Molecular Cloning and Gene Expression of Type I Suppressors of Cytokine Signaling 6 and 7 (SOCS6 and SOCS7) in Whiteleg Shrimp (Litopenaeus vannamei)
Abstract
1. Introduction
2. Materials and Methods
2.1. Shrimp Rearing and Tissue Collection
2.2. Immune Challenge
2.3. Total RNA Extraction and cDNA Synthesis
2.4. Cloning of LvSOCS6 and LvSOCS7 cDNA
2.5. In Silico Sequence Analysis and Molecular Phylogeny
2.6. Tissue Distribution and Expression Pattern Analysis
2.7. Statistical Analysis
3. Results
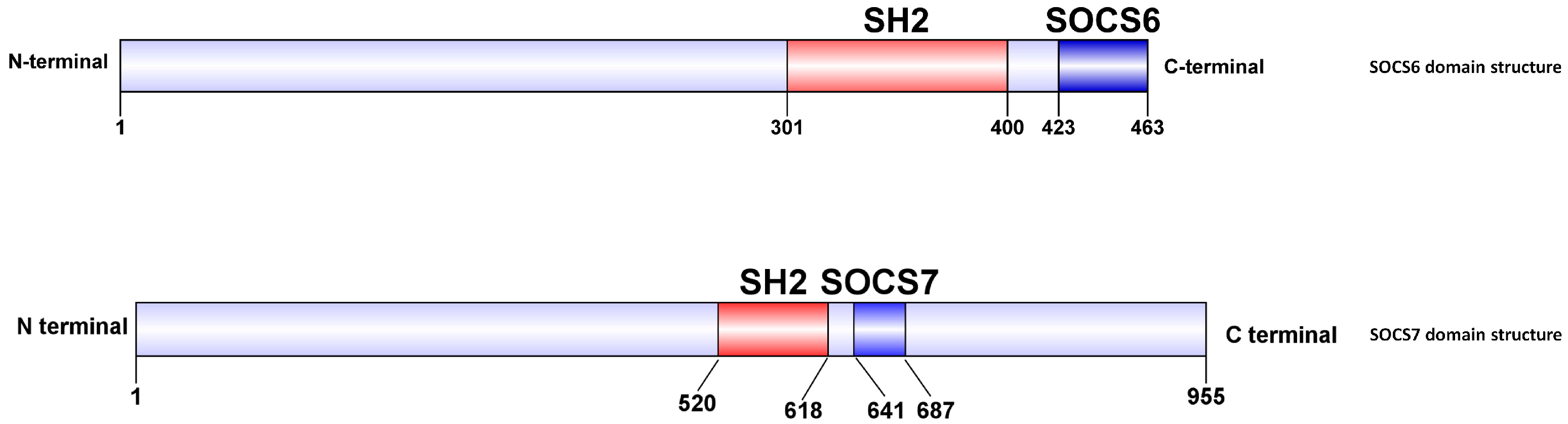
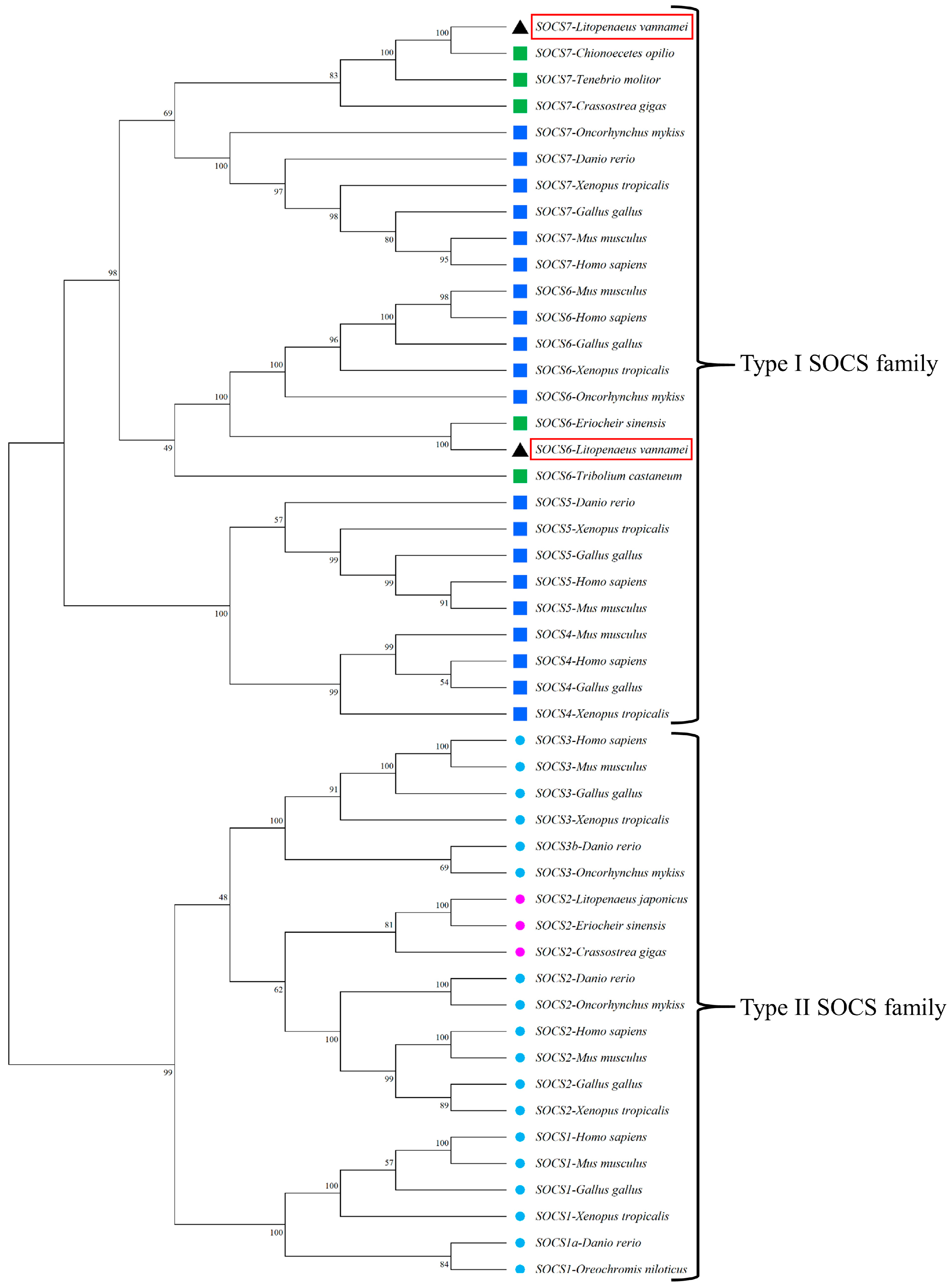
3.1. Molecular Characteristics and Phylogenetic Relationships of LvSOCS6 and LvSOCS7
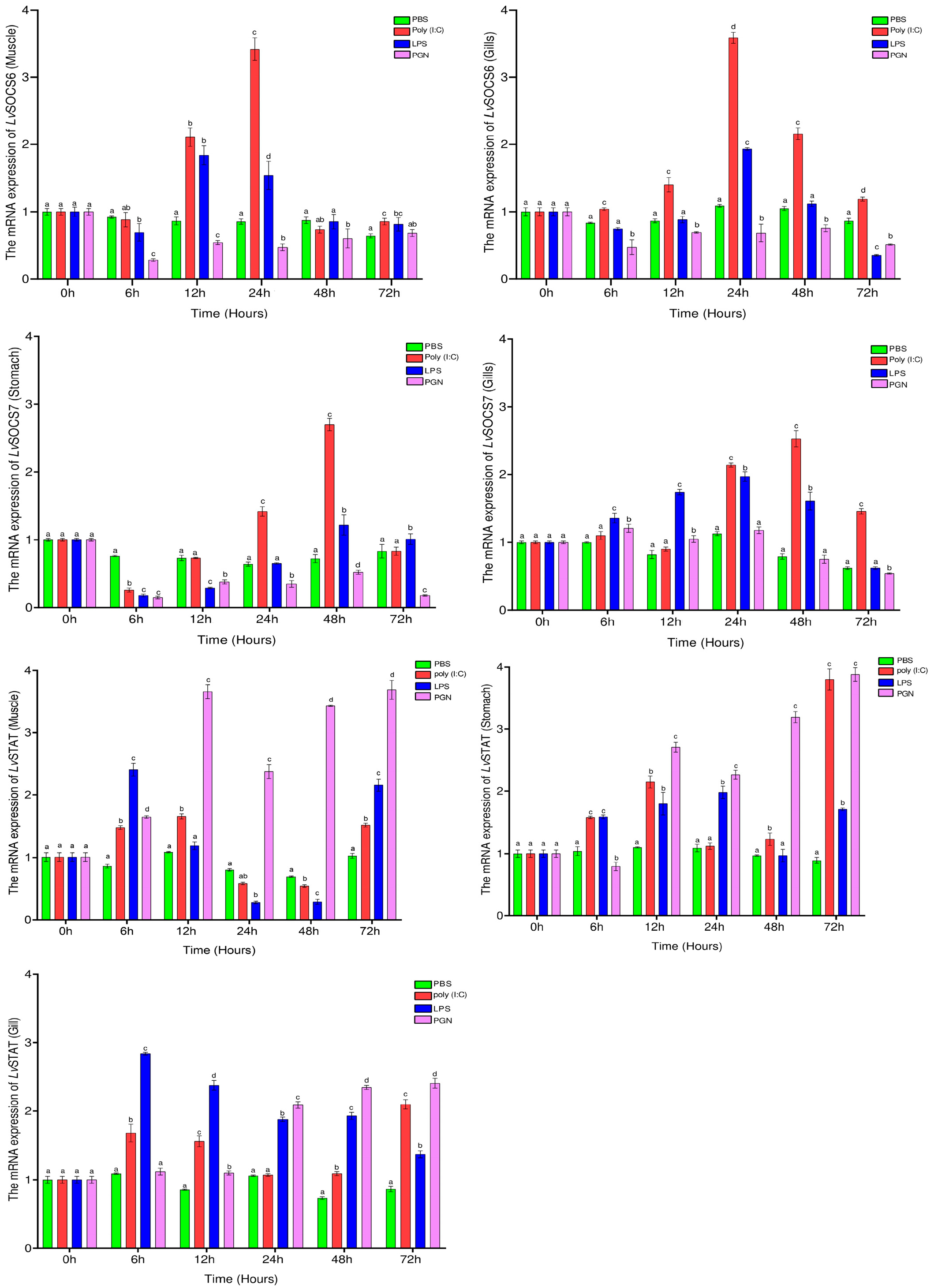
3.2. Tissue Distribution and mRNA Expression of LvSOCS6, LvSOCS7, and LvSTAT after LPS, Poly (I:C), and PGN Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Beschin, A.; Bilej, M.; Torreele, E.; De Baetselier, P. On the existence of cytokines in invertebrates. Cell. Mol. Life Sci. 2001, 58, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, R.A.; Cheng, T.; Visconti, R.; Frucht, D.M.; O’Shea, J.J. Janus kinases and signal transducers and activators of transcription: Their roles in cytokine signaling, development and immunoregulation. Arthritis Res. 2000, 2, 16–32. [Google Scholar] [CrossRef]
- Boudinot, P.; Bird, S.; Du Pasquier, L.; Collet, B. The repertoire of vertebrate STAT transcription factors: Origin and variations in fish. Dev. Comp. Immunol. 2021, 116, 103929. [Google Scholar] [CrossRef]
- Hou, S.X.; Zheng, Z.; Chen, X.; Perrimon, N. The JAK/STAT pathway in model organisms: Emerging roles in cell movement. Dev. Cell 2002, 3, 765–778. [Google Scholar] [CrossRef]
