaries Coasts 2022, 45, 2254–2273. [Google Scholar] [CrossRef]
- Cloern, J.E.; Barnard, P.L.; Beller, E.; Callaway, J.C.; Grenier, L.; Grosholz, D.; Grossinger, R.; Hieb, K.; Hollibaugh, J.T.; Knowles, N.; et al. Estuaries: Life on the Edge. In Ecosystems of California; University of California Press: Oakland, CA, USA, 2016; pp. 133–137. [Google Scholar]



| Variables | Measurement Units or Ordinal Scores | Data Source |
|---|---|---|
| Catchment area | Kilometres squared | Sandre |
| River length | Kilometres | Sandre |
| Estuary surface area | Kilometres squared | ArcGis software |
| Tidal influence limit | Kilometres | OFB |
| Substrate type | 1: muddy, 2: muddy/sandy, 3: sandy, 4: sandy/gravel, 5: rocky | Marine sediment maps, SHOM |
| Estuarine mouth width | Kilometres | Google Earth |
| Estuarine mouth depth | Metres | Marine Charts |
| Wave exposure | 1: very exposed, 2: moderately exposed, 3: protected from waves | Literature [35,38], SHOM |
| Maximum tidal rang | Meters | SHOM |
| Total intertidal area | 1: 0–20%, 2: 20–40%, 3: 40–60%, 4: 60–80%, 5: 80–100% | Literature [35,38] |
| Mean annual river discharge | Meters cube per second | Hydro.eaufrance.fr |
| Estuary latitude | Degree | Google Earth |
| N° Estuary | Estuary | Estuary Clusters | CPUE | Standardized CPUE |
|---|---|---|---|---|
| 1 | Slack | 1 | 2.29 ± 1.52 | 1.77 ± 1.17 |
| 2 | Wimereux | 1 | 7.22 ± 11.96 | 32.83 ± 54.35 |
| 3 | Liane | 1 | 8.68 ± 7.86 | 0.39 ± 0.36 |
| 4 | Canche | 2 | 1.85 ± 1.58 | 0.35 ± 0.30 |
| 5 | Authie | 2 | 2.64 ± 2.13 | 0.22 ± 0.18 |
| 6 | Somme | 2 | 2.74 ± 3.64 | 0.07 ± 0.09 |
| 7 | Seine | 4 | 1.38 ± 0.71 | 0.01 ± 0.01 |
| 8 | Orne | 2 | 7.38 ± 4.77 | 1.51 ± 0.97 |
| 9 | Veys bay | 2 | 0.81 ± 0.31 | 0.02 ± 0.01 |
| 10 | Mont St Michel bay | 2 | 0.69 ± 0.01 | 0.01 ± 0.01 |
| 11 | Trieux | 2 | 0.25 ± 0.01 | 0.03 ± 0.01 |
| 12 | Aber Wrach | 2 | 0.13 ± 0.01 | <0.01 ± 0.01 |
| 13 | Elorn | 1 | 1.19 ± 0.27 | 0.19 ± 0.04 |
| 14 | Aulne | 3 | 0.69 ± 0.44 | 0.04 ± 0.02 |
| 15 | Goyen | 1 | 1.75 ± 1.59 | 0.63 ± 0.57 |
| 16 | Pont l’Abbe | 1 | 3.50 ± 0.01 | 0.05 ± 0.01 |
| 17 | Odet | 1 | 10.75 ± 0.01 | 1.16 ± 0.01 |
| 18 | Aven | 1 | 2.31 ± 3.09 | 0.28 ± 0.38 |
| 19 | Belon | 1 | 5.00 ± 0.01 | 0.61 ± 0.01 |
| 20 | Laita | 1 | 8.00 ± 0.01 | 29.63 ± 0.01 |
| 21 | Scorff | 1 | 13.00 ± 0.01 | 4.39 ± 0.01 |
| 22 | Blavet | 3 | 0.88 ± 0.88 | 0.07 ± 0.07 |
| 23 | Vilaine | 3 | 2.25 ± 1.24 | 0.10 ± 0.06 |
