Abstract
In the inland waters of the Balkans, many brown trout populations have been severely depleted. Therefore, identifying potential threats to their continued survival and ranking populations based on their biological and evolutionary importance enables setting conservation priorities. To assess the sustainability of the brown trout populations in the territory of Serbia (central Balkans), a modification of the ESHIPPO model was performed. The main modification involves incorporating the investigated populations’ genetic structure into the model. Therefore, the new ESHIPPOsalmo model includes an analysis of biological parameters and the impact of multiple factors, including habitat alterations, invasive species, pollution, human population growth, and over-exploitation. In order to investigate individual levels of influence of the model’s analyzed parameters, a combination of supervised and unsupervised machine learning methods was used. The structure of the model is based on general and easily measurable indicators, which enables its application in any salmonid river in the world. By evaluating the parameters of the ESHIPPOsalmo model, we were able to establish that, of the analyzed populations from 46 localities, 37% have a moderate level of sustainability, 43% low, and 20% critically low.
Key Contribution:
In this work, we improved the ESHIPPO model used in Serbia in order to assess the sustainability of brown trout populations with the ultimate goal of proposing measures for their protection and sustainable management.
1. Introduction
Brown trout Salmo trutta L. is a widespread salmonid species with a natural range in Eurasia and North Africa [1]. It is characterized by the existence of numerous divergent local populations [2]. In response to the ecological heterogeneity of the environment, brown trout show remarkable morphological and ecological plasticity, even in a small geographical area [3].
The Balkan Peninsula is characterized by the most phenotypically diverse populations of Salmo spp. [4,5,6]. Moreover, the findings of several authors suggest that the brown trout lineages originated in the Eastern Mediterranean basin, possibly in the Balkans [7,8,9,10,11]. In the inland waters of the Balkans, salmonids play an important economic and social role in both commercial and recreational fisheries. Unfortunately, this has led to many of the stocks being severely depleted [12].
The hydrographic characteristics of Serbia, located in the central part of the Balkan Peninsula, are the result of paleogeographical, paleoclimatic, and geotectonic events [13] that caused the emergence of separate, locally specific populations of brown trout [14]. Solely based on mitochondrial DNA analysis, 38 haplotypes were detected on the territory of Serbia belonging to the Danube, Adriatic, and allochthonous (non-native) Atlantic phylogenetic lineages [11,14,15,16,17,18]. The high variability between watersheds in Serbia is also accompanied by a high interpopulation variability within watersheds, which is most likely a consequence of recent isolation and local differentiation within populations [19,20].
The extinction of local populations, i.e., the reduction or loss of intraspecies diversity of brown trout, is mainly the result of anthropogenic activities. The most pronounced adverse effects occur indirectly, due to habitat degradation, but also directly through excessive fishing and unplanned and inadequate stocking [21,22,23,24]. The introduction of allochthonous lineages of the brown trout complex and their hybridization with autochthonous populations is one of the main threats to the diversity of this taxon. Due to their easy cultivation and fast growth, the Atlantic domesticated strains have largely spread beyond their natural range to the Danube and Mediterranean basins [23]. Moreover, stocking with individuals from hatcheries of unknown origin is almost a regular practice in all parts of the area where brown trout populations are actively managed [23,24,25,26,27]. The first introductions, re-introduction, and translocation of salmonids in the Balkan Peninsula can be dated to the end of the 19th century [28]; these practices have considerably intensified during the 20th century, mainly to support recreational fishing [29].
In the aquatic ecosystems of Serbia, a multi-decade brown trout population decline has been recorded [30,31], with the main threatening factors being introgression (the entry or introduction of a gene from one gene complex into another), overfishing, and habitat destruction due to the construction of hydropower facilities. These facilities have led to changes in the water regime, habitat type, and quality.
Identifying potential threats to their continued survival and ranking populations based on their biological and evolutionary importance enable setting conservation priorities. In this way, the efficient allocation of limited resources and the targeted implementation of protection measures can be enabled [32]. Models such as ESHIPPO are designed to assess the extinction risk and conservation priorities of aquatic organisms at the national and local level [31,33,34,35,36], contributing to species’ long-term protection and recovery. The ESHIPPO model [33] is based on the quantitative measurement of two elements, the first of which refers to the Ecological Specialization (ES) of the taxon in relation to the habitat, diet, reproductive strategy, life cycle, body size, and level of endemism of the investigated taxon [37]. The second element refers to the impact of threatening factors on diversity, which are defined by the acronym “HIPPO”, which is derived from the initial letters of the following: H—Habitat alteration, I—Invasive species, P—Pollution, P—Population growth, and O—Over-exploitation [38].
This work aimed to improve the ESHIPPO model [34] by also incorporating genetic characterization of brown trout into the model. We applied and tested the improved model in Serbia in order to assess the sustainability of brown trout populations with the ultimate goal of proposing measures for their protection, conservation, and sustainable management.
2. Materials and Methods
Field research was carried out between 2014 and 2018. Brown trout individuals were sampled from 46 watercourses in the Danube and Aegean basins in the territory of Serbia (Table 5, Supplementary Table S1). Fish sampling was performed using the electrofishing method (Aquatech IG 1300, AquaTech, Kitzbühel, Austria; [39]), and subsequently, the standard body length (cm), total body length (cm), and body weight (g) of the caught individuals were measured.
The sustainability of brown trout populations and conservation priorities were assessed by modifying the ESHIPPO-fishing model [34]. The newly designed ESHIPPOsalmo model also incorporates the population’s Genetic Structure (GS). For that purpose, results of genetic characterization were retrieved from Veličković et al. [11] (Supplementary Table S1). Moreover, a partially modified evaluation system of the ILSFP element (Index of Local Sustainability of Fish Population [34]) was also included in the ESHIPPOsalmo model. In order to adequately assess the condition of brown trout populations, the ILSFP is estimated based on population density (number of individuals per m2), actual production (calculated as the ratio of real and potential production, obtained by Chapman [40] and Lager and Huet [41] methods, respectively), number of age classes, average length of individuals, and percentage of population present within the protected area. Finally, within the HIPPO element of the model, the invasive/non-native species (I) parameter was modified. Namely, in addition to data on invasive/non-native species in the habitat, information on the presence of allochthonous individuals that may hybridize with individuals from the studied populations is also included; in this case, the presence of individuals carrying allochthonous brown trout mitochondrial DNA (mtDNA) haplotypes for the territory of Serbia.