- Alexander, W.S.; Hilton, D.J. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 2004, 22, 503–529. [Google Scholar] [CrossRef]
- Wang, B.; Wangkahart, E.; Secombes, C.J.; Wang, T. Insights into the evolution of the suppressors of cytokine signaling (SOCS) gene family in vertebrates. Mol. Biol. Evol. 2019, 36, 393–411. [Google Scholar] [CrossRef]
- Delgado-Ortega, M.; Marc, D.; Dupont, J.; Trapp, S.; Berri, M.; Meurens, F. SOCS proteins in infectious diseases of mammals. Vet. Immunol. Immunopathol. 2013, 151, 1–19. [Google Scholar] [CrossRef]
- Yasukawa, H.; Misawa, H.; Sakamoto, H.; Masuhara, M.; Sasaki, A.; Wakioka, T.; Ohtsuka, S.; Imaizumi, T.; Matsuda, T.; Ihle, J.N.; et al. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999, 18, 1309–1320. [Google Scholar] [CrossRef]
- Sasaki, A.; Yasukawa, H.; Suzuki, A.; Kamizono, S.; Syoda, T.; Kinjyo, I.; Sasaki, M.; Johnston, J.A.; Yoshimura, A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 1999, 4, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Dalpke, A.; Heeg, K.; Bartz, H.; Baetz, A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology 2008, 213, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.P.; Chandrashekaran, I.R.; Low, A.; Speed, T.P.; Nicholson, S.E.; Norton, R.S. The N-terminal domains of SOCS proteins: A conserved region in the disordered N-termini of SOCS4 and 5. Proteins Struct. Funct. Bioinform. 2012, 80, 946–957. [Google Scholar] [CrossRef]
- Qu, C.; Xu, Q.; Lu, M.; Wang, F.; Liu, Z.; Liu, D.; Yang, W.; Yi, Q.; Wang, L.; Song, L. The involvement of suppressor of cytokine signaling 6 (SOCS6) in immune response of Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2018, 72, 502–509. [Google Scholar] [CrossRef]
