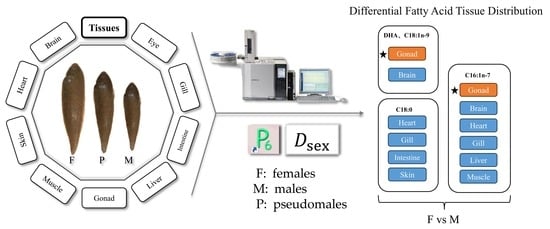
Sex Differences in Fatty Acid Composition of Chinese Tongue Sole (Cynoglossus semilaevis) Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Fish
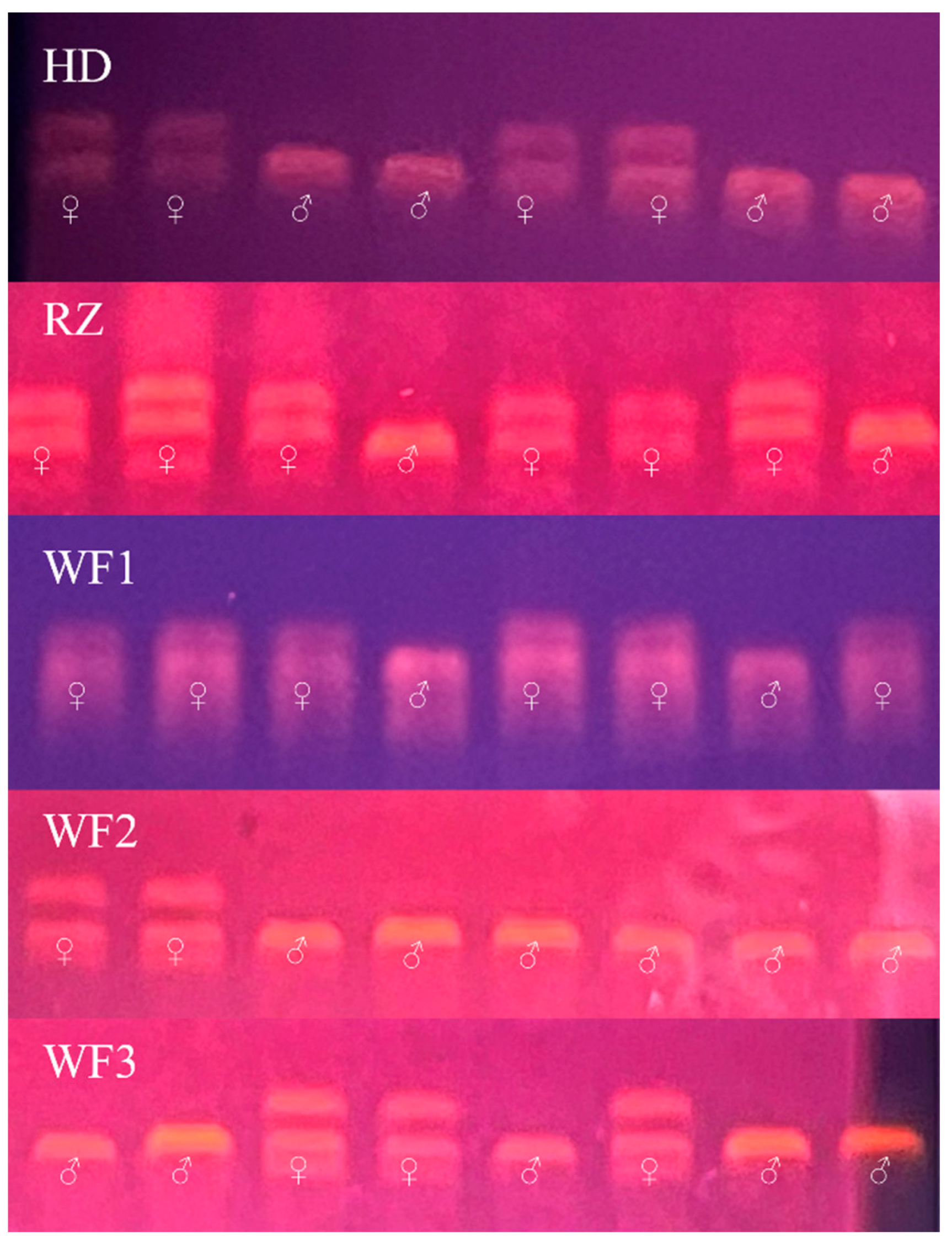
2.2. Sex Identification
2.3. Analysis of Total Lipid and Fatty Acid Compositions
2.4. Calculations and Statistical Methods
3. Results
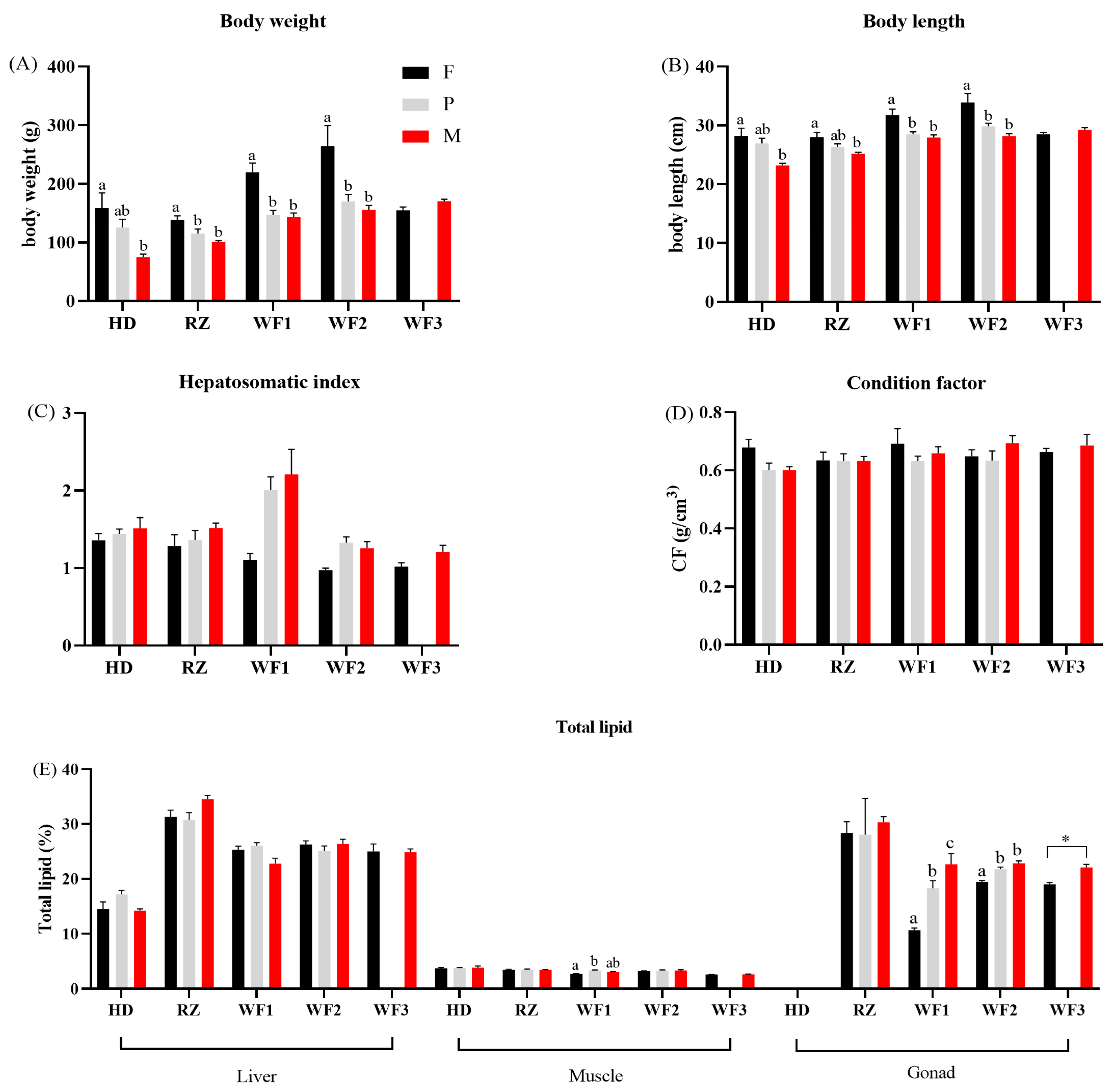
3.1. Somatic Indicators and Total Lipid Content
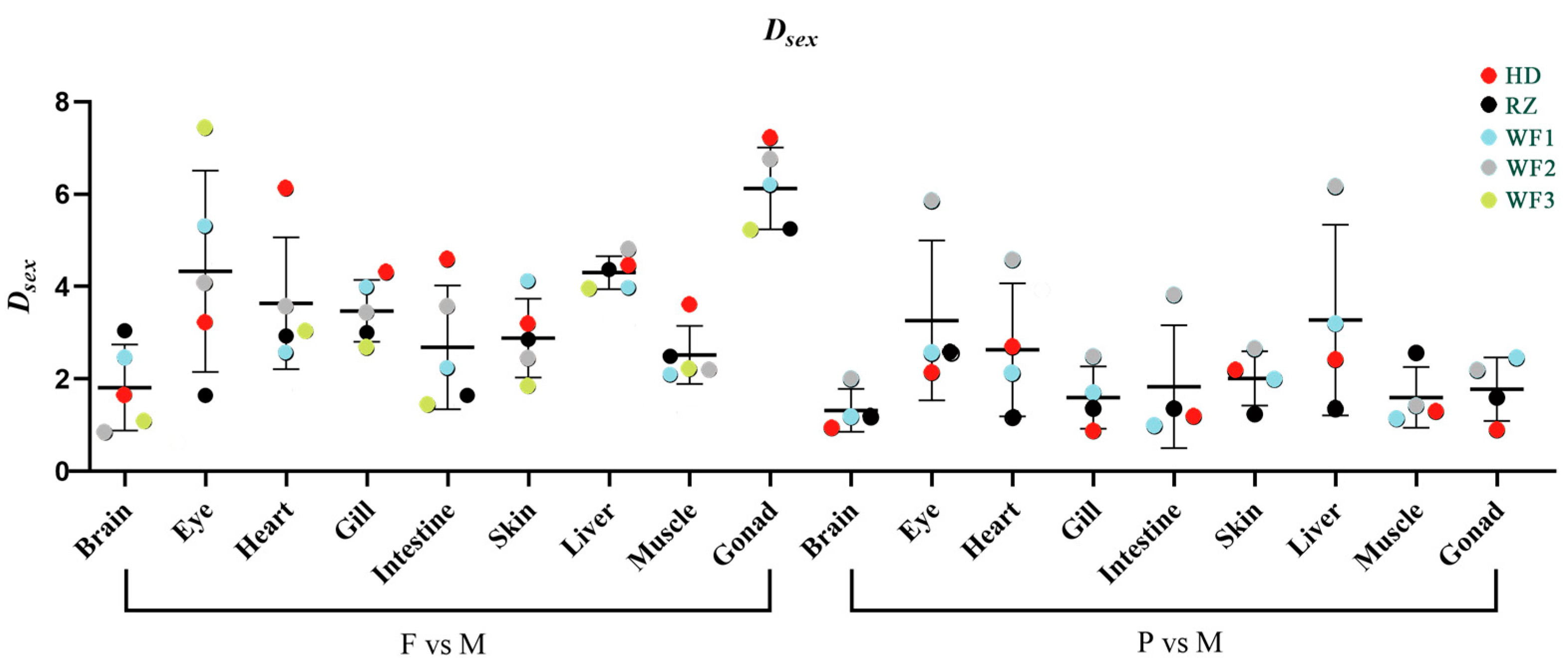
3.2. Dsex Values
3.3. SIMPER Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, D.A.; Engrola, S.; Morais, S.; Bandarra, N.; Coutinho, J.; Yúfera, M.; Conceição, L.E.C. Cortisol response to air exposure in Solea senegalensis post-larvae is affected by dietary arachidonic acid-to-eicosapentaenoic acid ratio. Fish Physiol. Biochem. 2011, 37, 733–743. [Google Scholar] [CrossRef]
- Martins, D.A.; Rocha, F.; Castanheira, F.; Mendes, A.; Pousão-Ferreira, P.; Bandarra, N.; Coutinho, J.; Morais, S.; Yúfera, M.; Conceição, L.E.C.; et al. Effects of dietary arachidonic acid on cortisol production and gene expression in stress response in Senegalese sole (Solea senegalensis) post-larvae. Fish Physiol. Biochem. 2013, 39, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Almatar, S.M.; James, C.M. Effects of varying dietary docosahexaenoic acid levels on growth, proximate composition and tissue fatty acid profile of juvenile silver pomfrets, Pampus argenteus (Euphrasen, 1788). Aquac. Res. 2012, 43, 1599–1610. [Google Scholar] [CrossRef]
- Cardona, E.; Segret, E.; Cachelou, Y. Effect of micro-algae Schizochytrium sp. supplementation in plant diet on reproduction of female rainbow trout (Oncorhynchus mykiss): Maternal programming impact of progeny. J. Anim. Sci. Biotechnol. 2022, 13, 33. [Google Scholar] [CrossRef]
- Xu, H.; Cao, L.; Zhang, Y.; Johnson, R.B.; Wei, Y.; Zheng, K.; Liang, M. Dietary arachidonic acid differentially regulates the gonadal steroidogenesis in the marine teleost, tongue sole (Cynoglossus semilaevis), depending on fish gender and maturation stage. Aquaculture 2017, 468, 378–385. [Google Scholar] [CrossRef]
- Xu, H.; Meng, X.; Wei, Y.; Ma, Q.; Liang, M.; Turchini, G.M. Arachidonic acid matters. Rev. Aquac. 2022, 14, 1912–1944. [Google Scholar] [CrossRef]