The concept and elements of the ESHIPPOsalmo model are presented in Table 1, Table 2 and Table 3. The specified parameters of the model elements are evaluated on a three-level point scale (1, 3, and 5 points). The priority of conservation and the population’s sustainability level depends on the sum of the values of the ES, GS, ILSFP, and HIPPO elements. Higher values of the sum of elements indicate a higher degree of sustainability of populations. Table 4 presents the point scale used to determine the level of sustainability and conservation priorities of brown trout populations, which are defined by the total number of points according to the ESHIPPOsalmo model.

Table 1.
Parameters and scoring system for the assessment of Ecological Specialization (ES) in the ESHIPPOsalmo model.

Table 2.
Parameters and scoring system for the assessment of the Genetic Structure (GS) and Index of Local Sustainability of Fish Population (ILSFP) of the ESHIPPOsalmo model.

Table 3.
The protocol of the HIPPO factors of the ESHIPPOsalmo model: parameters and scoring system.

Table 4.
Score scale for sustainability assessment and conservation priorities of brown trout populations according to the ESHIPPOsalmo model. The sustainability assessment and conservation priorities of populations are defined by the total number of points. The cell colors illustrate the level of population sustainability: critically low (red), low (yellow), moderate (green), and high (blue).
Additional data for assessment of the degree of sustainability of the populations were obtained from the BAES ex situ (Biodiversity of Aquatic Ecosystems of Serbia, ex situ protection [42]) database, which contains population monitoring data for the period between 2003 and 2022. Based on those data, all the mentioned parameters of the ESHIPPOsalmo model were estimated. In order to investigate individual levels of influence of the analyzed parameters of the ESHIPPOsalmo model and to visualize the obtained results, a combination of unsupervised machine learning methods using the UMAP model (Uniform Manifold Approximation and Projection [43]) and supervised learning using the decision tree classification algorithm in the BioVinci program v.3.0.9 was used (BioTuring Inc., San Diego, CA, USA) in order to provide more nuanced insights into the relationships between variables affecting brown trout sustainability.
The input matrix consisted of 46 rows (each row represented a population from one locality) and 19 columns that represented the analyzed parameters of the ESHIPPOsalmo model (Table 1, Table 2 and Table 3). Since the UMAP model is based on the Euclidean distance, the Hellinger transformation of the data was performed prior to the analysis to reduce the influence of the gradient length [44,45,46,47]. The determination of the most significant parameters that group populations into different subsets (clusters) was performed using algorithms that iteratively create all possible subsets and then, based on the classification algorithm, estimate which subset has the best performance [48,49]. The 2D-UMAP space was used to cluster the virtual sampling sites based on their similarities using hierarchical graph-based clustering. Additionally, we set the min_dust to 0.1 and spread to 1.0, which controls how dense UMAP is allowed to pack data points together. This approach leverages UMAP’s strength in capturing complex, non-linear relationships and effectively reducing dimensionality [50]. Finally, we applied a versatile supervised machine learning algorithm, decision tree, for classification.
3. Results
By applying the ordination process, the UMAP model’s main discriminating parameters of the ESHIPPOsalmo model for assessing the sustainable use and conservation priorities of the analyzed brown trout populations were isolated (Figure 1a). Populations from 46 localities were ordered by applying the UMAP model in two-dimensional space and distributed into four clusters using the decision tree algorithm (Figure 1b). Both Principal Components Analysis (PCA) [44] and UMAP are techniques for dimension reduction. In ecological and biodiversity studies (including genetic markers), PCA is commonly applied; however, as it is a linear projection tending to obscure final patterns [51] and lose important dependencies [46], we further explored our complex ecological and genetic datasets with UMAP. This relatively novel manifold learning technique, already successfully applied in the classification of biological indicators in aquatic ecology [45], should explore complex ecological and genetic datasets to retain as many variations as possible from the data. Moreover, combined unsupervised UMAP and supervised decision tree represent robust, highly predictive tools for exploring complex ecological datasets [50]. The division of populations into clusters and their position in the UMAP space was primarily influenced by threatening factors on diversity, which are defined by the acronym “HIPPO”. The presence of individuals carrying allochthonous mtDNA haplotypes for the territory of Serbia (parameter I; Figure 1a) is the main parameter that influenced the separation of populations into four clusters. In Figure 1a, the gradient is clearly defined: from the left side (Cluster 0-blue, which consists of 17 localities with a rating of 1, parameter I) to the right side (Clusters 1, 2, and 3, which make up 31 localities with a rating of 5, parameter I) of the UMAP space.
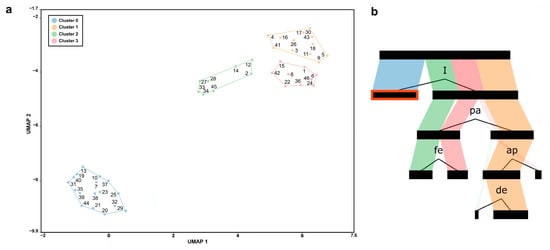
Figure 1.
(a) Grouping of analyzed populations using the ordination process of the UMAP model. The four identified clusters using the decision tree algorithm are marked with different colors. The analyzed populations are shown with ordinal numbers in the UMAP space, which correspond to both the ordinal number and name of the locality from Table 5 as well as the color coding in (b). Each cluster represents populations grouped by their vulnerability. The cluster along the lower left part of the UMAP space (blue) is characterized by the presence of allochthonous mtDNA haplotypes. A polygon is drawn through all the outer sample points for each location. (b) Schematic presentation of the results of the decision tree algorithm that detected the most important parameters for grouping the analyzed populations into clusters in (a). I—the presence of allochthonous species and/or brown trout mtDNA haplotypes (blue); pa—the percentage of population presence in the protected area (green); fe—habitat fragmentation (and isolation; red); ap—actual production; de—destruction of the habitat (orange).
In the lower left side of the UMAP space (Cluster 0), 17 populations with the recorded allochthonous mtDNA are grouped, in contrast to the upper right side, where the remaining three clusters are distinguished. Populations not within the protected area (or less than 50% of the population is in the protected area; parameter pa; Figure 1b) are grouped into Clusters 2 and 3, located in the upper right corner of the UMAP space. Cluster 1, located next to Clusters 2 and 3, consists of populations from 12 localities almost entirely within the protected area (Figure 1a). Also, habitat fragmentation (parameter fe; Figure 1b) was identified as one of the significant parameters for the distribution of populations into Clusters 2 (populations from eight localities where habitat fragmentation was recorded) and 3 (populations from nine localities where habitat fragmentation was not present) (Figure 1a). Actual production (parameter ap) and habitat destruction (parameter de) contributed to the distribution of populations in Cluster 2; however, they refer only to the additional differentiation of populations within the mentioned cluster (Figure 1b). Identifying these clusters is essential for prioritizing conservation efforts, as it highlights endangerment factors needing immediate intervention to prevent further decline or population. Cluster 0, for example, consists of populations with low or critically low sustainability, indicating a need for targeted management strategies to address the key factors driving these patterns.
