Long-Term Trends in Freshwater and Marine Growth Patterns in Three Sub-Arctic Atlantic Salmon Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
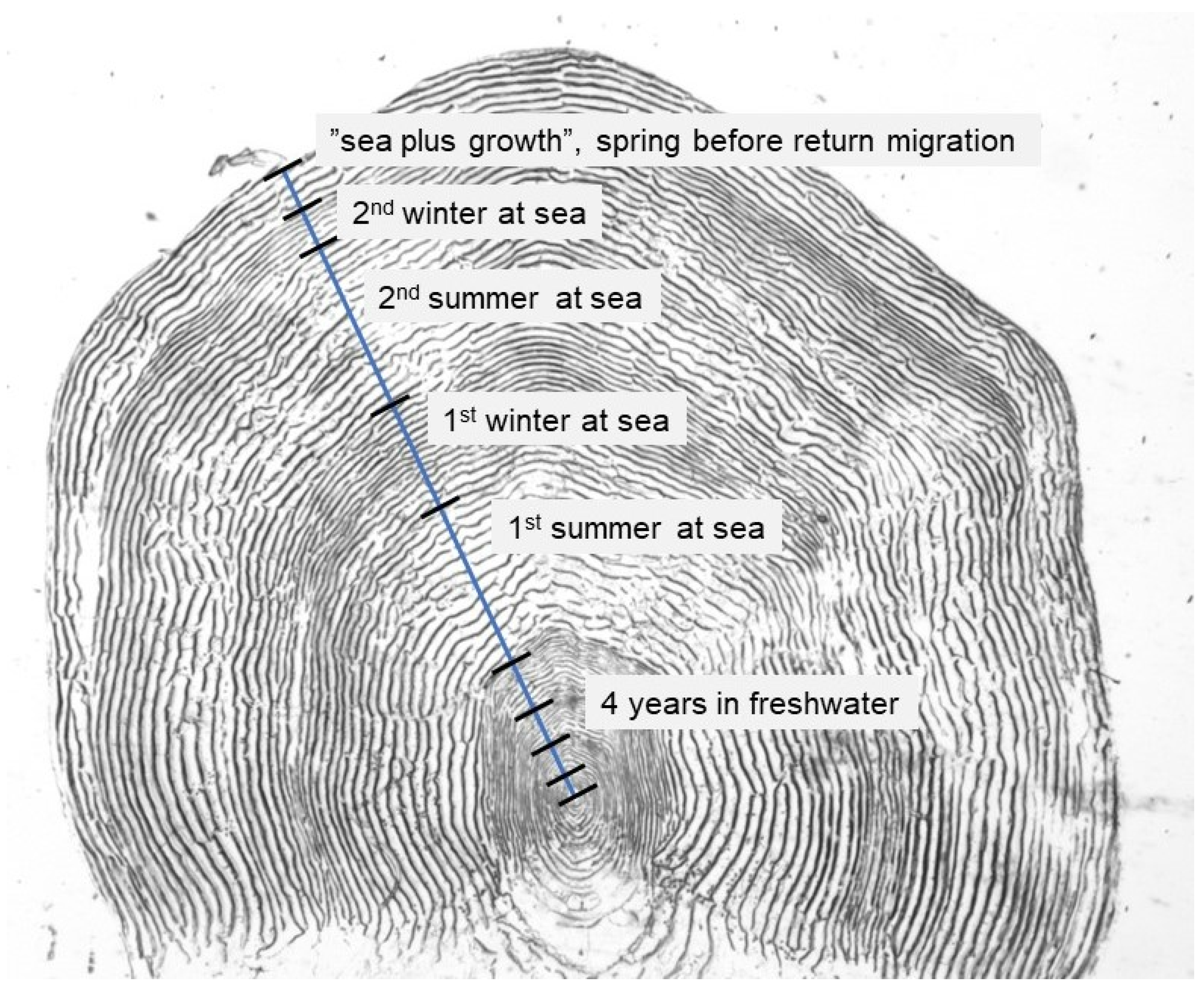
2.2. Scale Sampling and Analyses
2.3. Environmental Data and Curation
2.4. Statistical Analysis
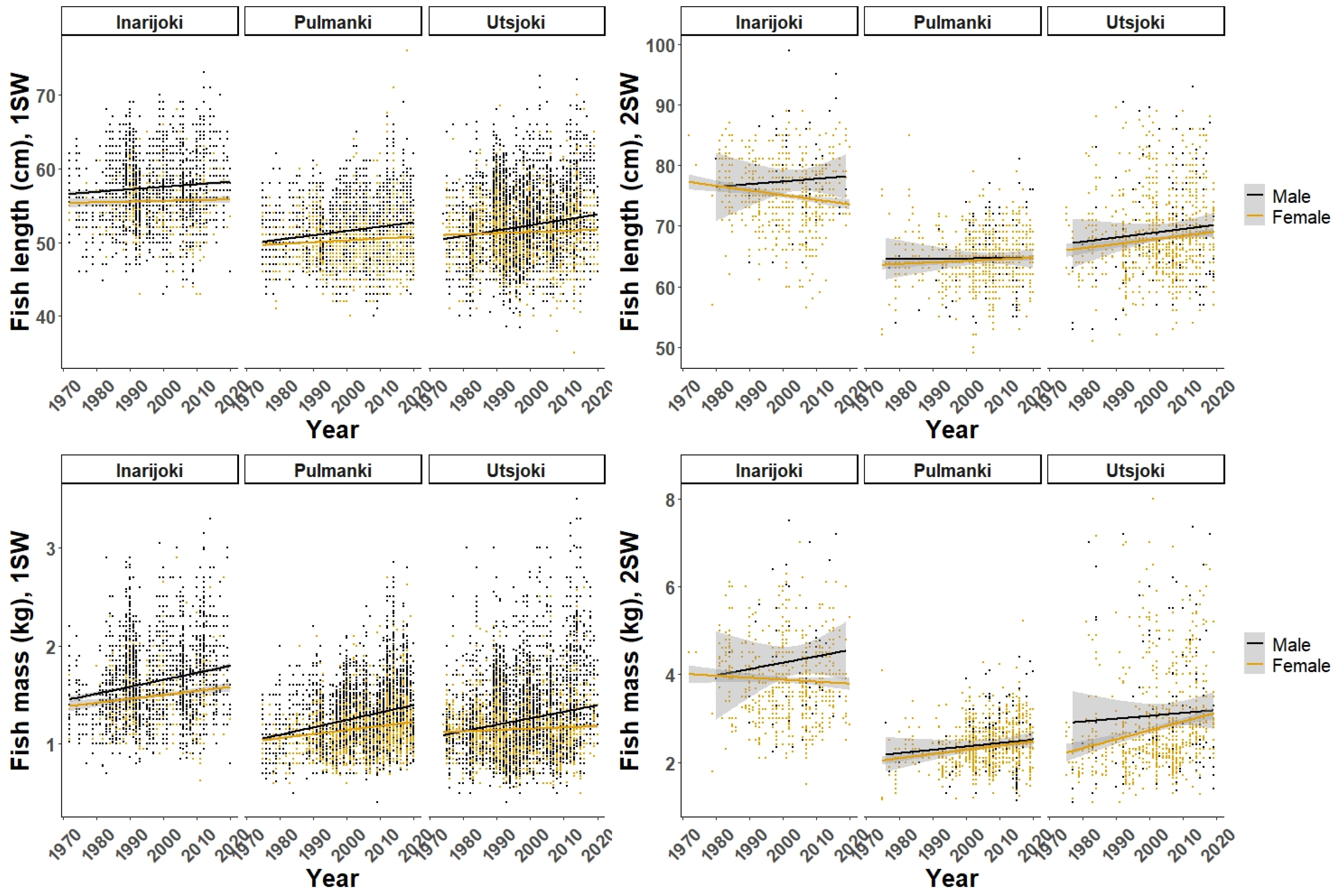
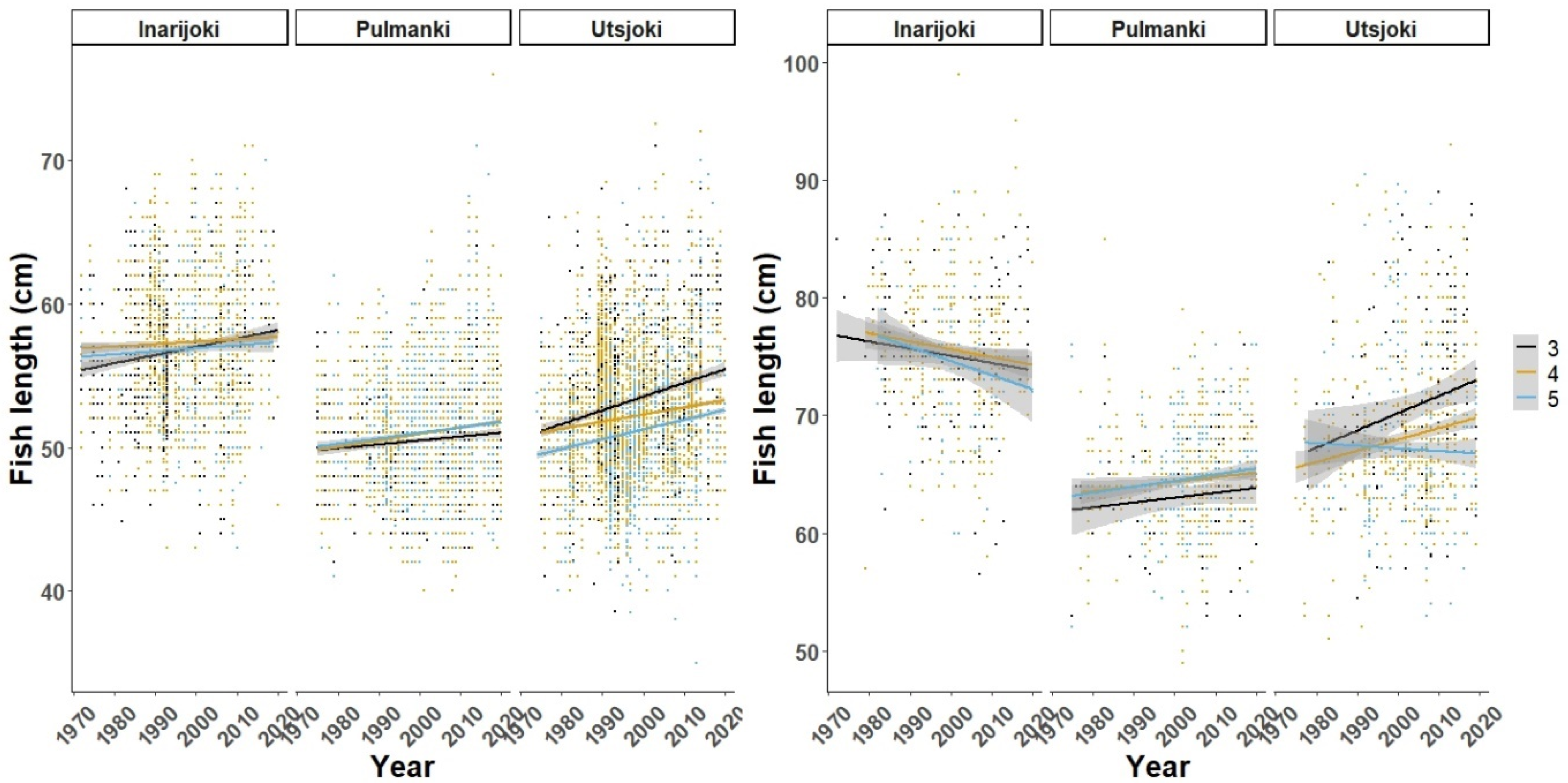
3. Results
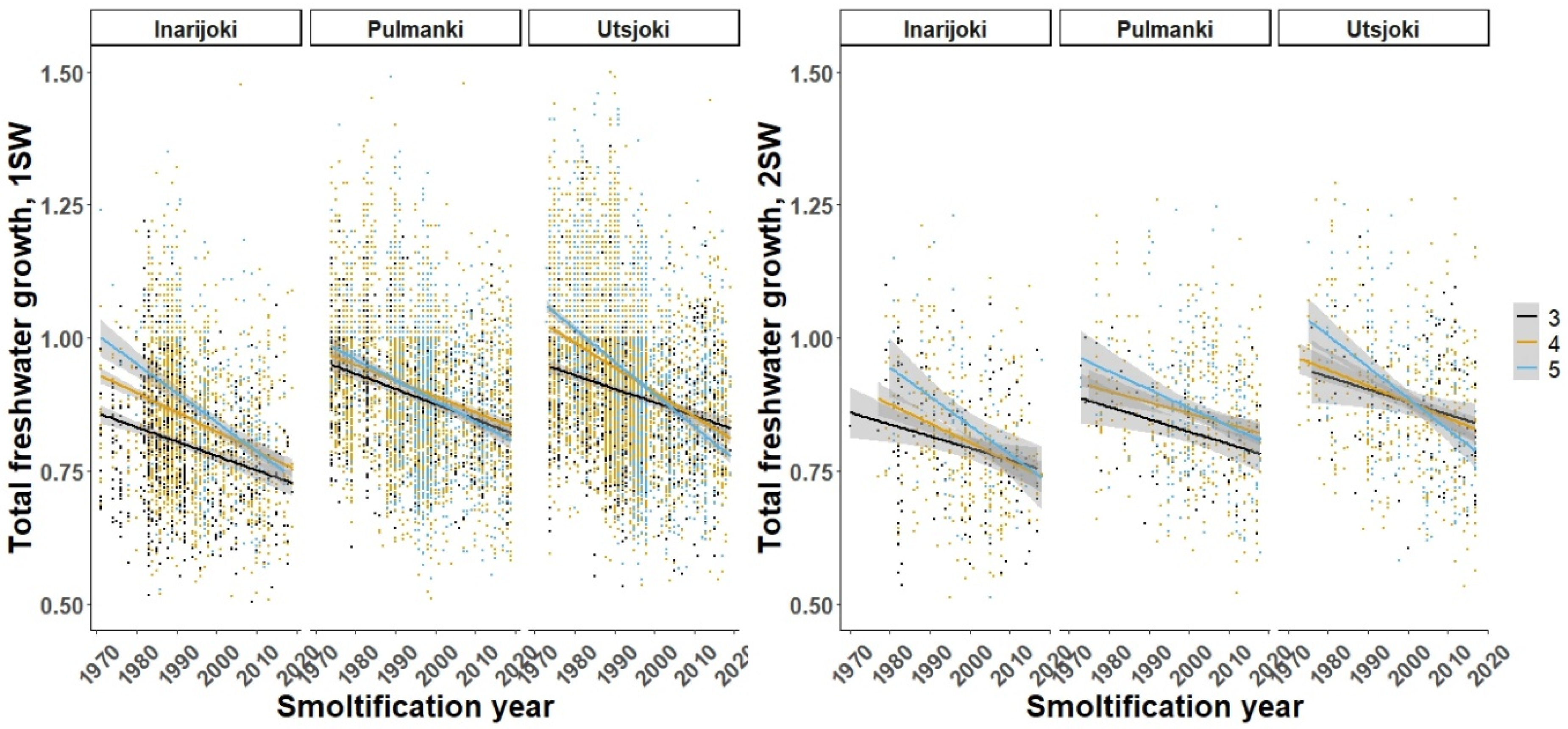
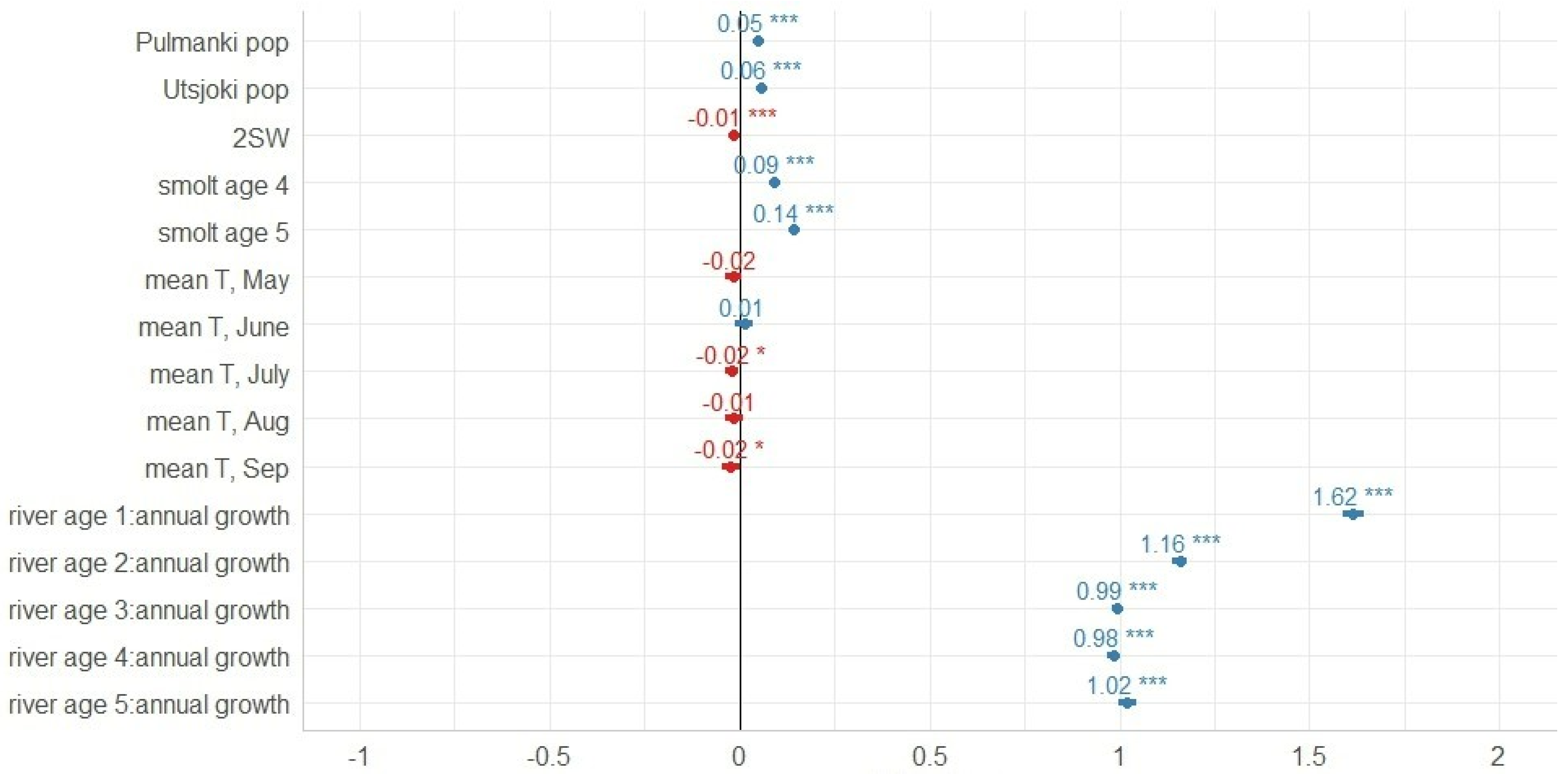
3.1. Freshwater Growth
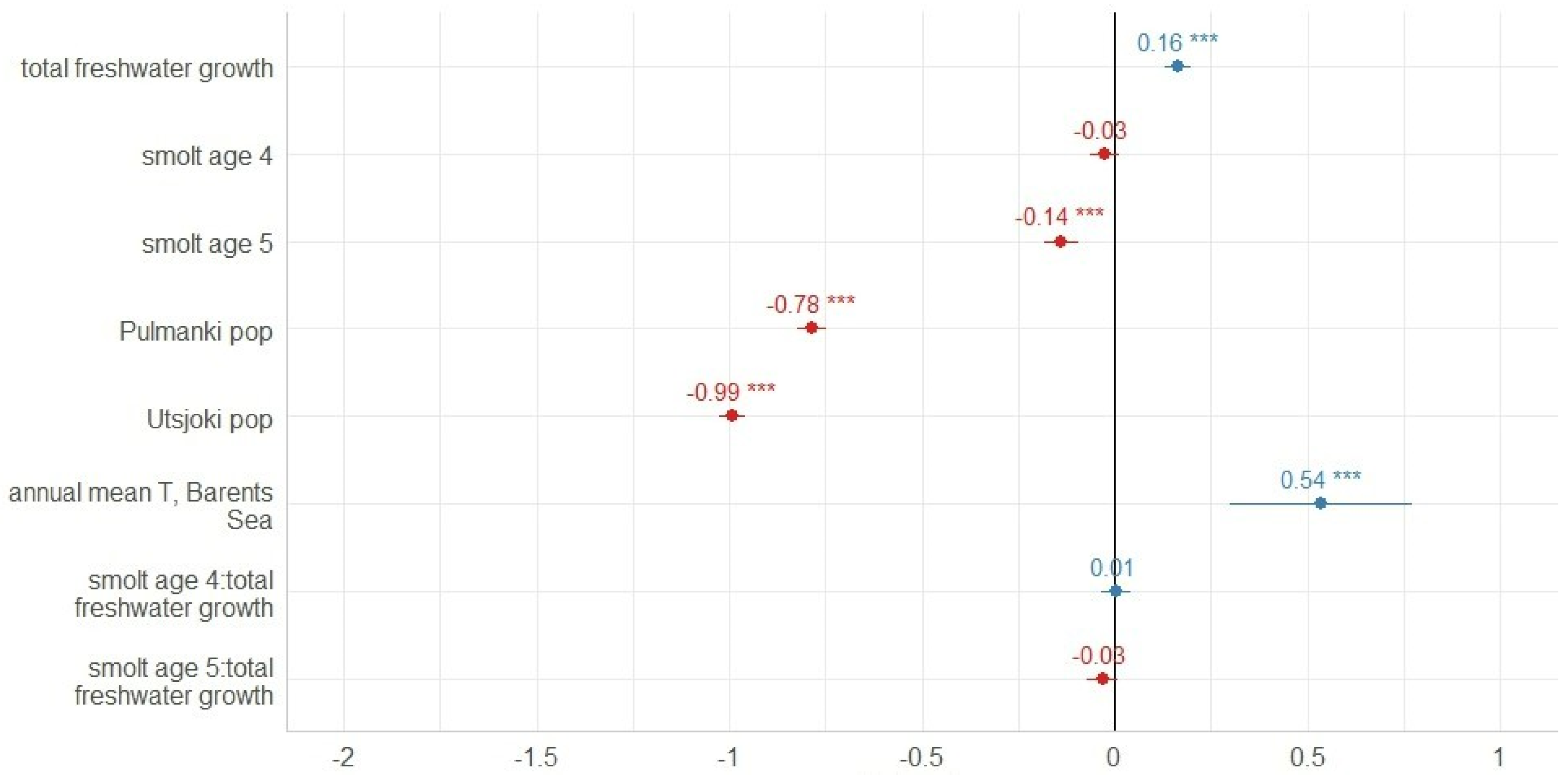
3.2. Marine Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rantanen, M.; Karpechko, A.Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K.; Vihma, T.; Laaksonen, A. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 2022, 3, 168. [Google Scholar] [CrossRef]
- Thomas, C.; Cameron, A.; Green, R.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; Visconti, P.; Butchart, S.H.M.; Watson, J.E.M.; Cassola, F.M.; Rondinini, C. Species’ traits influenced their response to recent climate change. Nat. Clim. Change 2017, 7, 205–208. [Google Scholar] [CrossRef]
- Beaugrand, G.; Kirby, R.R. How Do Marine Pelagic Species Respond to Climate Change? Theories and Observations. Annu. Rev. Mar. Sci. 2018, 10, 169–197. [Google Scholar] [CrossRef] [PubMed]
