Diversification of Aquaculture in the Sub-Saharan Region—The Obscure Snakehead
Abstract
1. Introduction
2. Growth Performance
3. Nutrition
4. Reproduction
5. Health
6. Water Parameters
7. Fisheries
8. Processing
9. Further Tasks
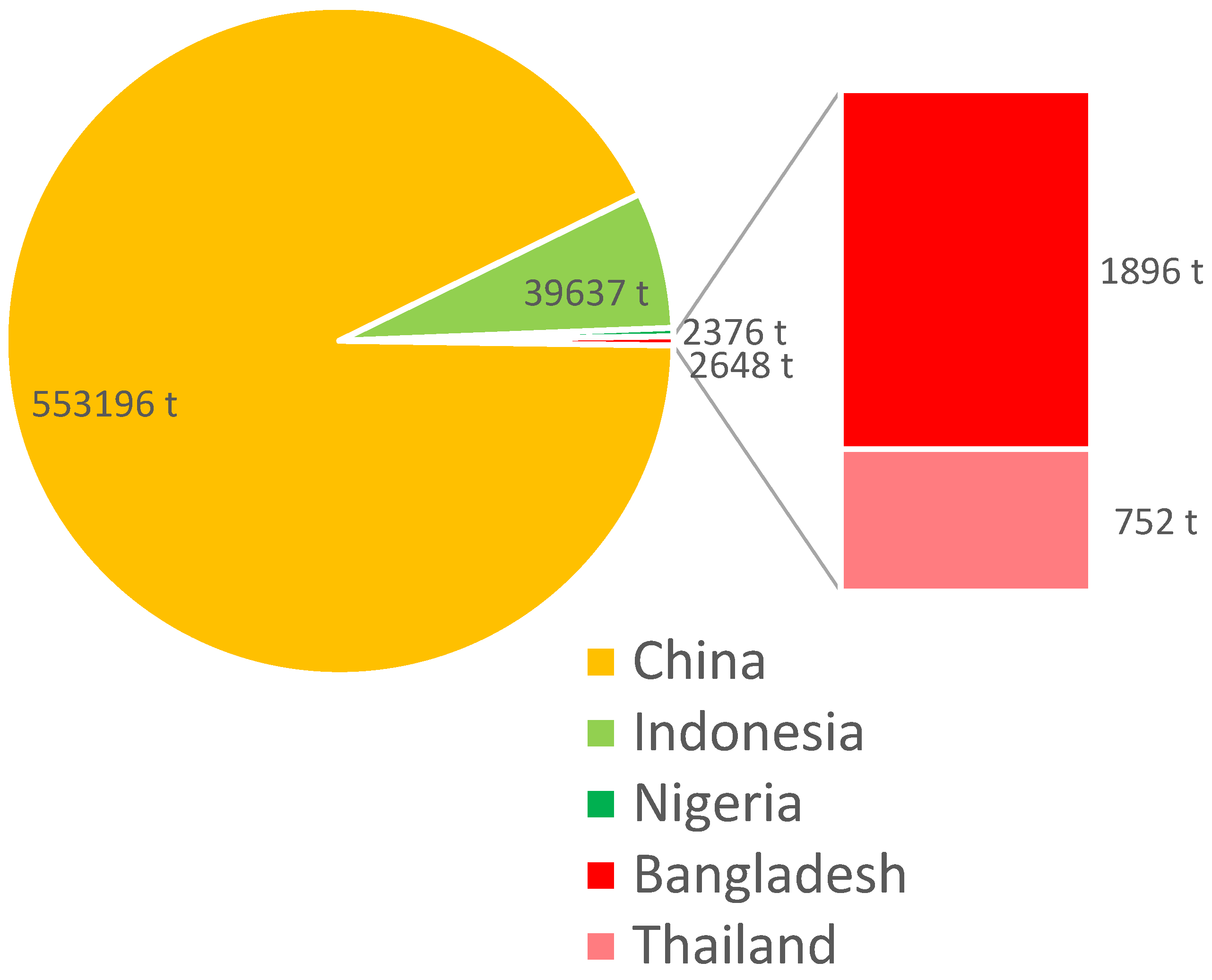
10. A Global Perspective on Snakehead Farming
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ragasa, C.; Charo-Karisa, H.; Rurangwa, E.; Tran, N.; Shikuku, K.M. Sustainable aquaculture development in sub-Saharan Africa. Nat. Food 2022, 3, 92–94. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO Fisheries and Aquaculture—FishStatJ—Software for Fishery and Aquaculture Statistical Time Series; FAO Fisheries and Aquaculture Division, FAO: Rome, Italy, 2024. [Google Scholar]
- Chan, C.Y.; Tran, N.; Pethiyagoda, S.; Crissman, C.C.; Sulser, T.B.; Phillips, M.J. Prospects and challenges of fish for food security in Africa. Glob. Food Secur. 2019, 20, 17–25. [Google Scholar] [CrossRef]
- Pullin, R.S.V. Diversification in aquaculture: Species, farmed types and culture systems. In Planning for Aquaculture Diversification: The Importance of Climate Change and Other Drivers FAO Technical Workshop, Proceedings of the FAO Fisheries and Aquaculture, Rome, Italy, 23–25 June 2016; Harvey, B., Soto, D., Carolsfeld, J., Beveridge, M., Bartley, D.M., Eds.; FAO: Rome, Italy, 2016. [Google Scholar]
- Oboh, A. Diversification of farmed fish species: A means to increase aquaculture production in Nigeria. Rev. Aquacult. 2022, 14, 2089–2098. [Google Scholar] [CrossRef]
- Teugels, G.G. Channidae. In Faune des Poissons D’eaux Douce et Saumâtres de l’Afrique de l’Ouest, Tome 2 Coll Faune et Flore Tropicales Musée Royal de l’Afrique Centrale, Tervuren, Belgique; Lévêque, C., Paugy, D., Teugels, G.G., Eds.; Museum National d’Histoire Naturalle: Paris, France; Institut de Recherche pour le Développement: Paris, France, 2003; pp. 443–446. [Google Scholar]
- Teugels, G.G.; Breine, J.J.; Thys van den Audernaerde, D.F.E. Channidae. In Checklist of the Freshwater Fishes of Africa (CLOFFA); Daget, J., Gosse, J.-P., van den Audenaerde, D.F.T., Eds.; ORSTOM: Paris, France; MRAC: Tervuren, Belgium, 1986; Volume 2, pp. 288–290. [Google Scholar]
- Bonou, C.A.; Teugels, G.G. Revision systematique du genre Parachanna (Teugels and Daget 1984) (Pisces: Channidae). Rev. Hydrobiol. Trop. 1985, 18, 267–280. [Google Scholar]
- Ishimatsu, A.; Glass, M.L.; Johansen, K. Aerial and aquatic respiration in the air-breathing fish Channa argus. Acta Physiol. Scan. 1985, 123, A46. [Google Scholar]
- Ishimatsu, A.; Itazawa, Y. Blood-oxygen levels and acid-base status following air exposure in an air-breathing fish, Channa argus—The role of air ventilation. Comp. Biochem. Physiol. A 1983, 74, 787–793. [Google Scholar]
- Itazawa, Y.; Ishimatsu, A. Gas-exchange in an air-breathing fish, the snakehead Channa argus, in normoxic and hypoxic water and in air. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 829–834. [Google Scholar] [CrossRef]
- Kpogue, D.N.S.; Mensah, G.A.; Fiogbe, E.D. A review of biology, ecology and prospect for aquaculture of Parachanna obscura. Rev. Fish Biol. Fish. 2013, 23, 41–50. [Google Scholar] [CrossRef]
- Arul, V. Effect of temperature on yolk utilization of Channa striatus. J. Therm. Biol. 1991, 16, 1–5. [Google Scholar] [CrossRef]