Based on the total number of points of the ESHIPPOsalmo model, the level of sustainability of the analyzed populations was estimated (Table 5). The spatial distribution and presentation of the sustainability of populations in the territory of Serbia, together with their geographical distribution, are shown in Figure 2.

Table 5.
The results of the ESHIPPOsalmo model for assessing the sustainability of the investigated populations of brown trout. UMAP model cluster—population ordination into clusters based on UMAP analysis (Figure 1). Species Ecological Specialization (ES): h—habitat, d—diet, rs—reproductive system, lc—life cycle, bs—body size, re—population isolation level; population genetic structures (GS): ps—population phylogeographic structure; Index of Local Sustainability of Fish Population (ILSFP): pd—population density, ap—actual production, nAge—number of age classes, Lmean—average recorded length of individuals in the population, pa—percentage of population presence in protected areas; HIPPO factors: H—habitat alterations, I—allochthonous species and/or brown trout individuals, P—pollution, P—Population growth, O—overexploitation. Cell colors illustrate the degree of population sustainability: critically low (red), low (yellow), and moderate (green).
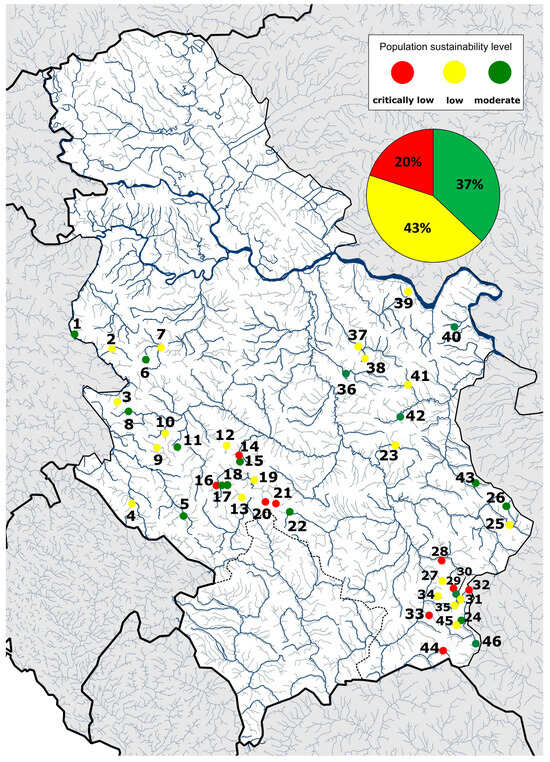
Figure 2.
The results of the sustainability assessment of the investigated brown trout populations using the ESHIPPOsalmo model. The nominal number and name of the locality from Table 5 correspond to the number of localities on the map. Circles next to the nominal numbers represent the sustainability assessment results of the brown trout population by locality (red—critically low, yellow—low, green—moderate). The circular chart in the top right corner shows the percentage distribution of results from the ESHIPPOsalmo model for all analyzed populations.
The scoring components of the model—ES, GS, ILSFP, and HIPPO—differ in how they influence the overall sustainability score. ES has the least influence; in contrast, ILSFP exerts the greatest impact on the overall score (Table 5). These elements are synthesized into a comprehensive sustainability score, which is then used to prioritize conservation actions based on the most critical endangerment factor for maintaining population viability and habitat integrity.
By evaluating the parameters of the ESHIPPOsalmo model, it was noted that none of the analyzed populations had a high level of sustainability (Table 5). Of the analyzed populations from 46 localities, 37% have a moderate level of sustainability, 43% low, and 20% critically low level of sustainability (Figure 2).
Based on the total number of points of Ecological Specialization, Genetic Structure, Index of Local Sustainability of Fish Populations, and HIPPO factors, 17 populations were found to be moderately sustainable (Table 5, Figure 2). The mentioned populations were detected in all analyzed river systems of the Danube basin and the Struma basin (Figure 2).
The sample’s highest value of the sum of analyzed parameters was recorded for the population from the Povlenska, Brevina, Polomska, and Janjska rivers (77 points each) (Table 5). A low level of sustainability was assessed for populations from 20 localities belonging to the Drina, Kolubara, West Morava, South Morava, and Timok river systems, including direct tributaries of the Danube (Figure 2).
Nine populations possessed a critically low level of sustainability based on the sum of the estimated parameters of the ESHIPPOsalmo model. By reviewing their distribution into clusters using the ordination process of the UMAP model, it was observed that habitat fragmentation was the main threatening factor to the populations from the Maglička, Bistrička, and Jelašnička rivers. The sustainability of the remaining six populations is questionable due to the presence of allochthonous individuals (Clusters 2, 0, respectively; Figure 1a, Table 5). In the sample, the lowest obtained value of the sum of the analyzed parameters is for the population from the Vlasina River (49 points; Table 5), on which nine mini hydropower plants have been built in the salmonid area in the last ten years.
4. Discussion
4.1. The Utility of the ESHIPPOsalmo Model for Assessing the Vulnerability and Sustainable Use of Brown Trout Populations
In the western Balkans, many salmonid stocks have been severely depleted and, in some cases, almost destroyed [12]. Moreover, extensive anthropogenic pressure has led to a reported reduction of nearly 50% in the region inhabited by salmonids in Serbia over the past two decades [31,34,52]; an assessment of conservation priorities is necessary so that conservation measures are first applied to critically endangered populations. This required the application of the ESHIPPO model and its modification, which was carried out in this work, to enable the detection of specific problems within each studied population in a simple and holistic way. Moreover, the model provides insights into population self-sustainability rather than the overall population conservation.
Data on the phylogenetic structure associated with the population endangerment level enable decisions on conservation priorities [53]. On the other hand, the analysis of the ecological characteristics of the habitat can provide information about potential environmental limitations of selection and the presence of alleles associated with adaptation to certain environmental conditions [54]. Therefore, the improved ESHIPPOsalmo model can be compared with the Population Adaptive Index (PAI; [55]) concept, based on which, in addition to the genetic diversity of populations, ecology and demographic characteristics of populations are taken into account for the formation of conservation strategies. However, despite the high genetic diversity recorded for the territory of Serbia [11], previous research indicates a declining trend in the number of brown trout populations in almost 80% of habitats in the territory of Serbia [31,34,52], additionally confirmed by the model in this work (Table 4 and Table 5).