- Biggs, R.; Carpenter, S.R.; Brock, W.A. Turning back from the brink: Detecting an impending regime shift in time to avert it. Proc. Natl. Acad. Sci. USA 2009, 106, 826–831. [Google Scholar] [CrossRef]
- Beaugrand, G.; Edwards, M.; Brander, K.; Luczak, C.; Ibanez, F. Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic. Ecol. Lett. 2008, 11, 1157–1168. [Google Scholar] [CrossRef]
- Foden, W.B.; Young, B.E.; Akçakaya, H.R.; Garcia, R.A.; Hoffmann, A.A.; Stein, B.A.; Thomas, C.D.; Wheatley, C.J.; Bickford, D.; Carr, J.A.; et al. Climate change vulnerability assessment of species. WIREs Clim. Change 2019, 10, e551. [Google Scholar] [CrossRef]
- Radchuk, V.; Reed, T.; Teplitsky, C.; van de Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P.; et al. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 2019, 10, 3109. [Google Scholar] [CrossRef]
- Todd, C.D.; Friedland, K.D.; MacLean, J.C.; Hazon, N.; Jensen, A.J. Getting into hot water? Atlantic salmon responses to climate change in freshwater and marine environments. In Atlantic Salmon Ecology; Blackwell Publishing: Hoboken, NJ, USA, 2011; pp. 409–443. [Google Scholar]