- Khatun, H.M.; Mostakim, G.M.; Islam, S.M. Acute responses of spotted snakehead (Channa punctata) to salinity stress: A study of a freshwater fish to salinity challenges during intrusion of saline water. Iran. J. Fish. Sci. 2020, 19, 2673–2687. [Google Scholar]
- O’ Bryen, P.J.; Lee, C.S. Discussion summary: Socioeconomic aspects of species and systems selection for sustainable aquaculture. In Species and System Selection for Sustainable Aquaculture; Blackwell Publishing: Oxford, UK, 2007; pp. 477–487. [Google Scholar]
- Bolaji, B.B.; Mfon, T.U.; Utibe, D.I. Preliminary study on the aspects of the biology of snakehead fish Parachanna obscura (Gunther) in a Nigerian wetland. Afr. J. Food Agri. Nutr. Dev. 2011, 11, 4708–4717. [Google Scholar] [CrossRef]
- Victor, R.; Akpocha, B.O. The biology of snakehead, Channa obscura (Gunther), in a Nigerian Pond under monoculture. Aquaculture 1992, 101, 17–24. [Google Scholar] [CrossRef]
- Amoutchi, A.I.; Kamelan, T.M.; Kargbo, A.; Gneho, D.; Kouamelan, E.P.; Mehner, T. Local fishers’ knowledge on the ecology, economic importance, and threats faced by populations of African snakehead fish, Parachanna obscura, within Côte d’Ivoire freshwater ecosystems. Aquac. Fish Fish. 2023, 3, 287–301. [Google Scholar] [CrossRef]
- Amoutchi, A.I.; Mehner, T.; Ugbor, O.N.; Kargbo, A.; Kouamelan, E.P. Fishermen’s perceptions and experiences toward the impact of climate change and anthropogenic activities on freshwater fish biodiversity in Côte d’Ivoire. Discov. Sustain. 2021, 2, 56. [Google Scholar] [CrossRef]
- Ali, A.B. Aspects of the reproductive biology of female snakehead (Bloch) obtained from irrigated rice agroecosystem, Malaysia. Hydrobiologia 1999, 411, 71–77. [Google Scholar] [CrossRef]
- Bich, T.T.N.; Tri, D.Q.; Yi-Ching, C.; Khoa, H.D. Productivity and economic viability of snakehead culture using an aquaponics approach. Aquac. Eng. 2020, 89, 102057. [Google Scholar] [CrossRef]
- Bassey, A.U.; Ajah, P.O. Effect of three feeding regimes on growth, condition factor and food conversion rate of pond cultured Parachanna obscura (Gunther, 1861) (Channidae) in Calabar, Nigeria. Turk. J. Fish. Aquat. Sci. 2010, 10, 195–202. [Google Scholar] [CrossRef]
- Jackson, P.B.N. Aquaculture in Africa. In Faune des Poissons d’eaux Douce et Saumâtres de l’Afrique de l’Ouest, Tome 2 Coll Faune et Flore tropicales Musée Royal de l’Afrique Centrale, Tervuren, Belgique; Lévêque, C., Paugy, D., Teugels, G.G., Eds.; Museum National d’Histoire Naturalle: Paris, French, 1988; pp. 459–475. [Google Scholar]
- Fagbenro, O.A. Recruitment control and production of Tilapia guineensis (Dumeril) with the predator, Channa obscura (Gunther). J. Aquat. Sci. 1989, 4, 7–10. [Google Scholar]
- King, R.P. Length-weight relationships and related statistics of 73 populations of fish occurring in inland waters of Nigeria. Naga ICLARM Q. 1996, 19, 49–52. [Google Scholar]
- Olaosebikan, B.D.; Raji, A. Field Guide to Nigerian Freshwater Fishes; Federal College of Freshwater Fisheries Technology: New Bussa, Nigeria, 1998. [Google Scholar]
- Fadekemi, I.T. Fillet quality and gut content analysis of Parachanna obscura and Clarias agboyiensis. J. Fish. Sci. 2022, 4, 1–8. [Google Scholar] [CrossRef]