- Manor, M.L.; Weber, G.M.; Cleveland, B.M.; Kenney, P.B. Effects of feeding level and sexual maturation on fatty acid composition of energy stores in diploid and triploid rainbow trout (Oncorhynchus mykiss). Aquaculture 2014, 418, 17–25. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.; Kang, M.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Wang, B.; et al. UPLC-QTOF/MS metabolomics and biochemical assays reveal changes in hepatic nutrition and energy metabolism during sexual maturation in female rainbow trout (Oncorhynchus mykiss). Biology 2022, 11, 1679. [Google Scholar] [CrossRef]
- Bhat, R.A.; Saini, S.; Saoca, C.; Maricchiolo, G.; Fazio, F. Analysis of fatty acids and sex steroid hormones in rainbow trout testes (Oncorhynchus mykiss) during the reproductive process. Aquac. Res. 2022, 53, 4426–4436. [Google Scholar] [CrossRef]
- Luzia, L.A.; Sampaio, G.R.; Castellucci, C.M.N.; Torres, E.A.F.S. The influence of season on the lipid profiles of five commercially important species of Brazilian fish. Food Chem. 2003, 83, 93–97. [Google Scholar] [CrossRef]
- Nogueira, N.; Fernandes, I.; Fernandes, T.; Cordeiro, N. A comparative analysis of lipid content and fatty acid composition in muscle, liver and gonads of Seriola fasciata Bloch 1793 based on gender and maturation stage. J. Food Compost. Anal. 2017, 59, 68–73. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Bi, Q.; Ma, Q.; Wei, Y.; Liang, M.; Xu, H. Sex difference in fatty acid composition of six marine teleosts. J. Fish Biol. 2022, 101, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Luczynska, J.; Tonska, E.; Krejszeff, S.; Zarski, D. Comparison of Fatty Acids in the Muscles and Liver of Pond-Cultured and Wild Perch, Perca fluviatilis (L.), in Poland. Turk. J. Fish Aquat. Sci. 2016, 16, 19–27. [Google Scholar] [CrossRef]
- Zarski, D.; Palinska-Zarska, K.; Luczynska, J.; Krejszeff, S. The type of spawning agent affects the egg composition during out-of season spawning but not during in-season spawning in Eurasian perch, Perca fluviatilis. Gen. Comp. Endocrinol. 2017, 245, 19–29. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Hu, Y.; Xu, W.; Yang, Y.; Chen, S.; Wang, N. Identification of lncRNA-miRNA-mRNA network involved in sexual size dimorphism of Chinese Tongue Sole (Cynoglossus semilaevis). Front. Mar. Sci. 2022, 9, 795525. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, M.; Liu, J.; Liu, Y.; Lu, J.; Sun, X. Sexually dimorphic gene expression associated with growth and reproduction of tongue sole (Cynoglossus semilaevis) revealed by brain transcriptome analysis. Int. J. Mol. Sci. 2016, 17, 1402. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, J.; Yang, Y.; Chen, Z.; Wang, Q.; Wang, J.; Zhang, T.; Xu, W.; Chen, S. Identification and expression pattern of cyp26b1 gene in gonad of the Chinese tongue sole (Cynoglossus semilaevis). Animals 2022, 12, 2652. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Turchini, G.M.; Francis, D.S.; De Silva, S.S. Finishing diets stimulate compensatory growth: Results of a study on Murray cod, Maccullochella peelii peelii. Aquacult. Nutr. 2007, 13, 351–360. [Google Scholar] [CrossRef]