- Klemetsen, A.; Amundsen, P.-A.; Dempson, J.B.; Jonsson, B.; Jonsson, N.; O’Connell, M.F.; Mortensen, E. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecol. Freshw. Fish 2003, 12, 1–59. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Ecology of Atlantic Salmon and Brown Trout. In Habitat as a Template for Life Histories; Fish & Fisheries Series; Springer: Berlin/Heidelberg, Germany, 2011; p. 708. [Google Scholar]
- Quinn, T.P. The Behavior and Ecology of Pacific Salmon and Trout; University of British Columbia Press: Vancouver, BC, Canada, 2018. [Google Scholar]
- Svenning, M.-A.; Falkegård, M.; Dempson, J.B.; Power, M.; Bårdsen, B.-J.; Gudbergsson, G.; Fauchald, P. Temporal changes in the relative abundance of anadromous Arctic charr, brown trout, and Atlantic salmon in northern Europe: Do they reflect changing climates? Freshw. Biol. 2022, 67, 64–77. [Google Scholar] [CrossRef]
- Gillis, C.-A.; Ouellet, V.; Breau, C.; Frechette, D.; Bergeron, N. Assessing climate change impacts on North American freshwater habitat of wild Atlantic salmon-urgent needs for collaborative research. Can. Water Resour. J. 2023, 48, 222–246. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Whoriskey, F.; Uglem, I.; Moore, A.; Rikardsen, A.H.; Finstad, B. A critical life stage of the Atlantic salmon Salmo salar: Behaviour and survival during the smolt and initial post-smolt migration. J. Fish Biol. 2012, 81, 500–542. [Google Scholar] [CrossRef]
- Otero, J.; L’Abée-Lund, J.H.; Castro-Santos, T.; Leonardsson, K.; Storvik, G.O.; Jonsson, B.; Dempson, B.; Russell, I.C.; Jensen, A.J.; Baglinière, J.-L.; et al. Basin-scale phenology and effects of climate variability on the global timing of initial seaward migration of Atlantic salmon (Salmo salar). Glob. Change Biol. 2014, 20, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Teichert, N.; Benitez, J.-P.; Dierckx, A.; Tétard, S.; de Oliveira, E.; Trancart, T.; Feunteun, E.; Ovidio, M. Development of an accurate model to predict the phenology of Atlantic salmon smolt spring migration. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1552–1565. [Google Scholar] [CrossRef]
- Vehanen, T.; Sutela, T.; Huusko, A. Potential impact of climate change on salmonid smolt ecology. Fishes 2023, 8, 332. [Google Scholar] [CrossRef]
- Mobley, K.B.; Aykanat, T.; Czorlich, Y.; House, A.; Kurko, J.; Miettinen, A.; Moustakas-Verho, J.; Salgado, A.; Sinclair-Waters, M.; Verta, J.-P.; et al. Maturation in Atlantic salmon (Salmo salar, Salmonidae): A synthesis of ecological, genetic, and molecular processes. Rev. Fish Biol. Fish. 2021, 31, 523–571. [Google Scholar] [CrossRef]