- Callus, B.A.; Mathey-Prevot, B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signaling in the imaginal wing disc. Oncogene 2002, 21, 4812–4821. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rennebeck, G.; Harrison, S.M.W.; Xi, R.; Harrison, D.A. Two Drosophila suppressors of cytokine signaling (SOCS) differentially regulate JAK and EGFR pathway activities. BMC Cell Biol. 2004, 5, 38. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, Y.; Liu, Y.; Xiang, Z.; Qu, F.; Yu, Z. Cloning and characterization of three suppressors of cytokine signaling (SOCS) genes from the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2015, 44, 525–532. [Google Scholar] [CrossRef]
- Zhu, B.; Dai, L.; Yu, Y.; Wang, D.; Peng, T.; Liu, C. A role of suppressor of cytokine signaling 2 in the regulation of ecdysteroid signaling pathway in Procambarus clarkii. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2016, 325, 441–452. [Google Scholar] [CrossRef]
- Wang, S.; Song, X.; Zhang, Z.; Li, H.; Lǚ, K.; Yin, B.; He, J.; Li, C. Shrimp with knockdown of LvSOCS2, a negative feedback loop regulator of JAK/STAT pathway in Litopenaeus vannamei, exhibit enhanced resistance against WSSV. Dev. Comp. Immunol. 2016, 65, 289–298. [Google Scholar] [CrossRef]
- Patnaik, B.B.; Kim, B.B.; Jo, Y.H.; Bang, I.S. Molecular cloning and expression analysis of three suppressors of cytokine signaling genes (SOCS5, SOCS6, SOCS7) in the Mealworm beetle Tenebrio molitor. Insects 2019, 10, 76. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Barrett, T.; Benson, D.A.; Bryant, S.H.; Canese, K.; Chetvernin, V.; Church, D.M.; Dicuccio, M.; Edgar, R.; Federhen, S.