- Hixson, S.M.; Parrish, C.C. Substitution of fish oil with camelina oil and inclusion of camelina meal in diets fed to Atlantic cod (Gadus morhua) and their effects on growth, tissue lipid classes, and fatty acids. J. Anim. Sci. 2014, 92, 1055–1067. [Google Scholar] [CrossRef]
- Ji, X.; Liu, H.; Chen, S.; Jiang, Y.; Tian, Y. Growth differences and dimorphic expression of growth hormone (GH) in female and male Cynoglossus semilaevis after male sexual maturation. Mar. Genom. 2011, 4, 9–16. [Google Scholar] [CrossRef]
- Martín, M.V.; Rodríguez, C.; Cejas, J.R.; Pérez, M.J.; Jerez, S.; Lorenzo, A. Body lipid and fatty acid composition in male gilthead seabream broodstock at different stages of the reproductive cycle: Effects of a diet lacking n-3 and n-6 HUFA. Aquacult. Nutr. 2009, 15, 60–72. [Google Scholar] [CrossRef]
- Bogevik, A.S.; Hayman, E.S.; Bjerke, M.T.; Dessen, J.E.; Rørvik, K.A.; Luckenbach, J.A. Phospholipid and LC-PUFA metabolism in Atlantic salmon (Salmo salar) testes during sexual maturation. PLoS ONE 2020, 15, e0233322. [Google Scholar] [CrossRef]
- Esmaeili, V.; Shahverdi, A.H.; Moghadasian, M.H.; Alizadeh, A.R. Dietary fatty acids affect semen quality: A review. Andrology 2015, 3, 450–461. [Google Scholar] [CrossRef]
- Hauville, M.R.; Rhody, N.R.; Resley, M.J.; Bell, J.G.; Main, K.L.; Migaud, H. Comparative study of lipids and fatty acids in the liver, muscle, and eggs of wild and captive common snook broodstock. Aquaculture 2015, 446, 227–235. [Google Scholar] [CrossRef]
- Nie, L.; Ren, Y.; Schulz, H. Identification and characterization of escherichia coli thioesterase III that functions in fatty acid beta-oxidation. Biochemistry 2008, 47, 7744–7751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Feng, C.; Liu, R.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Lu, H.; Zhang, T.; Zeng, W. Exogenous oleic acid and palmitic acid improve boar sperm motility via enhancing mitochondrial β-oxidation for ATP generation. Animals 2020, 10, 591. [Google Scholar] [CrossRef]
- Gholami, H.; Chamani, M.; Towhidi, A.; Fazeli, M.H. Effect of feeding a docosahexaenoic acid-enriched nutriceutical on the quality of fresh and frozen-thawed semen in Holstein bulls. Theriogenology 2010, 74, 1548–1558. [Google Scholar] [CrossRef]