- Jonsson, N.; Hansen, L.P.; Jonsson, B. Variation in age, size and repeat spawning of adult Atlantic salmon in relation to river discharge. J. Anim. Ecol. 1991, 60, 937–947. [Google Scholar] [CrossRef]
- Otero, J.; Jensen, A.J.; L’Abée-Lund, J.H.; Stenseth, N.C.; Storvik, G.O.; Vøllestad, L.A. Contemporary ocean warming and freshwater conditions are related to later sea age at maturity in Atlantic salmon spawning in Norwegian rivers. Ecol. Evol. 2012, 2, 2192–2203. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.E.; Chaput, G. Spawning history influence on fecundity, egg size, and egg survival of Atlantic salmon (Salmo salar) from the Miramichi River, New Brunswick, Canada. ICES J. Mar. Sci. 2012, 69, 1678–1685. [Google Scholar] [CrossRef]
- Erkinaro, J.; Czorlich, Y.; Orell, P.; Kuusela, J.; Länsman, M.; Falkegård, M.; Pulkkinen, H.; Primmer, C.; Niemelä, E. Life history variation across four decades in a diverse population complex of Atlantic salmon in a large subarctic river. Can. J. Fish. Aquat. Sci. 2019, 76, 42–55. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Finstad, A.G. Effects of temperature and food quality on age at maturity of ectotherms: An experimental test of Atlantic salmon. J. Anim. Ecol. 2013, 82, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Czorlich, J.; Aykanat, T.; Erkinaro, J.; Orell, P.; Primmer, C.R. Rapid evolution in salmon life-history induced by direct and indirect effects of fishing. Science 2022, 376, 420–423. [Google Scholar] [CrossRef]
- Vähä, J.P.; Erkinaro, J.; Falkegård, M.; Orell, P.; Niemelä, E. Genetic stock identification of Atlantic salmon and its evaluation in a large population complex. Can. J. Fish. Aquat. Sci. 2017, 74, 327–338. [Google Scholar] [CrossRef]
- Niemelä, E.; Erkinaro, J.; Julkunen, M.; Hassinen, E.; Länsman, M.; Brørs, S. Temporal variation in abundance, return rate and life histories of previously spawned Atlantic salmon in a large subarctic river. J. Fish Biol. 2006, 68, 1222–1240. [Google Scholar] [CrossRef]
- Tana Monitoring and Research Group. Status of the River Tana Salmon Populations 2022. Report of the Working Group on Salmon Monitoring and Research in the Tana River System. Available online: http://urn.fi/URN:NBN:fi-fe202301306407 (accessed on 15 May 2023).
