Narrowing the Horizon: Using Known Invasives and Propagule Pressure to Focus Risk Screening Efforts on Potential Invasives
Abstract
1. Introduction
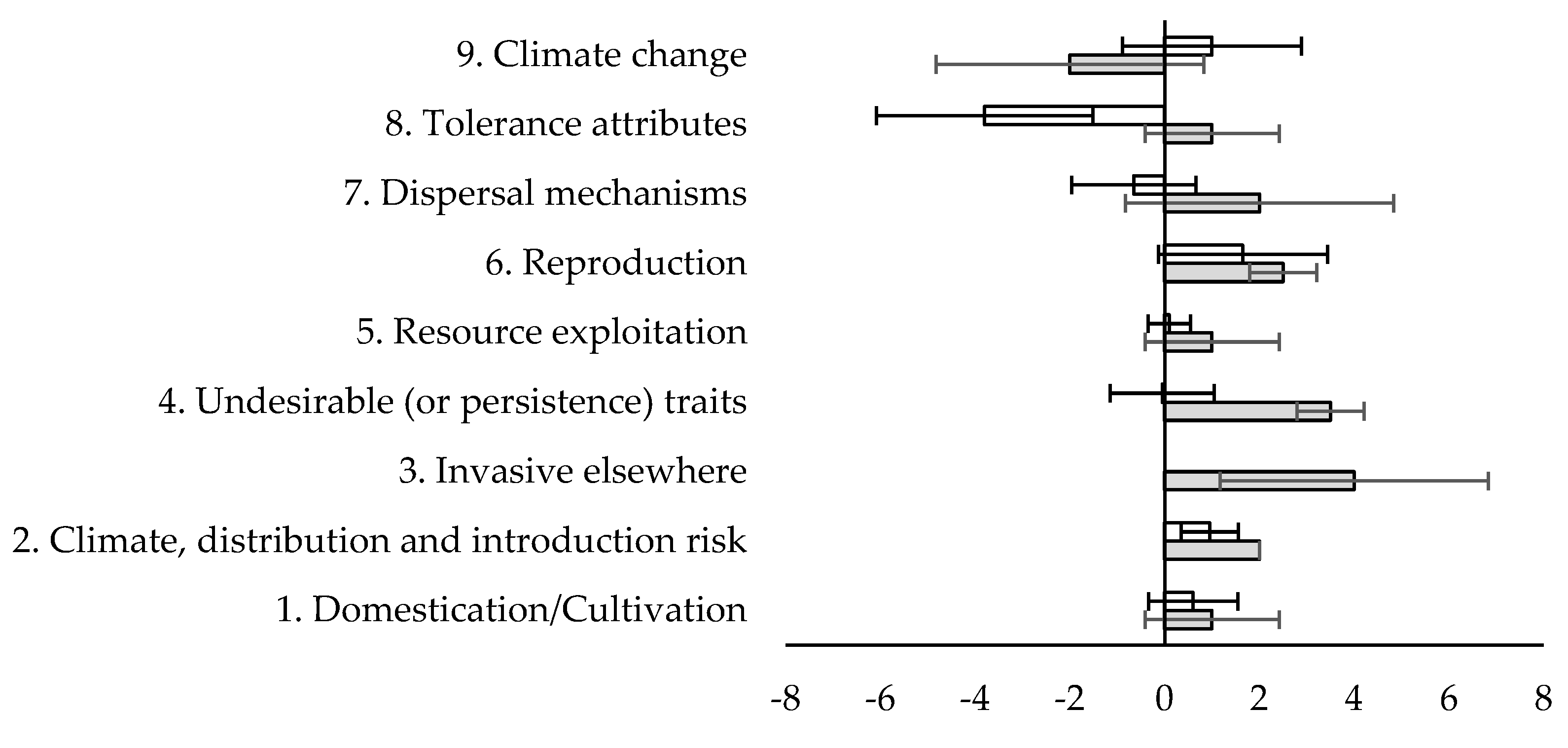
2. Materials and Methods
3. Results
4. Discussion
Management Recommendations
- Educating the marine aquarium hobbyist about releasing aquarium fish, including partnering with Land Grant and Sea Grant institutions, public aquaria, museums, zoological centers, and other organizations to multiply and coordinate efforts;
- Maintaining and promoting websites and applications for reporting non-native species, including non-native pomacentrids and other marine aquarium species;
- Monitoring coastal waters, especially known hotspot bridges, jetties, and reefs for released aquarium fishes (EDRR);
- Conducting removals of non-native pomacentrids or other marine aquarium species when detected (EDRR);
- Updating screens and assessments as new data of importance become available (e.g., new species establish).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Hazard—A risk source related to harm. Anything that can cause harm;
- Risk—A future activity or occurrence with potential negative consequences; the probability of an event occurring and the severity of the consequences.