- Kaeoket, K.; Sang-Urai, P.; Thamniyom, A.; Chanapiwat, P.; Techakumphu, M. Effect of docosahexaenoic acid on quality of cryopreserved boar semen in different breeds. Reprod. Domest. Anim. 2010, 45, 458–463. [Google Scholar] [CrossRef]
- Wassef, E.A.; Wahbi, O.M.; Shalaby, S.H. Effects of dietary vegetable oils on liver and gonad fatty acid metabolism and gonad maturation in gilthead seabream (Sparus aurata) males and females. Aquacult. Int. 2012, 20, 255–281. [Google Scholar] [CrossRef]
- Rudchenko, A.E.; Yablokov, N.O. Composition and content of fatty acids in the tissues of males and females of Eurasian perch Perca fluviatilis at the late stages of reproductive cycle. Contemp. Probl. Ecol. 2018, 11, 309–319. [Google Scholar] [CrossRef]
- Anderson, R.E. Lipids of ocular tissues. IV: A comparison of the phospholipids from the retina of six mammalian species. Exp. Eye Res. 1970, 10, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016, 69, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA effects in brain development and function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L.; Bi, Q.; Wei, Y.; Liang, M.; Xu, H. Lipid distribution and fatty acid profile of five benthic marine fish species in the yellow sea. Prog. Fish. Sci. 2023, 44, 97–110, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Asturiano, J.F.; Sorbera, L.A.; Zanuy, S.; Carrillo, M. Effects of polyunsaturated fatty acids and gonadotropin on prostaglandin series E production in a primary testis cell culture system for the European sea bass. J. Fish Biol. 2000, 57, 1563–1574. [Google Scholar] [CrossRef]
- Baeza, R.; Peñaranda, D.S.; Vílchez, M.C.; Tveiten, H.; Perez, L.; Asturiano, J.F. Exploring correlations between sex steroids and fatty acids and their potential roles in the induced maturation of the male European eel. Aquaculture 2015, 435, 328–335. [Google Scholar] [CrossRef]
- Burhans, M.S.; Flowers, M.T.; Harrington, K.R.; Bond, L.M.; Guo, C.A.; Anderson, R.M.; Ntambi, J.M. Hepatic oleate regulates adipose tissue lipogenesis and fatty acid oxidation. J. Lipid Res. 2015, 56, 304–318. [Google Scholar] [CrossRef]
- Burns, T.A.; Kadegowda, A.K.G.; Duckett, S.K.; Pratt, S.L.; Jenkins, T.C. Palmitoleic (16:1 cis-9) and cis-vaccenic (18:1 cis-11) acid alter lipogenesis in bovine adipocyte cultures. Lipids 2012, 47, 1143–1153. [Google Scholar] [CrossRef]
- Bu, S.Y.; Mashek, D.G. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. J. Lipid Res. 2010, 51, 3270–3280. [Google Scholar] [CrossRef]