- Niemelä, E.; Orell, P.; Erkinaro, J.; Dempson, J.B.; Brørs, S.; Svenning, M.A.; Hassinen, E. Previously spawned Atlantic salmon ascend a large subarctic river earlier than their maiden counterparts. J. Fish Biol. 2006, 69, 1151–1163. [Google Scholar] [CrossRef]
- Vähä, J.-P.; Erkinaro, J.; Niemelä, E.; Primmer, C.R.; Saloniemi, I.; Johansen, M.; Svenningm, M.; Brørs, S. Temporally stable population-specific differences in run timing of one-sea-winter Atlantic salmon returning to a large river system. Evol. Appl. 2010, 4, 39–53. [Google Scholar] [CrossRef] [PubMed]
- ICES. Report of the Workshop on Age Determination of Salmon (WKADS); ICES Document CM 2011/ACOM:44; ICES: Galway, Ireland, 2011. [Google Scholar]
- Hanson, N.N.; Smith, G.W.; Middlemas, S.J.; Todd, C.D. Precision and accuracy of Dahl-Lea back-calculated smolt lengths from adult scales of Atlantic salmon Salmo salar. J. Fish Biol. 2019, 94, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, B.; Jonsson, M.; Jonsson, N. Optimal size at seaward migration in an anadromous salmonid. Mar. Ecol. Prog. Ser. 2016, 559, 193–200. [Google Scholar] [CrossRef]
- Gonzalez-Pola, C.; Larsen, K.M.H.; Fratantoni, P.; Beszczynska-Möller, A. ICES Report on ocean climate 2020. ICES Coop. Res. Rep. CRR 2022, 356, 121. [Google Scholar] [CrossRef]
- ICES. Capelin (Mallotus villosus) in Subareas 1 and 2 (Northeast Arctic), Excluding Division 2.a West of 5° W (Barents Sea Capelin). In ICES Advice: Recurrent Advice; ICES: Copenhagen, Denmark, 2021. [Google Scholar] [CrossRef]
- Weisberg, S.; Spangler, G.; Richmond, L.S. Mixed effects models for fish growth. Can. J. Fish. Aquat. Sci. 2010, 67, 269–277. [Google Scholar] [CrossRef]
- Erkinaro, H.; Erkinaro, J. Feeding of Atlantic salmon, Salmo salar L., parr in the subarctic River Teno and three tributaries, northernmost Finland. Ecol. Freshw. Fish 1998, 7, 13–24. [Google Scholar] [CrossRef]
- Corey, E.; Linnansaari, T.; Cunjak, R.A. High-temperature events shape the broadscale distribution of juvenile Atlantic salmon (Salmo salar). Freshw. Biol. 2023, 68, 534–545. [Google Scholar] [CrossRef]
- Jonsson, B.; Forseth, T.; Jensen, A.J.; Næsje, T.F. Thermal performance of juvenile Atlantic salmon, Salmo salar L. Funct. Ecol. 2001, 15, 701–711. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J. Fish Biol. 2009, 75, 2381–2447. [Google Scholar] [CrossRef]
- Breau, C.; Cunjak, R.A.; Bremset, G. Age-specific aggregation of wild juvenile Atlantic salmon Salmo salar at cool water sites during high-temperature events. J. Fish Biol. 2007, 71, 1179–1191. [Google Scholar] [CrossRef]
- Fry, F.E.J. The Effect of Environmental Factors on the Physiology of Fish. Fish Physiol. 1971, 6, 1–98. [Google Scholar] [CrossRef]
- Erkinaro, J.; Dempson, J.B.; Julkunen, M.; Niemelä, E. Importance of ontogenetic habitat shifts to juvenile output and life history of Atlantic salmon in a large subarctic river: An approach based on analysis of scale characteristics. J. Fish Biol. 1997, 51, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A. Evolution of Life Histories; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Mills, K.E.; Pershing, A.J.; Sheehan, T.F.; Mountain, D. Climate and ecosystem linkages explain widespread declines in North American Atlantic salmon populations. Glob. Change Biol. 2013, 19, 3046–3061. [Google Scholar] [CrossRef]
- Capuzzo, E.; Lynam, C.P.; Barry, J.; Stephens, D.; Forster, R.M.; Greenwood, N.; McQuatters-Gollop, A.; Silva, T.; van Leeuwen, S.M.; Engelhard, G.H. A decline in primary production in the North Sea over 25 years, associated with reductions in zooplankton abundance and fish stock recruitment. Glob. Change Biol. 2018, 24, e352–e364. [Google Scholar] [CrossRef] [PubMed]
- Vollset, K.W.; Urdal, K.; Utne, K.; Thorstad, E.B.; Fiske, P. Ecological regime shift in the Northeast Atlantic Ocean revealed from the unprecedented reduction in marine growth of Atlantic salmon. Sci. Adv. 2022, 8, eabk2542. [Google Scholar] [CrossRef]
- Pasanen, L.; Laukkanen-Nevala, P.; Launonen, I.; Prusov, S.; Holmström, L.; Niemelä, E.; Erkinaro, J. Extraction of sea temperature in the Barents Sea by a scale-space multiresolution method–prospects for Atlantic salmon. J. Appl. Stat. 2017, 44, 2317–2336. [Google Scholar] [CrossRef]