- Hazard identification—A process whereby hazards are distinguished from non-hazards; determination of whether the non-native species of interest is likely to cause harm;
- Risk assessment—A systematic process to comprehend the nature of risk, and express and evaluate risk; estimation of the probability that a non-native species will be introduced, established, and spread; and evaluation of the impacts of the species in a region of interest (i.e., risk assessment area).
References
- Wood, E. Global Advances in Conservation and Management of Marine Ornamental Resources. Aquar. Sci. Conserv. 2001, 3, 65–77. [Google Scholar] [CrossRef]
- Leal, M.C.; Rocha, R.J.M.; Rosa, R.; Calado, R. Aquaculture of Marine Non-Food Organisms: What, Why and How? Rev. Aquac. 2018, 10, 400–423. [Google Scholar] [CrossRef]
- Rhyne, A.L.; Tlusty, M.F.; Schofield, P.J.; Kaufman, L.J.A.M., Jr.; Bruckner, A.W. Revealing the Appetite of the Marine Aquarium Fish Trade: The Volume and Biodiversity of Fish Imported into the United States. PLoS ONE 2012, 7, e35808. [Google Scholar] [CrossRef]
- Groover, E.; DiMaggio, M.; Cassiano, E. FA224/FA224: Overview of Commonly Cultured Marine Ornamental Fish. Available online: https://edis.ifas.ufl.edu/publication/FA224 (accessed on 29 November 2022).
- Biondo, M.V.; Burki, R.P. A Systematic Review of the Ornamental Fish Trade with Emphasis on Coral Reef Fishes—An Impossible Task. Animals 2020, 10, 2014. [Google Scholar] [CrossRef]
- Lyons, T.J.; Tuckett, Q.M.; Hill, J.E. Characterizing the US Trade in Lionfishes. PLoS ONE 2019, 14, e221272. [Google Scholar] [CrossRef]
- Zajicek, P.; Hardin, S.; Watson, C. A Florida Marine Ornamental Pathway Risk Analysis. Rev. Fish. Sci. 2009, 17, 156–169. [Google Scholar] [CrossRef]
- Rhyne, A.L.; Tlusty, M.F.; Szczebak, J.T.; Holmberg, R.J. Expanding Our Understanding of the Trade in Marine Aquarium Animals. PeerJ 2017, 5, e2949. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Cassey, P.; Blackburn, T.M. The More You Introduce the More You Get: The Role of Colonization Pressure and Propagule Pressure in Invasion Ecology. Divers. Distrib. 2009, 15, 904–910. [Google Scholar] [CrossRef]
- Schofield, P. Geographic Extent and Chronology of the Invasion of Non-Native Lionfish (Pterois Volitans [Linnaeus 1758] and P. Miles [Bennett 1828]) in the Western North Atlantic and Caribbean Sea. Aquat. Invasions 2009, 4, 473–479. [Google Scholar] [CrossRef]
- Campbell, M.D.; Pollack, A.G.; Thompson, K.; Switzer, T.; Driggers, W.B.; Hoffmayer, E.R.; Keenan, S.; Gardner, C.; Hanisko, D.; Rademacher, K.R.; et al. Rapid Spatial Expansion and Population Increase of Invasive Lionfish (Pterois Spp.) Observed on Natural Habitats in the Northern Gulf of Mexico. Biol. Invasions 2022, 24, 93–105. [Google Scholar] [CrossRef]
- Lyons, T.J.; Tuckett, Q.M.; Hill, J.E. Data Quality and Quantity for Invasive Species: A Case Study of the Lionfishes. Fish Fish. 2019, 20, 748–759. [Google Scholar] [CrossRef]