- Ducheix, S.; Montagner, A.; Polizzi, A.; Lasserre, F.; Regnier, M.; Marmugi, A.; Benhamed, F.; Bertrand-Michel, J.; Mselli-Lakhal, L.; Postic, C.; et al. Dietary oleic acid regulates hepatic lipogenesis through a liver X receptor-dependent signaling. PLoS ONE 2017, 12, e0181393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Mo, Z.; Li, Y.; Huang, L.; Yu, S.; Ge, L.; Hu, Y.; Shi, S.; Zhang, L.; Wang, L.; et al. Oleic acid reduces steroidogenesis by changing the lipid type stored in lipid droplets of ovarian granulosa cells. J. Anim. Sci. Biotechnol. 2022, 13, 27. [Google Scholar] [CrossRef]
- Zhou, H.; Leng, X.; Tan, Q.; Du, H.; Wu, J.; Liang, X.; Wei, Q. Identification of key nutrients for gonadal development by comparative analysis of proximate composition and fatty/amino acid profile in tissues and eggs of Chinese sturgeon (Acipenser sinensis Gray, 1835). J. Appl. Ichthyol. 2017, 33, 885–891. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Jeong, W.G.; Moon, S.K.; Ohshima, T. Preferential accumulation of fatty acids in the testis and ovary of cultured and wild sweet smelt Plecoglossus altivelis. Comp. Biochem. Phys. B 2002, 131, 251–259. [Google Scholar] [CrossRef] [PubMed]
| Reagent | Dosage | Temperature | Time | |
|---|---|---|---|---|
| 2 × A8 PCR MasterMix 1 | 12.5 μL | 95 °C | 3 min (pre-degeneration) | |
| Forward primer | 1 μL | 95 °C | 10 s | 35 cycles |
| Reverse primer | 1 μL | 55 °C | 15 s | |
| DNA | 2.5 μL | 72 °C | 1 min | |
| ddH2O | 8 μL | 72 °C | 5 min (Radical extension) |
| Sex | HD | RZ | WF1 | WF2 | WF3 |
|---|---|---|---|---|---|
| Female | 5 | 7 | 8 | 10 | 25 |
| Male | 7 | 17 | 9 | 11 | 3 |
| Pseudomale | 18 | 6 | 13 | 9 | 1 1 |
| Group | Tissue | 1st DFAsex 1 | 2nd DFAsex | 3rd DFAsex | 4th DFAsex | 5th DFAsex | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | Contrib% 2 | FA | Contrib% | FA | Contrib% | FA | Contrib% | FA | Contrib% | ||
| HD | brain | 22:6n-3 3 | 16.7 | 16:0 | 10.8 | 18:1n-9 | 10.6 | 16:1n-7 | 9.7 | 18:0 | 9.6 |
| eye | 22:6n-3 | 22.2 | 16:1n-7 | 16.5 | 20:5n-3 | 14.3 | 14:0 | 13.9 | 18:0 | 8.0 | |
| heart | 22:6n-3 | 21.7 | 20:5n-3 | 17.0 | 16:1n-7 | 16.8 | 18:0 | 10.3 | 14:0 | 8.4 | |
| gill | 16:1n-7 | 16.6 | 22:6n-3 | 13.9 | 20:5n-3 | 13.1 | 14:0 | 10.5 | 18:0 | 9.0 | |
| intestine | 22:6n-3 | 12.6 | 18:0 | 9.4 | 20:5n-3 | 9.2 | 16:1n-7 | 8.4 | 22:5n-3 | 8.1 | |
| skin | 16:1n-7 | 13.8 | 20:5n-3 | 11.7 | 18:2n-6 | 10.1 | 16:0 | 8.5 | 14:0 | 8.0 | |
| liver | 16:1n-7 | 23.4 | 18:0 | 14.5 | 22:6n-3 | 12.9 | 18:1n-9 | 11.0 | 22:5n-3 | 7.3 | |