Jakovljević et al. [46], for the first time, applied the UMAP model in combination with the decision tree classification algorithm to link the mutual influence of ecological and population parameters of fish species. Also, in the study by Milošević et al. [45], the UMAP model was applied to analyze an ecological data set, including the analysis of fish populations. The results of both studies confirmed that the dimensionality reductions obtained by the UMAP model are ecologically significant. Moreover, the UMAP model and the decision tree analysis as applied in this study enabled us to highlight the most critical factors that affect the sustainability of brown trout populations, offering deeper insights needed for better conservation strategies. These new insights, coupled with an extensive dataset on the vulnerabilities of brown trout populations in Serbia stemming from a long-term focus on conservation research of this species [11,14,30,31], helped us identify key vulnerability and sustainability parameters. These parameters (especially stocking and inadequate management practices) should be closely monitored in future studies for a better assessment of conservation efforts.
In this study, the presence of allochthonous individuals, a crucial engagement factor identified by the machine learning method (Cluster 0; Figure 1), results from decades of stocking or translocating individuals. Moreover, stocking, particularly if it has occurred recently, may further artificially elevate the points of the ILSFP index. Stocking as a practice is recorded in almost all parts of the brown trout distribution [56,57,58]. Moreover, in the inland waters of the Western Balkans, stocking with non-native brown trout has often resulted in genetic introgression into the autochthonous populations of Salmo trouts and loss of their genetic integrity [12]. Splendiani et al. [59] showed that the success of biological invasion and domesticated brown trout lineages is strongly related to the ecological characteristics of the habitat. Namely, aquatic ecosystems, which are characterized by unpredictable hydrological conditions, were originally inhabited by small trout populations; with the mass introduction of domesticated individuals, the limited autochthonous genetic diversity rapidly decreased [60]. This can be assumed for the population from upper Jerma, in which the autochthonous genetic structure was replaced entirely [11,30]. It is particularly important to take into consideration the state of populations containing certain Danubian haplotypes, as repeated stocking activities and translocations between river systems [30] have raised questions about the autochthony of these haplotypes in specific locations in Serbia. For example, the native origin of the Da2a haplotype was previously questioned [17]; therefore, the assumptions of it being the autochthonous haplotype in certain locations (e.g., the Boranjska and Povlenska rivers) needs to be further examined.
Along the brown trout distribution range, of particular concern is that inadequate population management also occurs within protected areas, e.g., [61,62]. The same situation is noted for Serbia (Table 5; Figure 1). Within the Kopaonik National Park, the Samokovska and Brzećka rivers possessed a sustainable population of brown trout until the construction of the mini-hydroelectric power plant and stocking with allochthonous individuals (Table 5). A similar situation was recorded in the Golija Nature Park brown trout populations [31,52]. The challenges in establishing effective protected areas in the Balkans, particularly for freshwater biodiversity, have been highlighted in recent studies. For instance, Stojanović et al. [63] discussed the distribution of two trichopteran species, emphasizing that existing protected areas may not adequately support the conservation of freshwater ecosystems. This example underscores the broader issue of whether current management practices and protected areas are sufficient for preserving freshwater biodiversity, which is also crucial for sustaining trout populations. In Serbia, other significant threats to brown trout populations include habitat degradation and fragmentation, which disrupt essential ecological processes. These threats diminish suitable habitats and impede the movement of trout between critical spawning and feeding areas. However, restoring natural connectivity along river systems is a promising solution. It is crucial to help re-establish the natural diversity patterns in brown trout as observed in salmon populations [64]. Such restoration efforts would likely have broader ecological benefits, positively impacting other freshwater species, including fish and invertebrates, such as crayfish.
Considering all factors, based on the ESHIPPOsalmo model, the first priority for protection must be assigned to nine populations with a critically low degree of sustainability. The 20 populations with a low level of sustainability should be given the second priority for protection within Serbia (see Table 5). On the other hand, for management purposes, it may be more effective to prioritize conservation efforts towards the populations that are already preserved both genetically and in terms of habitat quality, aiming to further enhance their sustainability to a high level (the 17 populations with moderate sustainability). The current state of the analyzed populations indicates that sustainable use must be implemented in a planned and selective manner. The potential for sustainable use within Serbia is most evident in the Aegean basin, particularly in the Struma River basin. The populations there are autochthonous and the watershed stands out for its high productivity, with registered finds of large individuals that are attractive for fishing (Table 5; [31]). Once the analyzed populations stabilize and habitat conditions improve, reducing the impact of factors threatening population diversity, sustainable use at the current level should be reconsidered and adapted to the specificities of the population. Conversely, populations in lower river reaches which are at least partially introgressed with allochthonous trout (e.g., Gradac, Veliki Rzav, Sokobanjska Moravica, Visočica, and Mlava rivers, Table 5) may be commercially valuable and attractive for fishing under-regulated practices. Based on the state of brown trout populations in Serbia, conservation efforts must focus on implementing measures necessary to preserve habitat, abundance, population structure, and genetic diversity. These activities require consistent implementation in future conservation and management practices.
4.2. Utility of ESHIPPOsalmo Model for Fisheries Management Guidelines
This study provides insights into the sustainability assessment of brown trout populations in Serbia and offers a practical framework for addressing similar management issues of salmonid waters worldwide, as well as of other organisms threatened by hybridization. Conservation decision frameworks are essential tools used by practitioners worldwide to guide actions and achieve conservation goals in specific projects [65,66]. Resource management agencies and non-governmental organizations widely adopt these frameworks, such as the Open Standards for the Practice of Conservation (CMP, [67]), which involve identifying conservation targets, assessing threats, and prioritizing actions with continuous monitoring to meet conservation objectives [68]. The brown trout is uniquely placed to inform freshwater fisheries and river ecosystem management in the 21st century. As a popular sport fish reliant on good water quality and functioning river ecosystems, brown trout enjoy enduring interest from anglers, the public, and government agencies [69]. Therefore, the model developed in this study of brown trout is an example of applying these standards. It includes detailed assessments of habitat requirements, threats from environmental changes and human activities, and prioritized conservation actions that may be applied in different parts of the brown trout geographical range. Moreover, continuous monitoring is a crucial component of this model, allowing for adjustments based on new data and changing conditions to ensure the long-term sustainability of brown trout populations.
Inland fisheries management in much of continental Europe is largely private, highly fragmented in spatial terms, and characterized by a heavy reliance on stocking programs [70,71]. Therefore, in the case of brown trout, any future breeding program strategy should aim to establish regional broodstocks in order to mitigate the effect of stocking with non-native individuals. This would also reduce instances of genetic homogenization, the risk of losing local adaptations, and the risk of replacing the recipient’s genetic background [72].