- Lyons, T.J.; Tuckett, Q.M.; Durland Donahou, A.; Hill, J.E. Risk Screen of Lionfishes, Pterois, Dendrochirus, and Parapterois, for Southeastern United States Coastal Waters of the Gulf of Mexico and Atlantic Ocean. Biol. Invasions 2020, 22, 1573–1583. [Google Scholar] [CrossRef]
- Hardin, S.; Hill, J.E. Risk Analysis of Barramundi Perch Lates Calcarifer Aquaculture in Florida. N. Am. J. Fish. Manag. 2012, 32, 577–585. [Google Scholar] [CrossRef]
- Hill, J.E.; Lawson, K.M. Risk Screening of Arapaima, a New Species Proposed for Aquaculture in Florida. N. Am. J. Fish. Manag. 2015, 35, 885–894. [Google Scholar] [CrossRef]
- Vaz, A.S.; Novoa, A.; Vicente, J.R.; Honrado, J.P.; Shackleton, R.T. Editorial: Invaders on the Horizon! Scanning the Future of Invasion Science and Management. Front. Ecol. Evol. 2021, 9, 756339. [Google Scholar] [CrossRef]
- Roy, H.E.; Peyton, J.; Aldridge, D.C.; Bantock, T.; Blackburn, T.M.; Britton, R.; Clark, P.; Cook, E.; Dehnen-Schmutz, K.; Dines, T.; et al. Horizon Scanning for Invasive Alien Species with the Potential to Threaten Biodiversity in Great Britain. Glob. Chang. Biol. 2014, 20, 3859–3871. [Google Scholar] [CrossRef]
- Tsiamis, K.; Azzurro, E.; Bariche, M.; Çinar, M.E.; Crocetta, F.; De Clerck, O.; Galil, B.; Gómez, F.; Hoffman, R.; Jensen, K.R.; et al. Prioritizing Marine Invasive Alien Species in the European Union through Horizon Scanning. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 794–845. [Google Scholar] [CrossRef]
- Matthews, J.; Beringen, R.; Creemers, R.; Hollander, H.; van Kessel, N.; van Kleef, H.; van de Koppel, S.; Lemaire, A.J.J.; Odé, B.; Verbrugge, L.N.H.; et al. A New Approach to Horizon-Scanning: Identifying Potentially Invasive Alien Species and Their Introduction Pathways. Manag. Biol. Invasions 2017, 8, 37–52. [Google Scholar] [CrossRef]
- CABI Horizon Scanning Tool. CAB International. Wallingford, UK. Available online: https://www.cabi.org/HorizonScanningTool (accessed on 29 March 2023).
- Gallien, L.; Carboni, M. The Community Ecology of Invasive Species: Where Are We and What’s Next? Ecography 2017, 40, 335–352. [Google Scholar] [CrossRef]
- Van Wilgen, N.J.; Richardson, D.M. The Roles of Climate, Phylogenetic Relatedness, Introduction Effort, and Reproductive Traits in the Establishment of Non-Native Reptiles and Amphibians. Conserv. Biol. 2012, 26, 267–277. [Google Scholar] [CrossRef]
- Azzurro, E.; Tuset, V.M.; Lombarte, A.; Maynou, F.; Simberloff, D.; Rodríguez-Pérez, A.; Solé, R.V. External Morphology Explains the Success of Biological Invasions. Ecol. Lett. 2014, 17, 1455–1463. [Google Scholar] [CrossRef]
- Duggan, I.C.; Rixon, C.A.M.; MacIsaac, H.J. Popularity and Propagule Pressure: Determinants of Introduction and Establishment of Aquarium Fish. Biol. Invasions 2006, 8, 377–382. [Google Scholar] [CrossRef]
- Bradie, J.; Chivers, C.; Leung, B.; Richardson, D. Importing Risk: Quantifying the Propagule Pressure-Establishment Relationship at the Pathway Level. Divers. Distrib. 2013, 19, 1020–1030. [Google Scholar] [CrossRef]
- USGS (U.S. Geological Survey). Nonindigenous Aquatic Species Database; USGS: Reston, VA, USA, 2022.