| muscle | 16:1n-7 | 20.4 | 18:0 | 18.9 | 22:6n-3 | 10.1 | 14:0 | 10.0 | 16:0 | 8.1 | |
| gonad | 16:1n-7 | 16.2 | 20:5n-3 | 15.7 | 16:0 | 15.4 | 18:0 | 11.8 | 18:2n-6 | 10.3 | |
| RZ | brain | 18:2n-6 | 20.2 | 22:6n-3 | 13.0 | 20:5n-3 | 12.7 | 16:1n-7 | 9.9 | 18:1n-9 | 9.5 |
| eye | 18:2n-6 | 18.4 | 22:5n-3 | 14.2 | 14:0 | 11.2 | 22:6n-3 | 11.0 | 16:1n-7 | 11.0 | |
| heart | 18:0 | 14.1 | 16:1n-7 | 13.5 | 22:6n-3 | 12.5 | 20:5n-3 | 11.5 | 18:1n-9 | 10.0 | |
| gill | 20:3n-3 | 12.9 | 16:1n-7 | 11.8 | 18:0 | 10.9 | 16:0 | 10.9 | 20:5n-3 | 9.0 | |
| intestine | 16:0 | 10.4 | 20:5n-3 | 10.3 | 18:0 | 10.0 | 20:2n-6 | 8.4 | 20:3n-6 | 8.1 | |
| skin | 18:0 | 13.5 | 14:0 | 13.5 | 16:0 | 11.5 | 20:2n-6 | 11.1 | 16:1n-7 | 8.8 | |
| liver | 16:1n-7 | 16.5 | 22:6n-3 | 15.2 | 18:0 | 12.5 | 20:5n-3 | 9.6 | 16:0 | 9.3 | |
| muscle | 16:1n-7 | 15.1 | 16:0 | 14.3 | 14:0 | 13.1 | 18:0 | 11.2 | 20:5n-3 | 8.6 | |
| gonad | 18:1n-9 | 16.9 | 16:1n-7 | 16.4 | 18:0 | 13.0 | 22:6n-3 | 10.4 | 14:0 | 8.3 | |
| WF1 | brain | 18:1n-9 | 18.1 | 18:0 | 11.8 | 22:6n-3 | 10.9 | 18:2n-6 | 9.4 | 16:1n-7 | 9.2 |
| eye | 22:6n-3 | 17.1 | 18:1n-9 | 15.6 | 18:0 | 13.3 | 16:1n-7 | 9.0 | 20:3n-3 | 8.9 | |
| heart | 18:1n-9 | 18.4 | 16:1n-7 | 15.5 | 18:0 | 10.9 | 20:5n-3 | 9.9 | 20:3n-3 | 8.6 | |
| gill | 18:0 | 15.8 | 14:0 | 12.5 | 16:1n-7 | 11.8 | 17:1n-7 | 9.5 | 20:3n-3 | 9.2 | |
| intestine | 18:0 | 13.9 | 16:1n-7 | 10.8 | 18:2n-6 | 9.8 | 18:1n-9 | 8.2 | 22:5n-3 | 8.1 | |
| skin | 18:0 | 20.7 | 16:1n-7 | 12.1 | 22:6n-3 | 9.4 | 18:3n-3 | 9.2 | 20:3n-3 | 9.1 | |
| liver | 18:0 | 21.2 | 22:6n-3 | 10.6 | 18:1n-9 | 9.9 | 16:1n-7 | 8.8 | 18:2n-6 | 7.4 | |
| muscle | 18:1n-9 | 16.9 | 14:0 | 15.6 | 22:5n-3 | 10.5 | 16:0 | 9.2 | 22:6n-3 | 8.6 | |
| gonad | 22:6n-3 | 22.3 | 22:5n-3 | 15.0 | 16:1n-7 | 9.8 | 18:1n-9 | 7.9 | 18:3n-3 | 7.2 | |
| WF2 | brain | 22:6n-3 | 15.8 | 18:1n-9 | 13.6 | 16:1n-7 | 12.9 | 22:5n-3 | 12.2 | 16:0 | 8.9 |
| eye | 22:6n-3 | 17.3 | 18:1n-9 | 14.5 | 20:5n-3 | 13.6 | 16:0 | 13.3 | 18:2n-6 | 12.7 | |
| heart | 16:0 | 13.1 | 18:2n-6 | 12.7 | 16:1n-7 | 11.1 | 18:0 | 10.8 | 22:6n-3 | 10.0 | |
| gill | 16:0 | 17.0 | 20:5n-3 | 12.2 | 22:6n-3 | 9.4 | 20:3n-3 | 8.5 | 18:0 | 8.4 | |
| intestine | 20:2n-6 | 15.4 | 18:2n-6 | 11.0 | 22:6n-3 | 10.9 | 22:5n-3 | 8.8 | 20:5n-3 | 8.0 | |
| skin | 18:0 | 11.0 | 18:2n-6 | 9.8 | 20:3n-3 | 8.6 | 16:0 | 8.3 | 14:0 | 7.5 | |
| liver | 16:0 | 17.9 | 18:0 | 15.6 | 18:2n-6 | 11.7 | 22:6n-3 | 9.3 | 18:1n-9 | 8.0 | |
| muscle | 18:2n-6 | 22.3 | 18:3n-3 | 13.0 | 22:5n-3 | 12.2 | 22:6n-3 | 11.6 | 18:0 | 8.0 | |