- Odo, G.E.; Onoja, S.U.; Onyishi, G.C. The biology of Parachanna obscura (Osteichthyes: Channidae) in Anambra River, Nigeria. Int. J. Fish. Aquac. 2012, 4, 154–169. [Google Scholar] [CrossRef]
- Ama-Abasi, D.E.; Affia, I.N. Aspects of the biology of snakehead Parachanna obscura (Gunther 1861) in the Cross river, Nigeria. Glob. J. Agric. Sci. 2010, 9, 7–13. [Google Scholar]
- Kpogue, D.N.S.; Fiogbe, E.D. Feeding rate requirements for Parachanna obscura fry reared under controlled environmental conditions. J. Appl. Biosci. 2012, 55, 3962–3972. [Google Scholar]
- Olasunkanmi, J.B.; Ipinmoroti, M.O. Food of the African snake head (Parachanna obscura) in a protected area. Int. J. Dev. Sustain. 2014, 3, 177–183. [Google Scholar]
- Fagbenro, O.; Adedire, O.; Fateru, O.; Owolabi, I.; Ogunlana, O.; Akanbi, B.; Fasanmi, T.; Ayo-Amu, P. Digestive enzyme assays in the gut of Oreochromis niloticus Linnaeus 1757, Parachanna (Channa) obscura Gunther 1861 and Gymnarchus niloticus Cuvier. Anim. Res. Int. 2005, 2, 292–296. [Google Scholar]
- Kori-Siakpere, O. Carbohydrases in the alimentary canal and associated organs of the African snakehead, Parachanna africans (Osteichthyes: Channidae). Isr. J. Aquac. BAMIGDEH 2004, 56, 22–28. [Google Scholar] [CrossRef]
- Ding, X.Q.; Yao, L.; Hou, Y.; Hou, Y.B.; Wang, G.L.; Fan, J.H.; Qian, L.C. Effects of different carbohydrate levels in puffed feed on digestive tract morphological function and liver tissue structure of snakeheads (Channa argus). Aquac. Res. 2020, 51, 557–568. [Google Scholar] [CrossRef]
- Li, W.F.; Zhang, Y.; Wang, Z.J.; Zhang, N.H.; Sheng, Z.Y.; Chen, N.S.; Li, S.L. Effects of dietary starch levels on growth performance, hepatic proximate composition and non-specific immunity of snakehead (Channa argus). Aquac. Res. 2022, 53, 5971–5978. [Google Scholar] [CrossRef]
- Diana, J.S.; Chang, W.Y.B.; Ottey, D.R.; Chuapoehuk, W. Production systems for commonly cultured freshwater fishes of Southeast Asia. Int. Program Rep. 1985, 7, 75–79. [Google Scholar]
- Gonella, H. Snakeheads are aquarium fish. Aquar. Live 2003, 3, 62–65. [Google Scholar]
- Kpoguè, D.N.S.; Ayanou, G.A.; Imorou Toko, I.; Mensah, G.A.; Fiogbé, E.D. Influence of dietary protein levels on growth, feed utilization and carcass composition of snakehead P. obscura (Günther, 1861) fingerlings. Int. J. Fish. Aquac. Acad. 2013, 5, 71–77. [Google Scholar]
- Kpogue, D.; Gangbazo, H.; Fiogbe, E. A preliminary study on the dietary protein requirement of Parachanna obscura (Gunther, 1861) larvae. Turk. J. Fish. Aquat. Sci. 2013, 13, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Vital, V.; Kpogue, D.N.; Apollinaire, D.G. Culture of earthworm (Eisenia fetida), production, nutritive value and utilization of its meal in diet for Parachanna obscura fingerlings reared in captivity. Int. J. Fish. Aquat. Stud. 2011, 4, 1–5. [Google Scholar]
- Liu, Y.Y.; Ding, X.Y.; Brown, P.B.; Bai, Y.H.; Liu, Z.Z.; Shen, J.F.; Liu, H.; Huang, Y. The digestive enzyme activity, intestinal microbiota and immune-related genes expression of snakehead fish (Channa argus) juveniles affected by dietary cricket (Cryllus testaceus) meal. Anim. Feed. Sci. Technol. 2023, 304, 115721. [Google Scholar] [CrossRef]
- Manh, H.N.; Suong, T.T.T.; Lan, P.T.P.; Tram, N.D.Q. Effect of inclusion of fresh or dried black soldier fly larvae in diets on snakehead fish’s growth performance and chemical composition (Channa sp.). Isr. J. Aquac. Bamidgeh 2024, 76, 92338. [Google Scholar] [CrossRef]