In the example of Serbia, it is evident that national legislation should be improved so that genetic differences between populations should also be taken into account for the effective conservation of brown trout. Moreover, effective population management includes measures for habitat preservation and monitoring population abundance, structure, and diversity [12,73]. Therefore, separate management of brown trout populations, based on regular genetic monitoring, should become a regular practice for better biodiversity protection. Although the use of genetic markers in fisheries is perceived as costly, their absence could be even more damaging in environmental, financial, and socio-economic terms.
Based on the experience gained in this work and leveraging 20 years of data [42] on brown trout populations (Table 5), several points should be taken into account for future studies and further implementation and improvement of the model. In addition to mtDNA, it is crucial to use markers that are inherited not uniparentally to assess the autochthony and genetic structure of populations. While the genetic results obtained provide valuable insights, the reliance on mtDNA alone has certain limitations. Mitochondrial DNA represents only the maternal lineage; therefore, including nuclear markers (e.g., microsatellites) inherited from both parents could offer a more comprehensive view of the genetic structure and origin of populations (wild versus domesticated). Microsatellites are highly polymorphic markers useful for examining genetic diversity, population structure, gene flow, and identifying genetic differentiation among populations [74]. Combining mtDNA and microsatellite or other more advanced nuclear markers would allow a more in-depth detection of introgression with allochthonous populations, offering a more comprehensive understanding of genetic dynamics and contributing to a better interpretation of results in the context of conservation and biodiversity management.
The ILSFP element may also be further improved. It is anticipated that the abundance of juveniles is primarily influenced by female fecundity, whereas the abundance of adult individuals is likely more dependent on habitat availability. Specifically, the size of the stream or river may play a crucial role due to competition for territory and the presence of a greater number of microhabitat refuges during periods of drought and thermal stress. For future studies, it may be more suitable to monitor the abundance of juvenile and adult individuals separately and correlate it to the stream size.
5. Conclusions
The conservation of species such as brown trout is layered and very complex. Therefore, the ESHIPPOsalmo model was conceived to introduce a systematic approach to biodiversity conservation and it represents an innovative approach that incorporates different aspects of brown trout research. Additionally, the obtained results that point out the most threatening factors for the species’ sustainability enable further improvement of the model in future studies.
It is also important to imply that machine learning techniques inherently rely on the quality and completeness of the input data. As a result, any deficiencies in the data, such as missing values or biases, can significantly impact the accuracy and generalizability of the model’s predictions, which need to be considered for any planned future studies.
This study underscores the dire consequences of fisheries management that still heavily relies on stocking programs and the urgent need for distinct management of each brown trout population in the central Balkans. Regular genetic monitoring needs to be a standard practice for biodiversity conservation. This call to action is urgent for their survival. The unfavorable state of trout populations is further threatened by the fact that adverse anthropogenic activities will intensify due to ongoing climate changes. This imposes the need for more intensive attention and urgent undertaking of concrete measures for species conservation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9110423/s1. Table S1: Sample locations, and geographical coordinates of brown trout populations. Sample sizes (N) and frequency distribution of brown trout mitochondrial DNA haplotypes in Serbia are retrieved from Veličković et al. [11]. GenBank Accession numbers of the haplotypes are presented in parentheses.
Author Contributions
Conceptualization, V.S. and T.V.; data analysis, D.S., M.R. and T.V.; writing—original draft preparation, T.V.; writing—review and editing, T.V., S.M., D.S., A.M., M.R., R.Š., J.V., S.Đ., N.K., M.J. and V.S.; supervision, S.M., D.S. and V.S.; funding acquisition, R.Š., J.V. and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
T.V., S.M. and V.S. were supported by the Ministry of Education (T.V. and V.S., grant no. 451-03-65/2024-03/200122; S.M., grant no. 451-03-65/2024-03/200178). A.M., M.R., S.Đ., N.K., and M.J. were supported by the Serbian Ministry of Science, Technological Development, and Innovation (A.M., grant no. 451-03-66/2024-03/200378; M.R., S.Đ., N.K., and M.J., grant no. 451-03-66/2024-03/200122). The study was co-financed by the Slovenian Research Agency (D.S., research program no. P1-0255 and research project no. J1-3015). R.Š. was supported by the Ministry of Culture of the Czech Republic (DKRVO 2024–2028/6. I.a, National Museum, 00023272), J.V. by institutional resources of the Ministry of Education, Youth, and Sports of the Czech Republic.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated and analyzed during this study are presented in this paper and in the Supplementary Materials. A part of the data is also available at http://baes.pmf.kg.ac.rs.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Behnke, R.J. Brown trout. Trout 1986, 27, 42–47. [Google Scholar]