| gonad | 18:1n-9 | 19.4 | 16:1n-7 | 17.0 | 22:6n-3 | 16.8 | 14:0 | 7.9 | 20:5n-3 | 5.9 | |
| WF3 | brain | 22:6n-3 | 16.6 | 18:1n-9 | 14.3 | 16:1n-7 | 11.2 | 16:0 | 9.6 | 18:0 | 8.4 |
| eye | 22:6n-3 | 25.9 | 18:1n-9 | 16.9 | 16:0 | 12.1 | 18:2n-6 | 11.4 | 14:0 | 8.5 | |
| heart | 18:0 | 23.2 | 20:3n-3 | 12.3 | 18:2n-6 | 10.7 | 22:6n-3 | 10.2 | 18:1n-9 | 8.2 | |
| gill | 18:2n-6 | 12.9 | 18:0 | 10.4 | 22:6n-3 | 10.1 | 20:3n-3 | 9.5 | 16:0 | 8.5 | |
| intestine | 22:5n-3 | 18.0 | 20:5n-3 | 13.4 | 16:0 | 10.1 | 18:0 | 8.2 | 22:6n-3 | 5.8 | |
| skin | 18:0 | 17.3 | 18:2n-6 | 9.3 | 22:5n-3 | 8.8 | 16:1n-7 | 7.6 | 17:1n-7 | 7.1 | |
| liver | 22:6n-3 | 15.6 | 18:2n-6 | 13.9 | 16:0 | 11.0 | 18:0 | 10.7 | 20:3n-3 | 7.8 | |
| muscle | 18:2n-6 | 16.4 | 22:5n-3 | 10.8 | 16:1n-7 | 10.0 | 22:6n-3 | 9.1 | 17:1n-7 | 9.1 | |
| gonad | 18:1n-9 | 18.1 | 16:1n-7 | 12.4 | 22:6n-3 | 11.0 | 16:0 | 10.2 | 20:4n-6 | 8.7 | |
| Group | Tissue | 1st DFAsex 1 | 2nd DFAsex | 3rd DFAsex | 4th DFAsex | 5th DFAsex | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | Contrib% 2 | FA | Contrib% | FA | Contrib% | FA | Contrib% | FA | Contrib% | ||
| HD | brain | 22:6n-3 3 | 16.8 | 16:1n-7 | 11.7 | 18:0 | 10.9 | 20:5n-3 | 9.5 | 22:5n-3 | 9.0 |
| eye | 22:6n-3 | 25.9 | 16:1n-7 | 17.3 | 14:0 | 12.0 | 18:1n-9 | 9.5 | 18:0 | 7.8 | |
| heart | 22:6n-3 | 19.5 | 16:1n-7 | 17.5 | 20:5n-3 | 11.7 | 18:0 | 10.3 | 14:0 | 9.3 | |
| gill | 22:6n-3 | 17.2 | 16:1n-7 | 15.6 | 14:0 | 9.2 | 18:0 | 8.9 | 20:5n-3 | 7.0 | |
| intestine | 16:1n-7 | 12.8 | 22:6n-3 | 12.6 | 14:0 | 10.2 | 18:0 | 8.8 | 20:5n-3 | 8.1 | |
| skin | 18:2n-6 | 10.8 | 22:6n-3 | 10.7 | 16:1n-7 | 9.9 | 20:5n-3 | 8.1 | 14:0 | 8.0 | |
| liver | 16:1n-7 | 22.1 | 22:6n-3 | 16.1 | 18:0 | 12.4 | 18:1n-9 | 12.4 | 20:3n-3 | 6.8 | |
| muscle | 18:0 | 20.9 | 16:1n-7 | 17.5 | 18:0 | 10.3 | 18:3n-3 | 7.2 | 16:0 | 7.0 | |
| gonad | 16:1n-7 | 13.5 | 18:2n-6 | 10.6 | 20:5n-3 | 10.3 | 16:0 | 9.4 | 18:0 | 9.1 | |
| RZ | brain | 18:2n-6 | 21.6 | 22:6n-3 | 15.3 | 18:1n-9 | 11.4 | 16:1n-7 | 10.7 | 18:0 | 9.6 |
| eye | 22:6n-3 | 18.4 | 18:2n-6 | 12.2 | 14:0 | 11.4 | 20:5n-3 | 11.3 | 18:0 | 10.9 | |
| heart | 22:6n-3 | 14.6 | 16:1n-7 | 14.3 | 20:5n-3 | 11.7 | 18:1n-9 | 11.4 | 18:0 | 10.7 | |
| gill | 20:5n-3 | 11.6 | 18:3n-3 | 10.6 | 18:2n-6 | 9.7 | 18:0 | 9.7 | 16:1n-7 | 8.7 | |
| intestine | 20:5n-3 | 10.4 | 18:0 | 10.0 | 16:0 | 8.6 | 20:2n-6 | 8.3 | 20:3n-6 | 7.8 | |
| skin | 18:0 | 11.5 | 18:3n-3 | 10.2 | 17:1n-7 | 9.6 | 20:2n-6 | 8.0 | 20:3n-3 | 7.4 | |
| liver | 16:1n-7 | 13.7 | 18:0 | 12.4 | 18:2n-6 | 11.0 | 16:0 | 9.5 | 18:1n-9 | 9.4 | |