- Hien, T.T.T.; Be, T.T.; Lee, C.M.; Bengtson, D.A. Development of formulated diets for snakehead (Channa striata and Channa micropeltes): Can phytase and taurine supplementation increase use of soybean meal to replace fish meal? Aquaculture 2015, 448, 334–340. [Google Scholar] [CrossRef]
- Fei, S.Z.; Ou, M.; Liu, H.Y.; Zhang, X.C.; Luo, Q.; Li, K.B.; Chen, K.C.; Chen, B.X.; Zhao, J. Transcriptomics and metabolomics reveal the regulation of reproductive performance and egg quality of blotched snakehead Channa maculata broodstock induced by a high protein diet. Aquac. Rep. 2024, 34, 101902. [Google Scholar] [CrossRef]
- Kpogue, D.N.S.; D’Almeida, A.F.M.; Houankanlin, N.; Dougnon, J.; Fiogbe, E.D. Influence of dietary lipid levels on growth performances, survival, feed utilization and carcass composition of African snakehead Parachanna obscura fingerlings. South Asian J. Life Sci. 2018, 6, 36–40. [Google Scholar] [CrossRef]
- Aliyu-Paiko, M.; Hashim, R. Effects of substituting dietary fish oil with crude palm oil and palm fatty acid distillate on growth, muscle fatty acid composition and the activities of hepatic lipogenic enzymes in snakehead (Channa striatus, Bloch 1793) fingerling. Aquac. Res. 2012, 43, 767–776. [Google Scholar] [CrossRef]
- Abdul-Halim, H.H.; Aliyu-Paiko, M.; Hashim, R. Partial replacement of fish meal with poultry by-product meal in diets for Snakehead (Bloch, 1793) fingerlings. J. World Aquac. Soc. 2014, 45, 233–241. [Google Scholar] [CrossRef]
- Hua, K.; Koppe, W.; Fontanillas, R. Effects of dietary protein and lipid levels on growth, body composition and nutrient utilization of Channa striata. Aquaculture 2019, 501, 368–373. [Google Scholar] [CrossRef]
- Ghaedi, A.; Kabir, M.A.; Hashim, R. Effect of lipid levels on the reproductive performance of Snakehead murrel, Channa striatus. Aquac. Res. 2016, 47, 983–991. [Google Scholar] [CrossRef]
- Kuah, M.K.; Jaya-Ram, A.; Shu-Chien, A.C. The capacity for long-chain polyunsaturated fatty acid synthesis in a carnivorous vertebrate: Functional characterisation and nutritional regulation of a Fads2 fatty acyl desaturase with Δ4 activity and an Elovl5 elongase in striped snakehead (Channa striata). Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 248–260. [Google Scholar]
- Ogunji, J.; Wuertz, S. Aquaculture development in Nigeria: The second biggest aquaculture producer in Africa. Water 2023, 15, 4224. [Google Scholar] [CrossRef]
- Hien, T.T.T.; Tam, B.M.; Tu, T.L.C.; Bengtson, D.A. Weaning methods using formulated feeds for snakehead (Channa striata and Channa micropeltes) larvae. Aquac. Res. 2017, 48, 4774–4782. [Google Scholar] [CrossRef]
- Kumari, R.; Srivastava, P.P.; Mohanta, K.N.; Das, P.; Kumar, R.; Sahoo, L.; Sharma, P.; Krishna, G.; Paul, A.; Siddaiah, G.M. Optimization of weaning age for striped murrel (Channa striata) based on expression and activity of proteases. Aquaculture 2024, 579, 740277. [Google Scholar] [CrossRef]
- Hien, T.T.T.; Trung, N.H.D.; Tâm, B.M.; Chau, V.M.Q.; Huy, N.H.; Lee, C.M.; Bengtson, D.A. Replacement of freshwater small-size fish by formulated feed in snakehead (Channa striata) aquaculture: Experimental and commercial-scale pond trials, with economic analysis. Aquac. Rep. 2016, 4, 42–47. [Google Scholar] [CrossRef][Green Version]