- González Gándara, C.; de la Cruz Francisco, V. Unusual Record of the Indo-Pacific Pomacentrid Neopomacentrus Cyanomos (Bleeker, 1856) on Coral Reefs of the Gulf of Mexico. BioInvasions Rec. 2014, 3, 49–52. [Google Scholar] [CrossRef]
- Robertson, D.R.; Dominguez-Dominguez, O.; Victor, B.; Simoes, N. An Indo-Pacific Damselfish (Neopomacentrus Cyanomos) in the Gulf of Mexico: Origin and Mode of Introduction. PeerJ 2018, 6, e4328. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Tidbury, H.; Stebbing, P.D.; Tarkan, A.S.; Miossec, L.; Goulletquer, P. Development of a Generic Decision-Support Tool for Identifying Potentially Invasive Aquatic Taxa: AS-ISK. Manag. Biol. Invasions 2016, 7, 343–350. [Google Scholar] [CrossRef]
- Vilizzi, L.; Copp, G.H.; Hill, J.E.; Adamovich, B.; Aislabie, L.; Akin, D.; Al-Faisal, A.J.; Almeida, D.; Azmai, M.N.A.; Bakiu, R.; et al. A Global-Scale Screening of Non-Native Aquatic Organisms to Identify Potentially Invasive Species under Current and Future Climate Conditions. Sci. Total Environ. 2021, 788, 147868. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Fong, J.D. Eschmeyer’s Catalog of Fishes—Genera/Species by Family/Subfamily. Available online: https://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp (accessed on 8 July 2022).
- Hill, J.E.; Tuckett, Q.M.; Lapham, L.; Asp, E. Pomacentrid Risk Screening Bioprofiles; Florida Fish and Wildlife Conservation Commission: Tallahassee, FL, USA, 2019. [Google Scholar]
- Vilizzi, L.; Copp, G.H.; Adamovich, B.; Almeida, D.; Chan, J.; Davison, P.I.; Dembski, S.; Ekmekçi, F.G.; Ferincz, Á.; Forneck, S.C.; et al. A Global Review and Meta-Analysis of Applications of the Freshwater Fish Invasiveness Screening Kit. Rev. Fish Biol. Fish. 2019, 29, 529–568. [Google Scholar] [CrossRef]
- Lawson, L.; Hill, J.; Hardin, S.; Vilizzi, L.; Copp, G. Evaluation of the Fish Invasiveness Screening Kit (FISK v2) for Peninsular Florida. Manag. Biol. Invasions 2015, 6, 413. [Google Scholar] [CrossRef]
- Lawson, L.L., Jr.; Hill, J.E.; Vilizzi, L.; Hardin, S.; Copp, G.H. Revisions of the Fish Invasiveness Screening Kit (FISK) for Its Application in Warmer Climatic Zones, with Particular Reference to Peninsular Florida. Risk Anal. 2013, 33, 1414–1431. [Google Scholar] [CrossRef]
- Roy, H.E.; Rabitsch, W.; Scalera, R.; Stewart, A.; Gallardo, B.; Genovesi, P.; Essl, F.; Adriaens, T.; Bacher, S.; Booy, O.; et al. Developing a Framework of Minimum Standards for the Risk Assessment of Alien Species. J. Appl. Ecol. 2018, 55, 526–538. [Google Scholar] [CrossRef]
- Bennett, C. First Record of the Non-Indigenous Indo-Pacific Damselfish, Neopomacentrus Cyanomos (Bleeker, 1856) in the Northern Gulf of Mexico. BioInvasions Rec. 2019, 8, 154–166. [Google Scholar] [CrossRef]
- Eme, J.; Bennett, W.A. Low Temperature as a Limiting Factor for Introduction and Distribution of Indo-Pacific Damselfishes in the Eastern United States. J. Therm. Biol. 2008, 33, 62–66. [Google Scholar] [CrossRef]
- Robertson, D.R.; Simoes, N.; Gutierrez Rodriguez, C.; Pineros, V.J.; Perez-Espana, H. An Indo-Pacific Damselfish Well Established in the SouthernGulf of Mexico: Prospects for a Wider, Adverse Invasion. J. Ocean Sci. Found. 2016, 19, 1–17. [Google Scholar]
- Tarnecki, J.H.; Garner, S.B.; Patterson, W.F. Non-Native Regal Demoiselle, Neopomacentrus Cyanomos, Presence, Abundance, and Habitat Factors in the North-Central Gulf of Mexico. Biol. Invasions 2021, 23, 1681–1693. [Google Scholar] [CrossRef]