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2008, 36, D13–D21. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Lu, S.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; DeWeese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; et al. CDD: A conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 2011, 39, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Sievers, F.; Higgins, D.G. Clustal omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kamura, T.; Sato, S.; Haque, D.; Liu, L.; Kaelin, W.G.; Conaway, R.C.; Conaway, J.W. The elongin BC complex interacts with the conserved SOCS-Box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998, 12, 3872–3881. [Google Scholar] [CrossRef]
- Bullock, A.N.; Rodriguez, M.C.; Debreczeni, J.É.; Songyang, Z.; Knapp, S. Structure of the SOCS4-elonginB/C complex reveals a distinct SOCS box interface and the molecular basis for SOCS-dependent EGFR degradation. Structure 2007, 15, 1493–1504. [Google Scholar] [CrossRef]
- Abbas, M.N.; Kausar, S.; Sun, Y.X.; Tian, J.W.; Zhu, B.J.; Liu, C.L. Suppressor of cytokine signaling 6 can enhance epidermal growth factor receptor signaling pathway in Bombyx mori (Dazao). Dev. Comp. Immunol. 2018, 81, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gao, Q.; Nie, P.; Secombes, C.J. Identification of suppressor of cytokine signalling (SOCS) 6, 7, 9 and CISH in rainbow trout Oncorhynchus mykiss and analysis of their expression in relation to other known trout SOCS. Fish Shellfish Immunol. 