- Grimm-Greenblatt, J.; Pomeroy, R.; Bravo-Ureta, B.; Sinh, L.X.; Hien, H.V.; Getchis, T. Economic analysis of alternative snakehead Channa striata feed. Aquac. Econ. Manag. 2015, 19, 192–209. [Google Scholar] [CrossRef]
- Li, M.; Fang, Q.Y.; Xiu, L.; Yu, L.H.; Peng, S.B.; Wu, X.Q.; Chen, X.M.; Niu, X.T.; Wang, G.Q.; Kong, Y.D. The molecular mechanisms of alpha-lipoic acid on ameliorating aflatoxin B1-induced liver toxicity and physiological dysfunction in northern snakehead (Channa argus). Aquat. Toxicol. 2023, 257, 106466. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.D.; Lai, Y.Q.; Wu, X.Q.; Zhang, J.W.; Niu, X.T.; Wang, G.Q. The effects of dietary supplementation of α-lipoic acid on the growth performance, antioxidant capacity, immune response, and disease resistance of northern snakehead, Channa argus. Fish Shellfish Immunol. 2022, 126, 57–72. [Google Scholar] [CrossRef]
- Olurin, K.B.; Savage, O.D. Reproductive biology, length–weight relationship and condition factor of the African snake head, Parachanna obscura, from River Oshun, South-west Nigeria. Int. J. Fish. Aquac. 2011, 3, 146–150. [Google Scholar]
- Kashyap, A.; Awasthi, M.; Serajuddin, M. Reproductive biology of the freshwater murrel Channa punctatus (Bloch, 1793) from River Gomti of Lucknow region, Uttar Pradesh, India. Indian J. Fish. 2016, 63, 126–130. [Google Scholar] [CrossRef]
- Belsare, D.K. Development of Gonads in Channa Punctatus Bloch (Osteichthyes—Channidae). J. Morphol. 1966, 119, 467–475. [Google Scholar] [CrossRef]
- Gogoi, N.; Hazarika, L.P.; Biswas, S.P. Studies on the reproductive biology and captive breeding of Channa aurantimaculata, an endemic fish from Assam. J. Environ. Biol. 2016, 37, 369–374. [Google Scholar]
- Damle, D.; Kumar, R.; Ahilan, B.; Pillai, B.R.; Chidambaram, P.; Swain, P.P.; Debbarma, J.; Sundaray, J.K. The Effect of habitat manipulation on early gonad maturation of Channa striata in captive condition. Indian J. Anim. Res. 2023, 57, 1462–1468. [Google Scholar] [CrossRef]
- Azon, M.T.C.; Sossoukpe, E.; Vodounnou, J.V.; Chikou, A.; Mensah, G.A.; Fiogbe, E.D. A review of the general aspects of reproduction of a threatened fresh water fishspecies in Benin: Parachanna obscura (Günter, 1861). Int. J. Agric. Environ. Biores. 2020, 5, 272–279. [Google Scholar]
- Huang, S.J.; Wu, Y.X.; Chen, K.C.; Zhang, X.T.; Zhao, J.; Luo, Q.; Liu, H.Y.; Wang, F.; Li, K.B.; Fei, S.Z.; et al. Gene expression and epigenetic modification of aromatase during sex reversal and gonadal development in Blotched snakehead (Channa maculata). Fishes 2023, 8, 129. [Google Scholar] [CrossRef]
- Ou, M.; Chen, K.C.; Luo, Q.; Liu, H.Y.; Wang, Y.P.; Chen, B.X.; Liang, X.Q.; Zhao, J. Performance evaluation of XY all-male hybrids derived from XX female Channa argus and YY super-males Channa maculata. Aquac. Rep. 2021, 20, 100768. [Google Scholar] [CrossRef]
- Kumar, R.; Gokulakrishnan, M.; Debbarma, J.; Damle, D.K. Advances in captive breeding and seed rearing of striped murrel Channa striata, a high value food fish of Asia. Anim. Reprod. Sci. 2022, 238, 106957. [Google Scholar] [CrossRef]
- Awal, M.R.; Pervin, R.; Rahman, M.A.; Bhadra, A.; Mahmud, Y.; Tanu, M.B.; Parvez, I. Effect of hormonal treatment on artificial propagation, spawning performance and embryonic development of striped snakehead Channa striata (Bloch, 1793). Anim. Reprod. Sci. 2024, 267, 107521. [Google Scholar] [CrossRef]