- Robertson, R.; Dominguez-Dominguez, O.; Solís-Guzmán, M.; Kingon, K. Origins of Isolated Populations of an Indo-Pacific Damselfish at Opposite Ends of the Greater Caribbean. Aquat. Invasions 2021, 16, 269–280. [Google Scholar] [CrossRef]
- Hoese, H.D.; Moore, R.H. Fishes of the Gulf of Mexico, 2nd ed.; Exas A & M University Press: College Station, TX, USA, 1998. [Google Scholar]
- Lawson, K.M.; Hill, J.E. Predicting Successful Reproduction and Establishment of Non-Native Freshwater Fish in Peninsular Florida Using Life History Traits. J. Vertebr. Biol. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Lawson, K.M.; Hill, J.E. Life History Strategies Differentiate Established from Failed Non-native Freshwater Fish in Peninsular Florida. Divers. Distrib. 2022, 28, 160–172. [Google Scholar] [CrossRef]
- Green, S.J.; Akins, J.L.; Maljković, A.; Côté, I.M. Invasive Lionfish Drive Atlantic Coral Reef Fish Declines. PLoS ONE 2012, 7, e32596. [Google Scholar] [CrossRef]
- Côté, I.M.; Green, S.J.; Hixon, M.A. Predatory Fish Invaders: Insights from Indo-Pacific Lionfish in the Western Atlantic and Caribbean. Biol. Conserv. 2013, 164, 50–61. [Google Scholar] [CrossRef]
- Chaudhary, C.; Richardson, A.J.; Schoeman, D.S.; Costello, M.J. Global Warming Is Causing a More Pronounced Dip in Marine Species Richness around the Equator. Proc. Natl. Acad. Sci. USA 2021, 118, e2015094118. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, K.F. The Regime Shift of the 1920s and 1930s in the North Atlantic. Prog. Oceanogr. 2006, 68, 134–151. [Google Scholar] [CrossRef]
- Urban, M.C.; Bocedi, G.; Hendry, A.P.; Mihoub, J.-B.; Pe’er, G.; Singer, A.; Bridle, J.R.; Crozier, L.G.; De Meester, L.; Godsoe, W.; et al. Improving the Forecast for Biodiversity under Climate Change. Science 2016, 353, aad8466. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the Global Threat of Invasive Species to Marine Biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Vilizzi, L.; Hill, J.E.; Piria, M.; Copp, G.H. A Protocol for Screening Potentially Invasive Non-Native Species Using Weed Risk Assessment-Type Decision-Support Tools. Sci. Total Environ. 2022, 832, 154966. [Google Scholar] [CrossRef] [PubMed]
- Bilge, G.; Filiz, H.; Yapici, S.; Tarkan, A.S.; Vilizzi, L. A risk screening study on the potential invasiveness of Lessepsian fishes in the south-western coasts of Anatolia. Acta Ichthyol. Piscat. 2019, 49, 23–31. [Google Scholar] [CrossRef]
- Lobel, P.S. Herbivory by Damselfishes and Their Role in Coral Reef Community Ecology. Bull. Mar. Sci. 1980, 30, 273–289. [Google Scholar]
- Figueira, W.F.; Lyman, S.J.; Crowder, L.B.; Rilov, G. Small-Scale Demographic Variability of the Biocolor Damselfish, Stegastes Partitus, in the Florida Keys USA. Environ. Biol. Fishes 2008, 81, 297–311. [Google Scholar] [CrossRef]
- Keller, R.P.; Springborn, M.R. Closing the Screen Door to New Invasions. Conserv. Lett. 2014, 7, 285–292. [Google Scholar] [CrossRef]
- Hayes, K.R.; Barry, S.C. Are There Any Consistent Predictors of Invasion Success? Biol. Invasions 2008, 10, 483–506. [Google Scholar] [CrossRef]
- Aven, T.; Ben-Haim, Y.; Andersen, H.B.; Cox, T.; Droguett, E.L.; Greenberg, M.; Guikema, S.; Kröger, W.; Renn, O.; Thompson, K.M.; et al. Society for Risk Analysis Glossary; Society for Risk Analysis: Herndon, VA, USA.
