Antimicrobial Effects of Plasma-Activated Simulated Seawater (PASW) on Total Coliform and Escherichia coli in Live Oysters during Static Depuration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Simulated Seawater (SS)
2.2. Plasma Generator Setting and Plasma-Activated Simulated Seawater (PASW) Generation
2.3. Analysis of Physicochemical Properties of Diluted PASW10
2.4. Oyster Preparation
2.5. Bacterial Culture Preparation and Bioaccumulation in Oysters
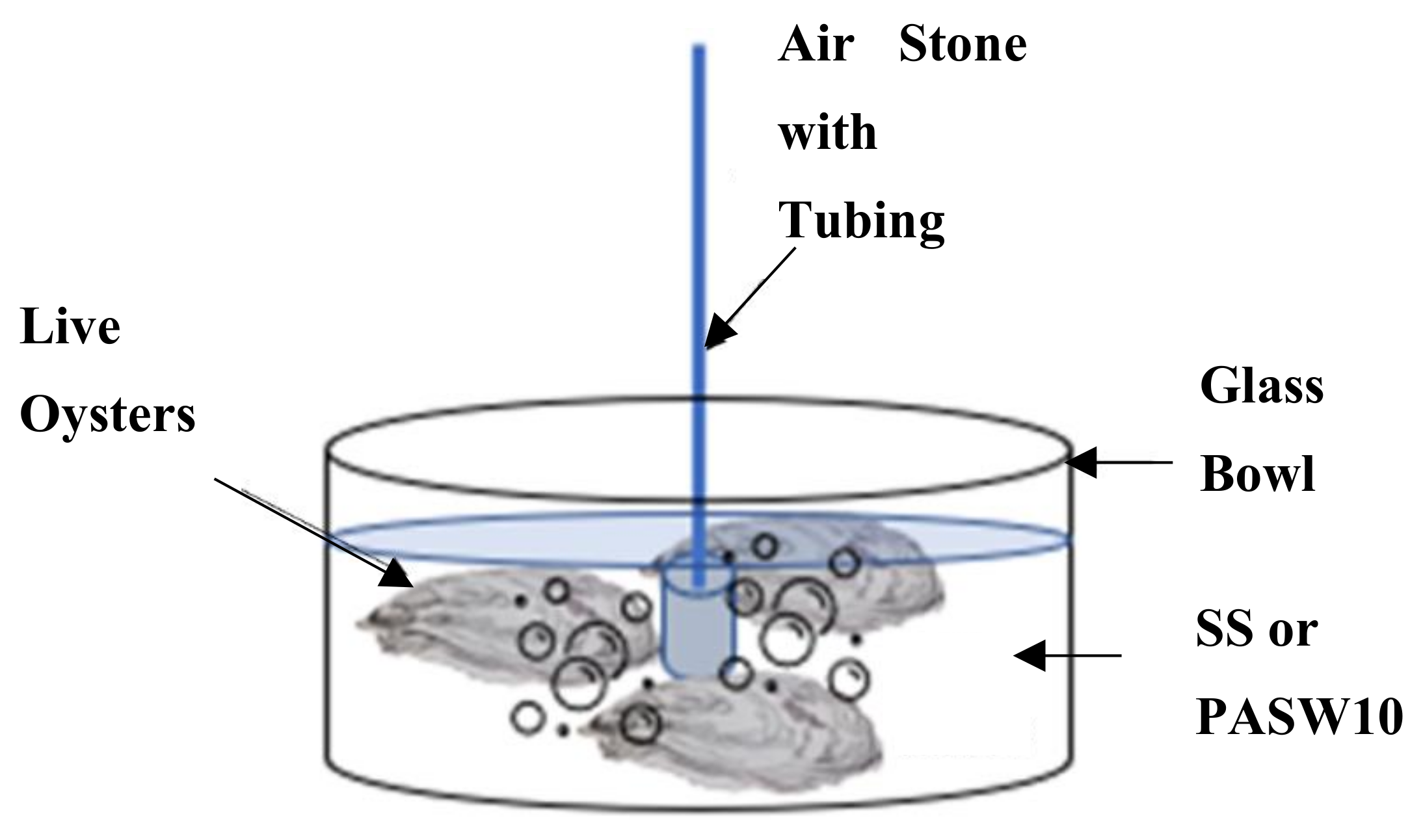
2.6. Depuration Setup and Processing
2.7. Determination of Microbial Inactivation Efficacy of PASW10 (1:1) against Total Coliform and E. coli in Oysters
2.8. Oyster Quality Testing
2.9. Data Analysis
3. Results
3.1. Physicochemical Properties of PASW10 (3:1 and 1:1)
3.2. Oyster Physical Properties and Mortality
3.3. Bacteria Bioaccumulation in Oysters
3.4. Microbial Inactivation Efficacy of PASW10 (1:1) against Total Coliform (TC) and E. coli (EC) in Oysters
3.5. Oyster Quality
4. Discussion
4.1. Physicochemical Properties of PASW10 (3:1 and 1:1)
4.2. Oyster Physical Properties and Mortality
4.3. Bacteria Bioaccumulation in Oysters
4.4. Microbial Inactivation Efficacy of PASW10 (1:1) against Total Coliform (TC) and E. coli (EC) in Oysters
4.5. Oyster Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| PASW | Plasma-activated simulated seawater |
| SS | Simulated seawater |
| PASW10 | SS activated by plasma for 10 min |
| PASW10 (1:1) | PASW10 diluted 1:1 with SS |
| PASW10 (3:1) | PASW10 diluted 3:1 with SS |
| PAW | Plasma-activated water |
| TC | Total coliform |
| EC | Escherichia coli |
References
- Sato, Y.; Yamada, S.; Takeda, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Hori, M.; Kodera, Y. Effect of Plasma-Activated Lactated Ringer’s Solution on Pancreatic Cancer Cells In Vitro and In Vivo. Ann. Surg. Oncol. 2018, 25, 299–307. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Evaluation of Plasma-Activated Water (PAW) as a Novel Disinfectant: Effectiveness on Escherichia coli and Listeria innocua, Physicochemical Properties, and Storage Stability. LWT 2021, 149, 111847. [Google Scholar] [CrossRef]
- Herianto, S.; Shih, M.-K.; Lin, C.-M.; Hung, Y.-C.; Hsieh, C.-W.; Wu, J.-S.; Chen, M.-H.; Chen, H.-L.; Hou, C.-Y. The Effects of Glazing with Plasma-Activated Water Generated by a Piezoelectric Direct Discharge Plasma System on Whiteleg Shrimp (Litopenaeus Vannamei). LWT 2022, 154, 112547. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, D.; Xiao, S.; Ning, M.; Zhou, R.; Zhang, S.; Chen, X.; Ostrikov, K.; Dai, X. InvivoPen: A Novel Plasma Source for in Vivo Cancer Treatment. J. Cancer 2020, 11, 2273. [Google Scholar] [CrossRef] [Green Version]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Narasimhan, S.L.; Salvi, D.; Schaffner, D.W.; Karwe, M.V.; Tan, J. Efficacy of Cold Plasma-Activated Water as an Environmentally Friendly Sanitizer in Egg Washing. Poult. Sci. 2023, 102, 102893. [Google Scholar] [CrossRef] [PubMed]