2010, 29, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Yamada, N.; Kumazaki, M.; Yasui, Y.; Iwasaki, J.; Naito, S.; Akao, Y. Socs7, a target gene of microRNA-145, regulates interferon-β induction through STAT3 nuclear translocation in bladder cancer cells. Cell Death Dis. 2013, 4, e482. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mishra, K.; Surolia, A.; Banerjee, K. Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition. PLoS ONE 2011, 6, e26674. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. Jak-Stat signaling: A double-edged sword of immune regulation and cancer progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wikumpriya, G.C.; Prabhatha, M.W.S.; Lee, J.; Kim, C.-H. Molecular Cloning and Gene Expression of Type I Suppressors of Cytokine Signaling 6 and 7 (SOCS6 and SOCS7) in Whiteleg Shrimp (Litopenaeus vannamei). Fishes 2023, 8, 416. https://doi.org/10.3390/fishes8080416
Wikumpriya GC, Prabhatha MWS, Lee J, Kim C-H. Molecular Cloning and Gene Expression of Type I Suppressors of Cytokine Signaling 6 and 7 (SOCS6 and SOCS7) in Whiteleg Shrimp (Litopenaeus vannamei). Fishes. 2023; 8(8):416. https://doi.org/10.3390/fishes8080416
Chicago/Turabian StyleWikumpriya, Gunasekara Chathura, Madhuranga Walawedurage Srinith Prabhatha, Jiye Lee, and Chan-Hee Kim. 2023. "Molecular Cloning and Gene Expression of Type I Suppressors of Cytokine Signaling 6 and 7 (SOCS6 and SOCS7) in Whiteleg Shrimp (Litopenaeus vannamei)" Fishes 8, no. 8: 416. https://doi.org/10.3390/fishes8080416
APA StyleWikumpriya, G. C., Prabhatha, M. W. S., Lee, J., & Kim, C.-H. (2023). Molecular Cloning and Gene Expression of Type I Suppressors of Cytokine Signaling 6 and 7 (SOCS6 and SOCS7) in Whiteleg Shrimp (Litopenaeus vannamei). Fishes, 8(8), 416. https://doi.org/10.3390/fishes8080416