- Baric, S.; Riedl, A.; Meraner, A.; Medgyesy, N.; Lackner, R.; Pelster, B.; Via, J.D. Alpine headwater streams as reservoirs of remnant populations of the Danubian clade of brown trout. Freshw. Biol. 2010, 55, 866–880. [Google Scholar] [CrossRef]
- Lobón-Cerviá, J. Introduction: Princess of the streams: The brown trout Salmo trutta L. as aquatic royalty. In Brown Trout: Biology, Ecology and Management; Lobón-Cerviá, J., Sanz, N., Eds.; Wiley: New York, NY, USA, 2018; pp. 1–13. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland, 2007; p. 646. [Google Scholar]
- Simonović, P.; Marić, S.; Nikolić, V. Trout Salmo spp. complex in Serbia and adjacent regions of the western Balkans: Reconstruction of evolutionary history from external morphology. J. Fish Biol. 2007, 70, 359–380. [Google Scholar] [CrossRef]
- Apostolidis, A.P.; Stoumboudi, M.T.; Kalogianni, E.; Cote, G.; Bernatchez, L. Genetic divergence among native trout Salmo trutta populations from southern Balkans based on mitochondrial DNA and microsatellite variation. J. Fish Biol. 2011, 79, 1950–1960. [Google Scholar] [CrossRef]
- Snoj, A.; Marić, S.; Sušnik Bajec, S.; Berrebi, P.; Janjani, S.; Schöffmann, J. Phylogeographic structure and demographic patterns of brown trout in North-West Africa. Mol. Phylogenet. Evol. 2011, 61, 203–211. [Google Scholar] [CrossRef]
- Snoj, A.; Bravničar, J.; Marić, S.; Sušnik Bajec, S.; Benaissa, H.; Schöffmann, J. Nuclear dna reveals multiple waves of colonisation, reticulate evolution and a large impact of stocking on trout in north-west Africa. Hydrobiologia 2021, 848, 3389–3405. [Google Scholar] [CrossRef]
- Berrebi, P.; Tougard, C.; Dubois, S.; Shao, Z.; Koutseri, I.; Petkovski, S.; Crivelli, A.J. Genetic diversity and conservation of the Prespa trout in the Balkans. Int. J. Mol. Sci. 2013, 14, 23454–23470. [Google Scholar] [CrossRef]
- Sanz, N. Phylogeographic history of brown trout: A review. In Brown Trout: Biology, Ecology and Management; Lobón-Cerviá, J., Sanz, N., Eds.; Wiley: New York, NY, USA, 2018; pp. 15–63. [Google Scholar]
- Veličković, T.; Snoj, A.; Simić, V.; Šanda, R.; Vukić, J.; Barcytė, D.; Stanković, D.; Marić, S. A new perspective on the molecular dating of the brown trout complex with an extended phylogeographic information on the species in Serbia. Contrib. Zool. 2023, 92, 362–389. [Google Scholar] [CrossRef]
- Schöffmann, J.; Marić, S. Salmonid fish species—Opportunities for sustainable use under multiple pressures and current climatic change. In Ecological Sustainability of Fish Resources of Inland Waters of the Western Balkans. Freshwater Fish Stocks, Sustainable Use and Conservation; Simić, V., Simić, S., Pešić, V., Eds.; Springer: Berlin, Germany, 2024; pp. 375–410. [Google Scholar]
- Stevanović, M.P. Istorijska Geologija; Rudarsko–Geološki Fakultet, Univerzitet u Beogradu: Beograd, Serbia, 1982; p. 604. (In Serbian) [Google Scholar]
- Marić, S.; Sušnik, S.; Simonović, P.; Snoj, A. Phylogeographic study of brown trout from Serbia, based on mitochondrial DNA control region analysis. Genet. Sel. Evol. 2006, 38, 411–430. [Google Scholar] [CrossRef][Green Version]
- Tošić, A.; Škraba, D.; Nikolić, V.; Mrdak, D.; Simonović, P. New mitochondrial DNA haplotype of brown trout Salmo trutta L. from Crni Timok drainage area in Serbia. Turk. J. Fish. Aquat. Sci. 2014, 14, 37–42. [Google Scholar]
- Tošić, A.; Škraba, D.; Nikolić, V.; Čanak Atlagić, J.; Mrdak, D.; Simonović, P. Haplotype diversity of brown trout Salmo trutta (L.) in the broader Iron Gate area. Turk. J. Zool. 2016, 40, 655–662. [Google Scholar] [CrossRef]
- Simonović, P.; Tošić, A.; Škraba Jurlina, D.; Nikolić, V.; Piria, M.; Tomljanović, T.; Šprem, N.; Mrdak, D.; Milošević, D.; Bećiraj, A.; et al. Diversity of brown trout Salmo cf. trutta in the River Danube basin of western Balkans as assessed from the structure of their mitochondrial control region haplotypes. J. Ichthyol. 2017, 57, 603–616. [Google Scholar]
- Kanjuh, T.; Tomić, S.; Marić, A.; Škraba Jurlina, D.; Nikolić, V.; Simonović, P. Trout Salmo spp. (Salmoniformes: Salmonidae) molecular diversity in streams on the southern slopes of the Stara Planina Mts. in Serbia. Acta Zool. Bulg. 2021, 7, 425–429. [Google Scholar]
- Marić, S. Evolucijska Istorija Kompleksa Potočne Pastrmke Salmo trutta L. 1758 na Području Republike Srbije i Značaj za Ribarstvo. Ph.D. Thesis, Biološki Fakultet, Univerzitet u Beogradu, Beograd, Srbija, 2005. (In Serbian). [Google Scholar]
- Veličković, T. Koncipiranje Modela za Održivo Korišćenje Populacija Kompleksa Potočne Pastrmke (Salmo spp.) na Području Srbije. Ph.D. Thesis, Prirodno-matematički Fakultet, Univerzitet u Kragujevcu, Kragujevac, Srbija, 2023. (In Serbian). [Google Scholar]
- Allendorf, F.W. Conservation biology of fishes. Conserv. Biol. 1988, 2, 145–148. [Google Scholar] [CrossRef]
- Laikre, L.; Ryman, N. Effects on intraspecific biodiversity from harvesting and enhancing natural populations. Ambio 1996, 25, 504–509. [Google Scholar]
- Laikre, L.; Antunes, A.; Apostolidis, A.; Berrebi, P.; Dugid, A.; Ferguson, A.; García-Marín, J.L.; Guyomard, R.; Hansen, M.M.; Hindar, K.; et al. Conservation Genetic Management of Brown Trout (Salmo trutta) in Europe; Report by the concerted action on identification, management and exploitation of genetic resources in the brown trout (Salmo trutta), ”TROUTCONCERT”; EU FAIR CT97–3882. Silkeborg, Danmarks fiskeri undersrgelser; Stockholm University: Stockholm, Sweden, 1999. [Google Scholar]
- Berrebi, P.; Horvath, Á.; Splendiani, A.; Palm, S.; Bernaś, R. Genetic diversity of domestic brown trout stocks in Europe. Aquaculture 2021, 544, 737043. [Google Scholar] [CrossRef]
- Araguas, R.M.; Sanz, N.; Fernandez, R.; Utter, F.M.; Pla, C.; García-Marín, J.L. Genetic refuges for a self-sustained fishery: Experience in wild brown trout populations in the eastern Pyrenees. Ecol. Freshw. Fish 2008, 17, 610–616. [Google Scholar] [CrossRef]