- Date, M.B.; Rivero, W.C.; Tan, J.; Specca, D.; Simon, J.E.; Salvi, D.A.; Karwe, M.V. Growth of Hydroponic Sweet Basil (O. basilicum L.) Using Plasma-Activated Nutrient Solution (PANS). Agriculture 2023, 13, 443. [Google Scholar] [CrossRef]
- Shah, U.; Wang, Q.; Kathariou, S.; Salvi, D. Optimization of Plasma-Activated Water and Validation of a Potential Surrogate for Salmonella for Future Egg Washing Processes. J. Food Prot. 2023, 86, 100029. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, Q.; Rai, R.; Salvi, D.; Nitin, N. DNA-Based Surrogates for the Validation of Microbial Inactivation Using Cold Atmospheric Pressure Plasma and Plasma-Activated Water Processing. J. Food Eng. 2023, 339, 111267. [Google Scholar] [CrossRef]
- Rivero, W.C.; Wang, Q.; Salvi, D. Impact of Plasma-Activated Water Washing on the Microbial Inactivation, Color, and Electrolyte Leakage of Alfalfa Sprouts, Broccoli Sprouts, and Clover Sprouts. Innov. Food Sci. Emerg. Technol. 2022, 81, 103123. [Google Scholar] [CrossRef]
- Campbell, V.; Wang, Q.; Hall, S.G.; Salvi, D. Physicochemical Properties and Antimicrobial Impacts of Plasma-Activated Simulated Seawater (PASW) on Escherichia coli. JSFA Rep. 2022, 2, 228–235. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Recent Progress in the Application of Plasma-Activated Water (PAW) for Food Decontamination. Curr. Opin. Food Sci. 2021, 42, 51–60. [Google Scholar] [CrossRef]
- Mumtaz, S.; Khan, R.; Rana, J.N.; Javed, R.; Iqbal, M.; Choi, E.H.; Han, I. Review on the Biomedical and Environmental Applications of Nonthermal Plasma. Catalysts 2023, 13, 685. [Google Scholar] [CrossRef]
- Patange, A.; Lu, P.; Boehm, D.; Cullen, P.J.; Bourke, P. Efficacy of Cold Plasma Functionalised Water for Improving Microbiological Safety of Fresh Produce and Wash Water Recycling. Food Microbiol. 2019, 84, 103226. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Karwe, M.V. Inactivation and Removal of Enterobacter Aerogenes Biofilm in a Model Piping System Using Plasma-Activated Water (PAW). Innov. Food Sci. Emerg. Technol. 2021, 69, 102664. [Google Scholar] [CrossRef]