- Qin, J.G.; Fast, A.W. Size and feed dependent cannibalism with juvenile snakehead Channa striatus. Aquaculture 1996, 144, 313–320. [Google Scholar] [CrossRef]
- Ama-Abasi, D.; Oden, E. The pathology of nematode infection in Parachanna obscura (Pisces: Channidae Gunther, 1886) of the Cross River system, Nigeria. Brit. J. Appl. Sci. Technol. 2015, 9, 131–136. [Google Scholar] [CrossRef]
- Nack, J.; Messu Mandeng, F.D.; Yede, M.; Bilong Bilong, C.F. Spatial distribution of monogenean gill parasites of Parachanna obscura (Günther, 1861)—Channidae—In Lake Ossa (Edéa, Cameroon). Int. J. Biol. Chem. Sci. 2018, 12, 749–768. [Google Scholar] [CrossRef]
- Osho, F. Parasitic helminth fauna of Parachanna obscura in River Ogun, Southwest Nigeria. Afr. J. Fish. Aquat. Res. Manag. 2017, 2, 79–85. [Google Scholar]
- Allameh, S.K.; Yusoff, F.M.; Daud, H.M.; Ringo, E.; Ideris, A.; Saad, C.R. Characterization of a probiotic isolated from Snakehead, Channa striatus, stomach. J. World Aquac. Soc. 2013, 44, 835–844. [Google Scholar] [CrossRef]
- Kong, Y.D.; Gao, C.S.; Du, X.Y.; Zhao, J.; Li, M.; Shan, X.F.; Wang, G.Q. Effects of single or conjoint administration of lactic acid bacteria as potential probiotics on growth, immune response and disease resistance of snakehead fish (Channa argus). Fish Shellfish Immunol. 2020, 102, 412–421. [Google Scholar] [CrossRef]
- Pulpipat, T.; Heckman, T.I.; Boonyawiwat, V.; Kerddee, P.; Phatthanakunanan, S.; Soto, E.; Surachetpong, W. Concurrent infections of Streptococcus iniae and Aeromonas veronii in farmed Giant snakehead (Channa micropeltes). J. Fish Dis. 2023, 46, 629–641. [Google Scholar] [CrossRef]
- Siriyappagouder, P.; Shankar, K.M.; Kumar, B.T.N.; Patil, R.; Byadgi, O.V. Evaluation of biofilm of Aeromonas hydrophila for oral vaccination of Channa striatus. Fish Shellfish Immunol. 2014, 41, 581–585. [Google Scholar] [CrossRef]
- Guo, M.Y.; Zhang, L.W.; Ye, J.X.; He, X.; Cao, P.; Zhou, Z.C.; Liu, X.D. Characterization of the pathogenesis and immune response to a highly virulent Edwardsiella tarda strain responsible for mass mortality in the hybrid snakehead (Channa maculate × Channa argus). Microb. Pathog. 2022, 170, 105689. [Google Scholar] [CrossRef]
- Miles, D.J.C.; Polchana, J.; Lilley, J.H.; Kanchanakhan, S.; Thompson, K.D.; Adams, A. Immunostimulation of striped snakehead Channa striata against epizootic ulcerative syndrome. Aquaculture 2001, 195, 1–15. [Google Scholar] [CrossRef]
- FAO. What You Need to Know About Epizootic Ulcerative Syndrome (EUS)—An Extension Brochure for Africa; FAO: Rome, Italy, 2020. [Google Scholar]
- Lilley, J.H.; Callinan, B.; Chinabut, S.; Kanchanakhan, S.; Macrae, I.H.; Philips, M.J. Epizootic Ulcerative Syndrome (EUS) Technical Handbook; Aquatic Animal Health Research Institute: Bangkok, Thailand, 1998. [Google Scholar]
- Ma, H.X.; Du, H.; Wang, D.X.; Cao, Y.; Liu, J.; Liu, T.Q.; Liu, T.; Wang, G.X.; Wang, E.L. A subunit vaccine encoding glycoprotein holds potential to protect snakehead (Channa argus) from snakehead vesiculovirus infection. Aquaculture 2023, 577, 739947. [Google Scholar] [CrossRef]