- Berrebi, P.; Caputo Barucchi, V.; Splendiani, A.; Muracciole, S.; Sabatini, A.; Palmas, F.; Tougard, C.; Arculeo, M.; Marić, S. Brown trout (Salmo trutta L.) high genetic diversity around the Tyrrhenian Sea as revealed by nuclear and mitochondrial markers. Hydrobiologia 2019, 826, 209–231. [Google Scholar] [CrossRef]
- Berrebi, P.; Marić, S.; Snoj, A.; Hasegawa, K. Brown trout in Japan− introduction history, distribution and genetic structure. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 18. [Google Scholar] [CrossRef]
- Stanković, D.; Crivelli, A.J.; Snoj, A. Rainbow Trout in Europe: Introduction, Naturalization, and Impacts. Rev. Fish. Sci. Aquac. 2015, 23, 39–71. [Google Scholar] [CrossRef]
- Piria, M.; Simonović, P.; Kalogianni, E.; Vardakas, L.; Koutsikos, N.; Zanella, D.; Ristovska, M.; Apostolou, A.; Adrović, A.; Mrdak, D.; et al. Alien freshwater fish species in the Balkans—Vectors and pathways of introduction. Fish Fish. 2018, 19, 138–169. [Google Scholar] [CrossRef]
- Marić, S.; Stanković, D.; Sušnik Bajec, S.; Vukić, J.; Šanda, R.; Stefanov, T.; Nikolić, D.; Snoj, A. Perils of brown trout (Salmo spp.) mitigation-driven translocations: A case study from the Vlasina Plateau, Southeast Serbia. Biol. Invasions 2022, 24, 999–1016. [Google Scholar] [CrossRef]
- Simić, V.; Bănăduc, D.; Curtean-Bănăduc, A.; Petrović, A.; Veličković, T.; Stojković-Piperac, M.; Simić, S. Assessment of the ecological sustainability of river basins based on the modified the ESHIPPO fish model on the example of the Velika Morava basin (Serbia, Central Balkans). Front. Environ. Sci. 2022, 10, 952692. [Google Scholar] [CrossRef]
- Muhlfeld, C.C.; Dauwalter, D.C.; D’Angelo, V.S.; Ferguson, A.; Giersch, J.J.; Impson, D.; Koizumi, I.; Kovach, R.; McGinnity, P.; Schöffmann, J.; et al. Global status of trout and char: Conservation challenges in the twenty-first century. In Trout and Char of the World; Kershner, J., Williams, J., Gresswell, R., Lobón-Cerviá, J., Eds.; American Fisheries Society: Bethesda, MD, USA, 2019; pp. 717–760. [Google Scholar]
- Simić, V.; Simić, S.; Paunović, M.; Cakić, P. Model of the assessment of the critical risk of extinction and the priorities of protection of endangered aquatic species at the national level. Biodivers. Conserv. 2007, 16, 2471–2493. [Google Scholar] [CrossRef]
- Simić, V.; Simić, S.; Stojković-Piperac, M.; Petrović, A.; Milošević, D. Commercial fish species of inland waters: A model for sustainability assessment and management. Sci. Total Environ. 2014, 497, 642–650. [Google Scholar] [CrossRef]
- Simić, V.; Maguire, I.; Rajković, M.; Petrović, A. Conservation strategy for the endangered crayfish species of the family Astacidae: The ESHIPPO crayfish model. Hydrobiologia 2015, 760, 1–13. [Google Scholar] [CrossRef]
- Simić, V.; Simić, S.; Paunović, M.; Radojković, N.; Petrović, A.; Talevski, T.; Milošević, D. The Alburnus benthopelagic fish species of the Western Balkan Peninsula: An assessment of their sustainable use. Sci. Total Environ. 2016, 540, 410–417. [Google Scholar] [CrossRef]
- Fisher, O.D.; Owens, P.F.I. The comparative method in conservation biology. Trends Ecol. Evol. 2004, 9, 391–398. [Google Scholar] [CrossRef]
- Brennan, S.; Withgott, J. Biodiversity and conservation biology. In Environment; The Science behind the Stories; Pearson, Benjamin-Cummings: San Francisco, CA, USA, 2005. [Google Scholar]
- SRPS EN 14011; Water Quality—Sampling Fish Using Electricity. Institute for Standardization of Serbia: Belgrade, Serbia, 2008.
- Chapman, D.W. Production. In Methods for Assessment of Fish Production in Fresh Waters; Ricker, W.E., Ed.; Blackwell Scientific Publications: Oxford, UK; Edinburgh, UK, , 1971; pp. 199–214. [Google Scholar]
- Lager, K.; Huet, M. Dynamics of Fish Populations in Inland Waters; Academic Press: London, UK, 1964. [Google Scholar]
- Simić, V.; Simić, S.; Petrović, A.; Šorić, V.; Paunović, M.; Dimitrijević, V. Database: Biodiversity in Aquatic Ecosystems in Serbia (Ex Situ Conservation) BAES Ex Situ. 2006. Available online: http://baes.pmf.kg.ac.rs (accessed on 12 April 2023).
- McInnes, L.; Healy, J.; Melville, J. UMAP: Uniform manifold approximation and projection for dimension reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2010, 129, 271–280. [Google Scholar] [CrossRef]
- Milošević, D.; Medeiros, A.; Stojković-Piperac, M.; Cvijanović, D.; Soininen, J.; Milosavljević, A.; Predić, B. The application of Uniform Manifold Approximation and Projection (UMAP) for unconstrained ordination and classification of biological indicators in aquatic ecology. Sci. Total Environ. 2022, 815, 152365. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, M.; Nikolić, M.; Kojadinović, N.; Ðuretanović, S.; Radenković, M.; Veličković, T.; Simić, V. Population characteristics of spirlin Alburnoides bipunctatus (Bloch, 1782) in Serbia (Central Balkans): Implications for conservation. Diversity 2023, 15, 616. [Google Scholar] [CrossRef]
- Xu, B.; Li, J.; Pei, X.; Bian, L.; Zhang, T.; Yi, G.; Bie, X.; Peng, P. Dominance of topography on vegetation dynamics in the Mt. Qomolangma National Nature Reserve: A UMAP and PLS-SEM Analysis. Forests 2023, 14, 1415. [Google Scholar] [CrossRef]
- Hall, M.A. Correlation-Based Feature Subset Selection for Machine Learning. Ph.D. Thesis, University of Waikato, Hamilton, New Zealand, 1998. [Google Scholar]
- Arrighi, C.; Castelli, F. Prediction of ecological status of surface water bodies with supervised machine learning classifiers. Sci. Total Environ. 2023, 857, 159655. [Google Scholar] [CrossRef]
- Jakovljević, M.; Đuretanović, S.; Kojadinović, N.; Nikolić, M.; Petrović, A.; Simović, P.; Simić, V. Assessing spirlin Alburnoides bipunctatus (Bloch, 1782) as an early indicator of climate change and anthropogenic stressors using ecological modeling and machine learning. Sci. Total Environ. 2024, 951, 175723. [Google Scholar] [CrossRef]
- Diaz-Papkovich, A.; Anderson-Trocmé, L.; Gravel, S. A review of UMAP in population genetics. J. Hum. Genet. 2021, 68, 85–91. [Google Scholar] [CrossRef]