- Kostoláni, D.; Ndiffo Yemeli, G.B.; Švubová, R.; Kyzek, S.; Machala, Z. Physiological Responses of Young Pea and Barley Seedlings to Plasma-Activated Water. Plants 2021, 10, 1750. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on Formation of Cold Plasma Activated Water (PAW) and the Applications in Food and Agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef]
- Hashizume, H.; Kitano, H.; Mizuno, H.; Abe, A.; Yuasa, G.; Tohno, S.; Tanaka, H.; Ishikawa, K.; Matsumoto, S.; Sakakibara, H.; et al. Improvement of Yield and Grain Quality by Periodic Cold Plasma Treatment with Rice Plants in a Paddy Field. Plasma Process. Polym. 2021, 18, 2000181. [Google Scholar] [CrossRef]
- Kang, C.; Xiang, Q.; Zhao, D.; Wang, W.; Niu, L.; Bai, Y. Inactivation of Pseudomonas Deceptionensis CM2 on Chicken Breasts Using Plasma-Activated Water. J. Food Sci. Technol. 2019, 56, 4938–4945. [Google Scholar] [CrossRef]
- Andrasch, M.; Stachowiak, J.; Schlüter, O.; Schnabel, U.; Ehlbeck, J. Scale-up to Pilot Plant Dimensions of Plasma Processed Water Generation for Fresh-Cut Lettuce Treatment. Food Packag. Shelf Life 2017, 14, 40–45. [Google Scholar] [CrossRef]
- Wong, K.S.; Lim, W.T.H.; Ooi, C.W.; Yeo, L.Y.; Tan, M.K. In Situ Generation of Plasma-Activated Aerosols via Surface Acoustic Wave Nebulization for Portable Spray-Based Surface Bacterial Inactivation. Lab Chip 2020, 20, 1856–1868. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of Plasma-Activated Water with Biofilms: Inactivation, Dispersal Effects and Mechanisms of Action. NPJ Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Wu, R.A.; Liao, X.; Liu, D.; Cullen, P.J.; Zhou, R.-W.; Ding, T. Diagnostic Analysis of Reactive Species in Plasma-Activated Water (PAW): Current Advances and Outlooks. J. Phys. D Appl. Phys. 2021, 55, 23002. [Google Scholar] [CrossRef]
- Kulawik, P.; Kumar Tiwari, B. Recent Advancements in the Application of Non-Thermal Plasma Technology for the Seafood Industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 3199–3210. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The Importance of ATP-Related Compounds for the Freshness and Flavor of Post-Mortem Fish and Shellfish Muscle: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- Xu, D.; Cui, Q.; Xu, Y.; Wang, B.; Tian, M.; Li, Q.; Liu, Z.; Liu, D.; Chen, H.; Kong, M.G. Systemic Study on the Safety of Immuno-Deficient Nude Mice Treated by Atmospheric Plasma-Activated Water. Plasma Sci. Technol. 2018, 20, 44003. [Google Scholar] [CrossRef] [Green Version]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- SOP MB-27-03; Standard Operating Procedure for Germicidal and Detergent Sanitizing Action of Disinfectants Tests. US Environmental Protection Agency Office of Pesticide Programs: Washington, DC, USA, 2019.