- Liu, X.D.; Wen, Y.; Hu, X.Q.; Wang, W.W.; Liang, X.F.; Li, J.; Vakharia, V.; Lin, L. Breaking the host range: Mandarin fish is susceptible to a vesiculovirus derived from snakehead fish. J. Gen. Virol. 2015, 96, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Teugels, G.G.; Reid, G.M.; King, R.P. Fishes of the Cross River basin (Cameroon Nigeria) taxonomy, zoogeography, ecology and conservation. AGRIS—Int. Syst. Agric. Sci. Technol. 1992, 266, 116–124. [Google Scholar]
- Ly, T.H.; Huang, C.T.; Lee, P.T.; Vo, V.T.; Diep, D.X. Hatching success and growth of Snakehead (Channa lucius Cuvier, 1831) larvae and fry at different pH levels. Aquat. Living Resour. 2024, 37, 1. [Google Scholar] [CrossRef]
- Saha, T.K.; Das, A.B. Effect of ammonia stress on the total autolytic levels of proteins in tissues of an air-breathing fish, Channa punctatus (Bloch). J. Biosci. 1994, 19, 301–306. [Google Scholar] [CrossRef]
- Ansari, I.A. Acute toxicity of sodium nitrite on Channa punctatus (Bloch) and Mystus vittatus (Bloch). Acta Hydrochim. Hydrobiol. 1987, 15, 175–178. [Google Scholar] [CrossRef]
- Das, A.B. Physiological and biochemical effects of sublethal ambient ammonia on Channa punctatus (Bloch). Indian J. Biochem. 1981, 18, 5. [Google Scholar]
- Tah, L.; Gouli, G.B.; Da Costa, K.S. Length-weight relationships for 36 freshwater fish species from two tropical reservoirs: Ayamé i and Buyo, Côte d’Ivoire. Rev. Biol. Trop. 2012, 60, 1847–1856. [Google Scholar] [CrossRef]
- Adou, Y.E.; Blahoua, K.G.; Bamba, M.; Yao, S.S.; Kouamelan, E.P.; N’douba, V. Premières données sur l’inventaire du peuplement ichtyologique d’un lac ouest Africain situé entre deux barrages hydroélectriques: Lac d’Ayamé 2 (Côte d’Ivoire). J. Appl. Biosci. 2017, 110, 10808–10818. [Google Scholar] [CrossRef][Green Version]
- Kien, K.B.; Ndiaye, A.; N’Da, A.S.; Kouamelan, E.P. Production and specific diversity of fish at the level of the Taabo dam lake (Ivory Coast, West Africa). World J. Adv. Res. Rev. 2022, 15, 244–256. [Google Scholar] [CrossRef]
- Laë, R.; Williams, S.; Massou, M.; Morand, P.; Mikolasek, O. Review of the present state of the environment, fish stocks and fisheries of the River Niger (West Africa). In Proceedings of the Second International Symposium on the Managment of Large Rivers for Fisheries, Phnom Penh, Cambodia, 11–14 February 2003; pp. 199–228. [Google Scholar]
- Ugbor, O.N.; Omoigberale, M.O.; Amoutchi, A.I.; Affian, K.; Mehner, T. Environmental and spatial determinants of fish community structure in an Afro-tropical river ecosystem. Ecol. Freshw. Fish 2023, 32, 852–863. [Google Scholar] [CrossRef]
- Ren, M.T.; Yin, T.; You, J.; Liu, R.; Huang, Q.L.; Xiong, S.B. Comparative study of the nutritional composition and antioxidant ability of soups made from wild and farmed snakehead fish (Channa argus). Foods 2022, 11, 3294. [Google Scholar] [CrossRef]
- Chawafambira, T.A.; Dang, H.T.T.; Nguyen, D.T.; Nguyen, M.V. Effects of ascorbic acid sodium citrate treatments on the sensory quality lipid stability of fresh snakehead fish (Channa striata) fillets during 14 days chilled storage at, 2–4 °C. Iran. J. Fish. Sci. 2022, 21, 1472–1494. [Google Scholar]
- Huynh, T.K.D.; Nguyen, L.A.D.; Nguyen, T.N.H.; Nguyen, Q.T.; Tomoaki, H.; Tran, M.P. Effects of green tea (Camellia sinensis) and guava (Psidium guajava) extracts on the quality of snakehead (Channa striata) fillets during ice storage. J. Food Process. Preserv. 2022, 46, e16194. [Google Scholar] [CrossRef]