- Tréhin, C.; Rivot, E.; Lamireau, L.; Meslier, L.; Besnard, A.-L.; Gregory, S.D.; Nevoux, M. Growth during the first summer at sea modulates sex-specific maturation schedule in Atlantic salmon. Can. J. Fish. Aquat. Sci. 2021, 78, 659–669. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Albretsen, J. Environmental change influences the life history of salmon Salmo salar in the North Atlantic Ocean. J. Fish Biol. 2016, 88, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.D.; Hanson, N.N.; Boehme, L.; Revie, C.W.; Marques, A.R. Variation in post-smolt growth pattern of wild one sea-winter salmon (Salmo salar L.), and its linkage to surface warming in the eastern North Atlantic Ocean. J. Fish Biol. 2020, 98, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Min, S.-K.; Gillett, N.P.; Notz, D.; Malinina, E. Observationally-constrained projections of an ice-free Arctic even under a low emission scenario. Nat. Commun. 2023, 14, 3139. [Google Scholar] [CrossRef]
- Friedland, K.D.; Todd, C.D. Changes in Northwest Atlantic Arctic and Subarctic conditions and the growth response of Atlantic salmon. Polar Biol. 2012, 35, 593–609. [Google Scholar] [CrossRef]
- Friedland, K.D.; Chaput, G.; MacLean, J.C. The emerging role of climate in post-smolt growth of Atlantic salmon. ICES J. Mar. Sci. 2005, 62, 1338–1349. [Google Scholar] [CrossRef]
- Peyronnet, A.; Friedland, K.D.Ó.; Maoiléidigh, N.; Manning, M.; Poole, W.R. Links between patterns of marine growth and survival of Atlantic salmon (Salmo salar, L.). J. Fish Biol. 2007, 71, 684–700. [Google Scholar] [CrossRef]
- Friedland, K.D.; Moore, D.; Hogan, F. Retrospective growth analysis of Atlantic salmon (Salmo salar) from the Miramichi River, Canada. Can. J. Fish. Aquat. Sci. 2009, 66, 1294–1308. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Rountos, K.J.; Essington, T.E.; Santora, C.; Pauly, D.; Watson, R.; Sumaila, U.R.; Boersma, P.D.; Boyd, I.L.; Conover, D.O.; et al. The global contribution of forage fish to marine fisheries and ecosystems. Fish Fish. 2014, 15, 43–64. [Google Scholar] [CrossRef]
- Hjerman, D.Ø.; Ottersen, G.; Stenseth, N.C. Competition among fishermen and fish causes the collapse of Barents Sea capelin. Proc. Natl. Acad. Sci. USA 2004, 101, 11679–11684. [Google Scholar] [CrossRef] [PubMed]
- Utne, R.U.; Diaz Pauli, B.; Haugland, M.; Jacobsen, J.A.; Maoileidigh, N.; Melle, W.; Broms, C.T.; Nøttestad, L.; Holm, M.; Thomas, K.; et al. Poor feeding opportunities and reduced condition factor for salmon post-smolts in the Northeast Atlantic Ocean. ICES J. Mar. Sci. 2021, 78, 2844–2857. [Google Scholar] [CrossRef]
- Jacobsen, J.A.; Hansen, L.P. Feeding habits of wild and escaped farmed Atlantic salmon, Salmo salar L., in the Northeast Atlantic. ICES J. Mar. Sci. 2001, 58, 916–933. [Google Scholar] [CrossRef]
- Aykanat, T.; Rasmussen, M.; Ozerov, M.; Niemelä, E.; Paulin, L.; Vähä, J.-P.; Hindar, K.; Wennevik, V.; Pedersen, T.; Svenning, M.-A.; et al. Life-history genomic regions explain differences in Atlantic salmon marine diet specialization. J. Anim. Ecol. 2020, 89, 2677–2691. [Google Scholar] [CrossRef] [PubMed]
- Prowse, T.D.; Wrona, F.J.; Reist, J.D.; Gibson, J.J.; Hobbie, J.E.; Levesque, L.M.J.; Vincent, W.F. Climate change effects on hydroecology of Arctic freshwater ecosystems. Ambio 2006, 35, 347–358. [Google Scholar] [CrossRef]
- Heino, J.; Culp, J.M.; Erkinaro, J.; Goedkoop, W.; Lento, J.; Ruhland, K.M.; Smol, J.P. Abruptly and irreversibly changing Arctic freshwaters urgently require standardized monitoring. J. Appl. Ecol. 2020, 57, 1192–1198. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alioravainen, N.; Orell, P.; Erkinaro, J. Long-Term Trends in Freshwater and Marine Growth Patterns in Three Sub-Arctic Atlantic Salmon Populations. Fishes 2023, 8, 441. https://doi.org/10.3390/fishes8090441
Alioravainen N, Orell P, Erkinaro J. Long-Term Trends in Freshwater and Marine Growth Patterns in Three Sub-Arctic Atlantic Salmon Populations. Fishes. 2023; 8(9):441. https://doi.org/10.3390/fishes8090441
Chicago/Turabian StyleAlioravainen, Nico, Panu Orell, and Jaakko Erkinaro. 2023. "Long-Term Trends in Freshwater and Marine Growth Patterns in Three Sub-Arctic Atlantic Salmon Populations" Fishes 8, no. 9: 441. https://doi.org/10.3390/fishes8090441
APA StyleAlioravainen, N., Orell, P., & Erkinaro, J. (2023). Long-Term Trends in Freshwater and Marine Growth Patterns in Three Sub-Arctic Atlantic Salmon Populations. Fishes, 8(9), 441. https://doi.org/10.3390/fishes8090441