- Simić, V.; Miljanović, B.; Petrović, A.; Radenković, M.; Stojković-Piperac, M.; Veličković, T.; Jakovljević, M.; Simić, S. Inland fisheries in Serbia: Historical aspect, fish resources, management and conservation. In Ecological Sustainability of Fish Resources of Inland Waters of the Western Balkans. Freshwater Fish Stocks, Sustainable Use and Conservation; Simić, V., Simić, S., Pešić, V., Eds.; Springer: Berlin, Germany, 2024; pp. 113–200. [Google Scholar]
- Owen, C.L.; Bracken-Grissom, H.; Stern, D.; Crandall, K.A. A synthetic phylogeny of freshwater crayfish: Insights for conservation. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140009. [Google Scholar] [CrossRef]
- Marić, S.; Sušnik Bajec, S.; Schöffmann, J.; Kostov, V.; Snoj, A. Phylogeography of stream-dwelling trout in the Republic of Macedonia and a molecular genetic basis for revision of the taxonomy proposed by S. Karaman. Hydrobiologia 2017, 785, 249–260. [Google Scholar] [CrossRef]
- Bonin, A.; Nicole, F.; Pompanon, F.; Miaud, C.; Taberlet, P. Population adaptive index: A new method to help measure intraspecific genetic diversity and prioritize populations for conservation. Conserv. Biol. 2007, 21, 697–708. [Google Scholar] [CrossRef]
- Lobón-Cerviá, J.; Elvira, B.; Rincón, P.A. Historical changes in the fish fauna of the River Duero basin. In Historical Change of Large Alluvial Rivers: Western Europe; Petts, G.E., Moller, H., Roux, A.L., Eds.; John Wiley & Sons: Chichester, UK, 1989; pp. 221–232. [Google Scholar]
- De Sostoa, A.; Lobón-Cerviá, J. Fish and fisheries of the River Ebro: Actual state and recent history. In Historical Change of Large Alluvial Rivers: Western Europe; Petts, G.E., Moller, H., Roux, A.L., Eds.; John Wiley & Sons: Chichester, UK, 1989; pp. 233–247. [Google Scholar]
- Vøllestad, L.A.; Hesthagen, T. Stocking of freshwater fish in Norway: Management goals and effects. Nord. J. Freshw. Res. 2001, 75, 143–152. [Google Scholar]
- Splendiani, A.; Ruggeri, P.; Giovannotti, M.; Caputo Barucchi, V. Role of environmental factors in the spread of domestic trout in Mediterranean streams. Freshw. Biol. 2013, 58, 2089–2101. [Google Scholar] [CrossRef]
- Splendiani, A.; Ruggeri, P.; Giovannotti, M.; Pesaresi, S.; Occhipinti, G.; Fioravanti, T.; Lorenzoni, M.; Nisi Cerioni, P.; Caputo Barucchi, V. Alien brown trout invasion of the Italian peninsula: The role of geological, climate and anthropogenic factors. Biol. Invasions 2016, 18, 2029–2044. [Google Scholar] [CrossRef]
- Splendiani, A.; Giovannotti, M.; Righi, T.; Fioravanti, T.; Cerioni, P.N.; Lorenzoni, M.; Carosi, A.; La Porta, G.; Barucchi, V.C. Introgression despite protection: The case of native brown trout in Natura 2000 network in Italy. Conserv. Genet. 2019, 20, 343–356. [Google Scholar] [CrossRef]
- Vera, M.; Aparicio, E.; Heras, S.; Abras, A.; Casanova, A.; Roldán, M.I.; García-Marin, J.L. Regional environmental and climatic concerns on preserving native gene pools of a least concern species: Brown trout lineages in Mediterranean streams. Sci. Total Environ. 2023, 862, 160739. [Google Scholar] [CrossRef]
- Stojanović, K.; Milić, D.; Ranković Perišić, M.; Miličić, M.; Živić, I. Destiny of two caddisfly species under global climate change. Diversity 2023, 15, 995. [Google Scholar] [CrossRef]
- Perrier, C.; Le Gentil, J.; Ravigne, V.; Gaudin, P.; Salvado, J.C. Origins and genetic diversity among Atlantic salmon recolonizing upstream areas of a large South European river following restoration of connectivity and stocking. Conserv. Genet. 2014, 15, 1095–1109. [Google Scholar] [CrossRef]
- Schwartz, M.; Cook, C.; Pressey, R.; Pullin, A.; Runge, M.; Salafsky, N.; Sutherland, W.; Williamson, M. Decision support frameworks and tools for conservation. Conserv. Lett. 2018, 11, e12385. [Google Scholar] [CrossRef]
- Zin, T.T.; Morimoto, T.; Suanyuk, N.; Itami, T.; Tantikitti, C. Image technology based detection of infected shrimp in adverse environments. Songklanakarin J. Sci. Technol. 2022, 44, 112–118. [Google Scholar]
- Conservation Measures Partnership. The Open Standards for the Practice of Conservation. Ver. 3.0. 2013. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.iai.int/admin/site/sites/default/files/uploads/2015/08/CMP_Open_Standards_Version_3.0_April_2013.pdf&ved=2ahUKEwi_quGQkKGJAxWxj68BHRiGJT4QFnoECBkQAQ&usg=AOvVaw28-9_AGuEYhdOXYW1_u8Iq (accessed on 16 April 2024).
- Bernos, T.A.; Jeffries, K.M.; Mandrak, N.E. Linking genomics and fish conservation decision making: A review. Rev. Fish Biol. Fish. 2020, 30, 587–604. [Google Scholar] [CrossRef]
- Young, K.A.; Gaskell, P.; Jacklin, T.; Williams, J.E. Brown trout management for the 21st century. In Brown Trout: Biology, Ecology and Management; Lobón-Cerviá, J., Sanz, N., Eds.; Wiley: New York, NY, USA, 2018; pp. 735–769. [Google Scholar]
- Pinter, K.; Epifanio, J.; Unfer, G. Release of hatchery-reared brown trout (Salmo trutta) as a threat to wild populations? A case study from Austria. Fish. Res. 2019, 219, 105296. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Riepe, C.; Theis, S.; Pagel, T.; Fujitani, M. Dysfunctional information feedbacks cause the emergence of management panaceas in social-ecological systems: The case of fish stocking in inland recreational fisheries. J. Outdoor Recreat. Tour. 2022, 38, 100475. [Google Scholar] [CrossRef]
- Weeks, A.R.; Sgro, C.M.; Young, A.G.; Frankham, R.; Mitchell, N.J.; Miller, K.A.; Byrne, M.; Coates, D.J.; Eldridge, M.D.; Sunnucks, P.; et al. Assessing the benefits and risks of translocations in changing environments: A genetic perspective. Evol. Appl. 2011, 4, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Cowx, I.G. Stocking strategies. Fish. Manag. Ecol. 1994, 1, 15–30. [Google Scholar] [CrossRef]
- Wenne, R. Microsatellites as molecular markers with applications in exploitation and conservation of aquatic animal populations. Genes 2023, 14, 808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).