- Laila, L.; Febriyenti, F.; Salhimi, S.M.; Baie, S. Wound healing effect of Haruan (Channa striatus) spray. Int. Wound J. 2011, 8, 484–491. [Google Scholar] [CrossRef]
- Lee, V.L.L.; Kumari, Y.; Shaikh, M.F. Haruan (Channa striatus) extract modulates post-seizure inflammatory response in a zebrafish model. Epilepsia 2019, 60, 62–63. [Google Scholar]
- Leng, W.J.; Wu, X.Y.; Xiong, Z.Y.; Shi, T.; Sun, Q.C.; Yuan, L.; Gao, R.C. Study on antibacterial properties of mucus extract of snakehead (Channa argus) against Escherichia coli and its application in chilled fish fillets preservation. LWT 2022, 167, 113840. [Google Scholar] [CrossRef]
- Duan, Z.P.; Zhang, C.Y.; Huang, L.L.; Lan, Q.; Hu, J.; Li, X.Q.; Leng, X.J. An evaluation of replacing fish meal with fermented soybean meal in diet of hybrid snakehead (Channa argus × Channa maculata): Growth, nutrient utilization, serum biochemical indices, intestinal histology, and microbial community. Aquac. Nutr. 2022, 2022, 2964779. [Google Scholar] [CrossRef]
- Landis, A.M.G.; Lapointe, N.W.R. First record of a Northern snakehead (Channa argus Cantor) nest in North America. Northeast Nat. 2010, 17, 325–332. [Google Scholar] [CrossRef]
- Landis, A.M.G.; Lapointe, N.W.R.; Angermeier, P.L. Individual growth and reproductive behavior in a newly established population of northern snakehead (Channa argus), Potomac River, USA. Hydrobiologia 2011, 661, 123–131. [Google Scholar] [CrossRef]
- Li, K.C.; Shieh, B.S.; Chiu, Y.W.; Huang, D.J.; Liang, S.H. Growth, diet composition and reproductive biology of the invasive freshwater fish chevron snakehead Channa striata on a subtropical island. Zool. Stud. 2016, 55, e53. [Google Scholar] [CrossRef]
- Li, X.; Meng, Q.; Xie, N. Snakehead culture. In Aquaculture in China: Success Stories and Modern Trends; Gui, J.-F., Tang, Q., Li, Z., Liu, J., De Silva, S.S., Eds.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Gustiano, R.; Kurniawan, K.; Kusmini, I.I. Bioresources and diversity of snakehead, Channa striata (Bloch 1793): A proposed model for optimal and sustainable utilization of freshwater fish. IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 012012. [Google Scholar] [CrossRef]
- Bin Mohd Khatib, M.A.; Mat Jais, A.M. A brief overview of the integrated fish farming of three commercially popular fish species (snakehead, tilapia and catfish) in Malaysia. Malays. J. Appl. Sci. 2021, 6, 105–112. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wuertz, S.; Amoutchi, A.I.; Ogunji, J. Diversification of Aquaculture in the Sub-Saharan Region—The Obscure Snakehead. Fishes 2024, 9, 526. https://doi.org/10.3390/fishes9120526
Wuertz S, Amoutchi AI, Ogunji J. Diversification of Aquaculture in the Sub-Saharan Region—The Obscure Snakehead. Fishes. 2024; 9(12):526. https://doi.org/10.3390/fishes9120526
Chicago/Turabian StyleWuertz, Sven, Amien Isaac Amoutchi, and Johnny Ogunji. 2024. "Diversification of Aquaculture in the Sub-Saharan Region—The Obscure Snakehead" Fishes 9, no. 12: 526. https://doi.org/10.3390/fishes9120526
APA StyleWuertz, S., Amoutchi, A. I., & Ogunji, J. (2024). Diversification of Aquaculture in the Sub-Saharan Region—The Obscure Snakehead. Fishes, 9(12), 526. https://doi.org/10.3390/fishes9120526