- Larsen, A.; Scott, F.; Walton, W.; Arias, C. Effective Reduction of Vibrio vulnificus in the Eastern Oyster (Crassostrea virginica) Using High Salinity Depuration. Food Microbiol. 2013, 34, 118–122. [Google Scholar] [CrossRef]
- Lawrence, D.R.; Scott, G.I. The Determination and Use of Condition Index of Oysters. Estuaries 1982, 5, 23–27. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 15 June 2022).
- Zhang, J.; Peng, S.; Zhang, X.; Fan, R.; Zhao, X.; Qi, M.; Liu, R.; Xu, D.; Liu, D. Investigation of Different Solutions Activated by Air Plasma Jet and Their Anticancer Effect. Appl. Phys. Lett. 2022, 120, 264102. [Google Scholar] [CrossRef]
- Baek, K.H.; Yong, H.I.; Yoo, J.H.; Kim, J.W.; Byeon, Y.S.; Lim, J.; Yoon, S.Y.; Ryu, S.; Jo, C. Antimicrobial Effects and Mechanism of Plasma Activated Fine Droplets Produced from Arc Discharge Plasma on Planktonic Listeria monocytogenes and Escherichia coli O157:H7. J. Phys. D Appl. Phys. 2020, 53, 124002. [Google Scholar] [CrossRef]
- Hadinoto, K.; Rao, N.R.H.; Astorga, J.B.; Zhou, R.; Biazik, J.; Zhang, T.; Cullen, P.J.; Prescott, S.; Henderson, R.K.; Trujillo, F.J. Hybrid Plasma-Bubble Discharges for Energy-Efficient Production of Plasma-Activated Water. SSRN 2022, 541, 4115167. [Google Scholar] [CrossRef]
- Epifanio, C.E.; Srna, R.F. Toxicity of Ammonia, Nitrite Ion, Nitrate Ion, and Orthophosphate to Mercenaria mercenaria and Crassostrea virginica. Mar. Biol. 1975, 33, 241–246. [Google Scholar] [CrossRef]
- Rybovich, M.; La Peyre, M.K.; Hall, S.G.; Peyre, J.F. Increased Temperatures Combined with Lowered Salinities Differentially Impact Oyster Size Class Growth and Mortality. J. Shellfish Res. 2016, 35, 101–113. [Google Scholar] [CrossRef]
- La Peyre, M.K.; Geaghan, J.; Decossas, G.; Peyre, J.F. Analysis of Environmental Factors Influencing Salinity Patterns, Oyster Growth, and Mortality in Lower Breton Sound Estuary, Louisiana, Using 20 Years of Data. J. Coast. Res. 2016, 32, 519–530. [Google Scholar] [CrossRef]
- Love, D.; Lovelace, G.; Sobsey, M. Removal of Escherichia coli, Enterococcus Fecalis, Coliphage MS2, Poliovirus, and Hepatitis A Virus from Oysters (Crassostrea Virginica) and Hard Shell Clams (Mercinaria mercinaria) by Depuration. Int. J. Food Microbiol. 2010, 143, 211–217. [Google Scholar] [CrossRef]
- Pace, J.; Wu, C.; Chai, T. Bacterial Flora in Pasteurized Oysters after Refrigerated Storage. J. Food Sci. 1988, 53, 325–327. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.-D.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Ren, T.; Su, Y.-C. Effects of Electrolyzed Oxidizing Water Treatment on Reducing Vibrio parahaemolyticus and Vibrio vulnificus in Raw Oysters. J. Food Prot. 2006, 69, 1829–1834. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Adams, R.; Farkas, D.; Morrissey, M. Use of High-pressure Processing for Oyster Shucking and Shelf-life Extension. J. Food Sci. 2002, 67, 640–645. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, K.B.; Shin, S.B.; Park, K.; Oh, E.G.; Son, K.T.; Yu, H.; Lee, H.J.; Mok, J.S. Comparison of Bioaccumulation and Elimination of Escherichia coli and Male-Specific Bacteriophages by Ascidians and Bivalves. Environ. Sci. Pollut. Res. 2017, 24, 28268–28276. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, S.; Mohamed, K.; Venkatesan, V.; Sharma, J.; Kripa, V. Depuration of Bacterial Populations in the Indian Backwater Oyster Crassostrea madrasensis (Preston, 1916): Effects on Surface and Bottom Held Oysters. J. Shellfish Res. 2014, 33, 409–415. [Google Scholar] [CrossRef]
- Csadek, I.; Paulsen, P.; Weidinger, P.; Bak, K.H.; Bauer, S.; Pilz, B.; Nowotny, N.; Smulders, F.J.M. Nitrogen Accumulation in Oyster (Crassostrea gigas) Slurry Exposed to Virucidal Cold Atmospheric Plasma Treatment. Life 2021, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-S.; Jeon, E.B.; Kim, J.Y.; Choi, E.H.; Lim, J.S.; Choi, J.; Park, S.Y. Application of Dielectric Barrier Discharge Plasma for the Reduction of Non-Pathogenic Escherichia coli and E. coli O157:H7 and the Quality Stability of Fresh Oysters (Crassostrea gigas). LWT 2022, 154, 112698. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Dielectric Barrier Discharge Cold Atmospheric Plasma: Bacterial Inactivation Mechanism. J. Food Saf. 2019, 39, e12705. [Google Scholar] [CrossRef]
- Campbell, V.; Chouljenko, A.; Hall, S. Depuration of Live Oysters to Reduce Vibrio parahaemolyticus and Vibrio vulnificus: A Review of Ecology and Processing Parameters. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3480–3506. [Google Scholar] [CrossRef]
- Ruano, F.; Ramos, P.; Quaresma, M.; Bandarra, N.M.; Da Fonseca, I.P. Evolution of Fatty Acid Profile and Condition Index in Mollusc Bivalves Submitted to Different Depuration Periods. Rev. Port. Cienc. Vet. 2012, 111, 75–84. [Google Scholar]
- Bagenda, D.K.; Nishikawa, S.; Kita, H.; Kinai, Y.; Terai, S.; Kato, M.; Kasai, H. Impact of Feeding on Oyster Depuration Efficacy Under Conditions of High Salinity and Low Temperature. Aquaculture 2019, 500, 135–140. [Google Scholar] [CrossRef]
- Bio, S.; Nunes, B. Twists and Turns of an Oyster’s Life: Effects of Different Depuration Periods on Physiological Biochemical Functions of Oysters. Environ. Sci. Pollut. Res. 2021, 28, 29601–29614. [Google Scholar] [CrossRef]
- Percy, J.A.; Aldrich, F.A.; Marcus, T.R. Influence of Environmental Factors on Respiration of Excised Tissues of American Oysters, Crassostrea virginica (Gmelin). Can. J. Zool. 1971, 49, 353–360. [Google Scholar] [CrossRef]
- Mudoh, M.F.; Parveen, S.; Schwarz, J.; Rippen, T.; Chaudhuri, A. The Effects of Storage Temperature on the Growth of Vibrio parahaemolyticus and Organoleptic Properties in Oysters. Front. Public Health 2014, 2, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Walton, W.C.; Wang, Y. Quantitative Quality Evaluation of Eastern Oyster (Crassostrea virginica) Cultured by Two Different Methods. Aquac. Res. 2017, 48, 2934–2944. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.-K.; Jo, D.-M.; Khan, F.; Yu, D.; Lee, J.-H.; Kang, M.-G.; Lee, D.; Sim, Y.-A.; Kim, Y.-M. Effectiveness of Depuration of Pacific Oyster (Crassostrea Gigas): Removal of Bioaccumulated Vibrio Vulnificus by UV-Treatment. Food Sci. Biotechnol. 2021, 30, 765–771. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Temperature (°C) | pH | Oxidation Reduction Potential (mV) | Conductivity (µS/cm) |