| muscle | 16:1n-7 | 21.6 | 18:0 | 15.2 | 22:6n-3 | 12.6 | 18:1n-9 | 9.0 | 14:0 | 8.2 | |
| gonad | 18:0 | 13.0 | 20:5n-3 | 12.7 | 18:2n-6 | 10.9 | 18:1n-9 | 10.2 | 16:1n-7 | 10.1 | |
| WF1 | brain | 22:5n-3 | 14.3 | 18:1n-9 | 11.3 | 22:6n-3 | 10.0 | 18:0 | 9.2 | 14:0 | 8.1 |
| eye | 18:2n-6 | 14.2 | 16:0 | 12.3 | 22:6n-3 | 11.9 | 16:1n-7 | 11.0 | 18:0 | 9.9 | |
| heart | 18:1n-9 | 18.7 | 22:6n-3 | 15.6 | 20:5n-3 | 12.3 | 16:1n-7 | 8.9 | 20:3n-3 | 7.9 | |
| gill | 18:0 | 15.9 | 16:1n-7 | 12.1 | 20:3n-3 | 11.8 | 14:0 | 10.3 | 18:1n-9 | 9.1 | |
| intestine | 16:0 | 11.7 | 16:1n-7 | 10.6 | 18:1n-9 | 9.9 | 18:2n-6 | 9.7 | 18:0 | 9.6 | |
| skin | 18:0 | 16.7 | 16:1n-7 | 10.2 | 20:3n-3 | 8.6 | 20:2n-6 | 7.7 | 14:0 | 7.5 | |
| liver | 14:0 | 17.9 | 16:0 | 12.2 | 18:1n-9 | 8.5 | 22:6n-3 | 8.0 | 18:0 | 7.6 | |
| muscle | 16:0 | 12.7 | 22:5n-3 | 11.1 | 18:0 | 11.0 | 18:2n-6 | 10.0 | 17:1n-7 | 9.1 | |
| gonad | 22:5n-3 | 20.2 | 22:6n-3 | 11.6 | 18:1n-9 | 8.0 | 14:0 | 7.8 | 16:1n-7 | 7.7 | |
| WF2 4 | brain | 22:6n-3 | 19.5 | 18:1n-9 | 14.1 | 18:0 | 11.6 | 18:2n-6 | 11.4 | 16:0 | 9.0 |
| eye | 22:6n-3 | 23.2 | 20:5n-3 | 16.0 | 18:2n-6 | 15.7 | 18:1n-9 | 13.2 | 16:1n-7 | 7.8 | |
| heart | 22:6n-3 | 20.9 | 18:2n-6 | 18.0 | 18:1n-9 | 9.8 | 16:1n-7 | 9.8 | 16:0 | 9.7 | |
| gill | 20:4n-6 | 12.6 | 20:5n-3 | 10.7 | 16:0 | 9.2 | 18:2n-6 | 9.0 | 18:1n-9 | 8.8 | |
| intestine | 20:2n-6 | 13.2 | 22:6n-3 | 12.4 | 20:5n-3 | 11.3 | 18:0 | 8.9 | 18:2n-6 | 8.7 | |
| skin | 16:0 | 13.0 | 22:6n-3 | 10.4 | 18:3n-3 | 9.2 | 18:2n-6 | 7.9 | 18:1n-9 | 7.8 | |
| liver | 22:6n-3 | 24.1 | 18:2n-6 | 15.8 | 20:5n-3 | 10.9 | 18:3n-3 | 10.2 | 16:0 | 9.0 | |
| muscle | 18:2n-6 | 19.4 | 18:0 | 15.3 | 16:0 | 10.0 | 22:6n-3 | 8.7 | 18:3n-3 | 8.5 | |
| gonad | 18:1n-9 | 17.9 | 22:6n-3 | 10.1 | 14:0 | 9.9 | 20:5n-3 | 9.5 | 20:3n-3 | 8.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, F.; Ma, Q.; Wei, Y.; Liang, M.; Xu, H. Sex Differences in Fatty Acid Composition of Chinese Tongue Sole (Cynoglossus semilaevis) Tissues. Fishes 2023, 8, 421. https://doi.org/10.3390/fishes8080421
Liu J, Zhang F, Ma Q, Wei Y, Liang M, Xu H. Sex Differences in Fatty Acid Composition of Chinese Tongue Sole (Cynoglossus semilaevis) Tissues. Fishes. 2023; 8(8):421. https://doi.org/10.3390/fishes8080421
Chicago/Turabian StyleLiu, Jiahao, Feiran Zhang, Qiang Ma, Yuliang Wei, Mengqing Liang, and Houguo Xu. 2023. "Sex Differences in Fatty Acid Composition of Chinese Tongue Sole (Cynoglossus semilaevis) Tissues" Fishes 8, no. 8: 421. https://doi.org/10.3390/fishes8080421