| 24 | Sevre Niortaise | 3 | 0.88 ± 0.88 | 0.02 ± 0.02 |
| 25 | Charente | 3 | 1.13 ± 0.71 | 0.05 ± 0.03 |
| 26 | Seudre | 1 | 1.42 ± 0.47 | 0.17 ± 0.06 |
| 27 | Gironde | 4 | 0.50 ± 0.25 | <0.01 ± 0.01 |
| 28 | Adour | 3 | 0.71 ± 0.12 | 0.06 ± 0.01 |
| 29 | Bidassoa | 3 | 3.25 ± 3.54 | 0.03 ± 0.04 |
| Estuary | Estuary Clusters | CPI | LIA | IH | AC | WC | WB | BS | DOT | DOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Canche | 2 | 16 | 9 | 3 | 1 | 0 | 1 | 1 | 1 | 0 |
| Authie | 2 | 13 | 9 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| Somme | 2 | 25 | 5 | 5 | 7 | 3 | 3 | 1 | 1 | 0 |
| Seine | 4 | 56 | 9 | 9 | 5 | 9 | 7 | 5 | 5 | 7 |
| Orne | 2 | 29 | 9 | 9 | 7 | 1 | 1 | 1 | 1 | 0 |
| Trieux | 2 | 21 | 5 | 5 | 7 | 1 | 1 | 1 | 1 | 0 |
| Goyen | 1 | 27 | 7 | 7 | 7 | 5 | 0 | 0 | 1 | 0 |
| Odet | 1 | 22 | 3 | 5 | 7 | 3 | 1 | 1 | 1 | 1 |
| Aven | 1 | 10 | 5 | 1 | 3 | 0 | 0 | 0 | 1 | 0 |
| Belon | 1 | 10 | 3 | 1 | 5 | 0 | 0 | 0 | 1 | 0 |
| Laita | 1 | 7 | 3 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Scorff | 1 | 18 | 5 | 7 | 5 | 0 | 0 | 0 | 1 | 0 |
| Blavet | 3 | 13 | 3 | 5 | 3 | 0 | 0 | 1 | 1 | 0 |
| Sevre Niortaise | 3 | 33 | 9 | 7 | 7 | 5 | 3 | 1 | 1 | 0 |
| Charente | 3 | 26 | 7 | 3 | 7 | 1 | 1 | 3 | 3 | 1 |
| Seudre | 1 | 29 | 9 | 3 | 7 | 3 | 1 | 5 | 1 | 0 |
| Gironde | 4 | 50 | 5 | 7 | 9 | 7 | 9 | 3 | 3 | 7 |
| Adour | 3 | 44 | 9 | 5 | 9 | 5 | 5 | 5 | 3 | 3 |
| Bidassoa | 3 | 35 | 9 | 9 | 7 | 3 | 3 | 3 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denis, J.; Lepage, M.; Gruselle, M.-C.; Amara, R. The Influence of Natural and Anthropogenic Environmental Pressures on European Eel Abundances in French Estuaries. Fishes 2024, 9, 44. https://doi.org/10.3390/fishes9020044
Denis J, Lepage M, Gruselle M-C, Amara R. The Influence of Natural and Anthropogenic Environmental Pressures on European Eel Abundances in French Estuaries. Fishes. 2024; 9(2):44. https://doi.org/10.3390/fishes9020044
Chicago/Turabian StyleDenis, Jérémy, Mario Lepage, Marie-Christine Gruselle, and Rachid Amara. 2024. "The Influence of Natural and Anthropogenic Environmental Pressures on European Eel Abundances in French Estuaries" Fishes 9, no. 2: 44. https://doi.org/10.3390/fishes9020044
APA StyleDenis, J., Lepage, M., Gruselle, M.-C., & Amara, R. (2024). The Influence of Natural and Anthropogenic Environmental Pressures on European Eel Abundances in French Estuaries. Fishes, 9(2), 44. https://doi.org/10.3390/fishes9020044