|---|---|---|---|---|
| PASW10 (3:1) | ||||
| Before activation Immediately after activation | 18.7 ± 0.6 Bc | 9.06 ± 0.0 Bc | −116.6 ± 0.3 Bc | 44.0 ± 0.1 Bc |
| 55.3 ± 1.5 Cb | 2.93 ± 0.0 Db | 249.8 ± 2.1 Cb | 57.5 ± 0.6 Db | |
| After dilution with SS | 23.0 ± 0.0 Ea | 5.49 ± 0.0 Fa | 76.30 ± 0.6 Ea | 46.4 ± 1.4 ACa |
| PASW10 (1:1) | ||||
| Before activation Immediately after activation | 19.0 ± 0.0 Bc | 9.00 ± 0.0 Cc | −114.0 ± 0.8 Bc | 44.6 ± 0.0 BCc |
| 48.3 ± 1.5 Db | 2.99 ± 0.0 Eb | 243.0 ± 0.6 Db | 52.2 ± 1.0 Eb | |
| After dilution with SS | 27.0 ± 0.0 Aa | 3.43 ± 0.0 Aa | 201.4 ± 0.3 Aa | 47.2 ± 0.4 Aa |
| Treatment | NO3− (mM) | NO2− (mM) |
|---|---|---|
| SS (control) | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| PASW10 (3:1) | 5.21 ±1.84 c | 6.71 ± 0.01 c |
| PASW10 (1:1) | 34.3 ±1.43 a | 11.3 ± 0.11 a |
| # of Oysters | Mass (g) | Length (cm) | Width (cm) | |
|---|---|---|---|---|
| Processing tests | 30 | 33.8 ± 6.96 | 5.84 ± 0.68 | 3.38 ± 0.44 |
| Quality tests | 13 | 30.8 ± 6.70 | 5.00 ± 0.54 | 3.15 ± 0.59 |
| Average | 32.9 ± 6.94 | 5.57 ± 0.75 | 3.30 ± 0.49 |
| Treatment | CI | ∆ CI |
|---|---|---|
| Control (0 h) | 5.51 ± 2.49 | – |
| Refrigeration | 3.65 ± 1.11 | −1.86 |
| SS depuration | 5.15 ± 1.49 | −0.36 |
| PASW10 depuration | 3.35 ± 0.87 | −2.16 |
| Treatment | Peak Force (g/mm) | ∆ Peak Force (g/mm) |
|---|---|---|
| Control (0 h) | 177 ± 3.54 | — |
| Refrigeration | 339 ± 147 | +162 |
| SS depuration | 117 ± 7.72 | −60.0 |
| PASW10 depuration | 198 ± 49.0 | +21.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, V.M.; Hall, S.; Salvi, D. Antimicrobial Effects of Plasma-Activated Simulated Seawater (PASW) on Total Coliform and Escherichia coli in Live Oysters during Static Depuration. Fishes 2023, 8, 396. https://doi.org/10.3390/fishes8080396
Campbell VM, Hall S, Salvi D. Antimicrobial Effects of Plasma-Activated Simulated Seawater (PASW) on Total Coliform and Escherichia coli in Live Oysters during Static Depuration. Fishes. 2023; 8(8):396. https://doi.org/10.3390/fishes8080396
Chicago/Turabian StyleCampbell, Vashti M., Steven Hall, and Deepti Salvi. 2023. "Antimicrobial Effects of Plasma-Activated Simulated Seawater (PASW) on Total Coliform and Escherichia coli in Live Oysters during Static Depuration" Fishes 8, no. 8: 396. https://doi.org/10.3390/fishes8080396
APA StyleCampbell, V. M., Hall, S., & Salvi, D. (2023). Antimicrobial Effects of Plasma-Activated Simulated Seawater (PASW) on Total Coliform and Escherichia coli in Live Oysters during Static Depuration. Fishes, 8(8), 396. https://doi.org/10.3390/fishes8080396