- Pheloung, P.C.; Williams, P.A.; Halloy, S.R. A Weed Risk Assessment Model for Use as a Biosecurity Tool Evaluating Plant Introductions. J. Environ. Manag. 1999, 57, 239–251. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Mumford, J.; Fenwick, G.V.; Godard, M.J.; Gozlan, R.E. Calibration of FISK, an Invasiveness Screening Tool for Nonnative Freshwater Fishes. Risk Anal. Int. J. 2009, 29, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Marr, S.M.; Ellender, B.R.; Woodford, D.J.; Alexander, M.E.; Wasserman, R.J.; Ivey, P.; Tsungai, Z.; Weyl, O.L.F. Evaluating Invasion Risk for Freshwater Fishes in South Africa. Bothalia Afr. Biodivers. Conserv. 2017, 47, 1–10. [Google Scholar] [CrossRef]
- Hill, J.E.; Copp, G.H.; Hardin, S.; Lawson, K.M.; Lawson, L.L., Jr.; Tuckett, Q.M.; Vilizzi, L.; Watson, C.A. Comparing Apples to Oranges and Other Misrepresentations of the Risk Screening Tools FISK and AS-ISK—A Rebuttal of Marcot et al. (2019). Manag. Biol. Invasions 2020, 11, 325–341. [Google Scholar] [CrossRef]
- Marcot, B.G.; Hoff, M.H.; Martin, C.D.; Jewell, S.D.; Givens, C.E. A Decision Support System for Identifying Potentially Invasive and Injurious Freshwater Fishes. Manag. Biol. Invasions 2019, 10, 200–226. [Google Scholar] [CrossRef]
- USFWS (U.S. Fish and Wildlife Service). Standard Operating Procedures: How to Prepare an “Ecological Risk Screening Summary”; USFWS: Bailey’s Crossroads, VA, USA, 2020; p. 132.
- Bayon, A.; Vila, M. Horizon Scanning to Identify Invasion Risk of Ornamental Plants Marketed in Spain. NeoBiota 2019, 52, 47–87. [Google Scholar] [CrossRef]
| Common Name | Scientific Name | High Trade Volume |
|---|---|---|
| Spiny Chromis Damselfish | Acanthochromis polyacanthus | No (collected in Miami Beach) |
| Clown Anemonefish | Amphiprion ocellaris | Yes |
| Orange Clownfish | Amphiprion percula | Yes |
| Green Chromis | Chromis viridis | Yes |
| Sapphire Devil | Chrysiptera cyanea | Yes |
| Azure Demoiselle | Chrysiptera hemicyanea | Yes |
| Goldtail Demoiselle | Chrysiptera parasema | Yes |
| Whitetail Humbug | Dascyllus aruanus | Yes |
| Blacktail Dascyllus | Dascyllus melanurus | Yes |
| Threespot Dascyllus | Dascyllus trimaculatus | Yes |
| Regal Demoiselle | Neopomacentrus cyanomos | No (established in Gulf of Mexico) |
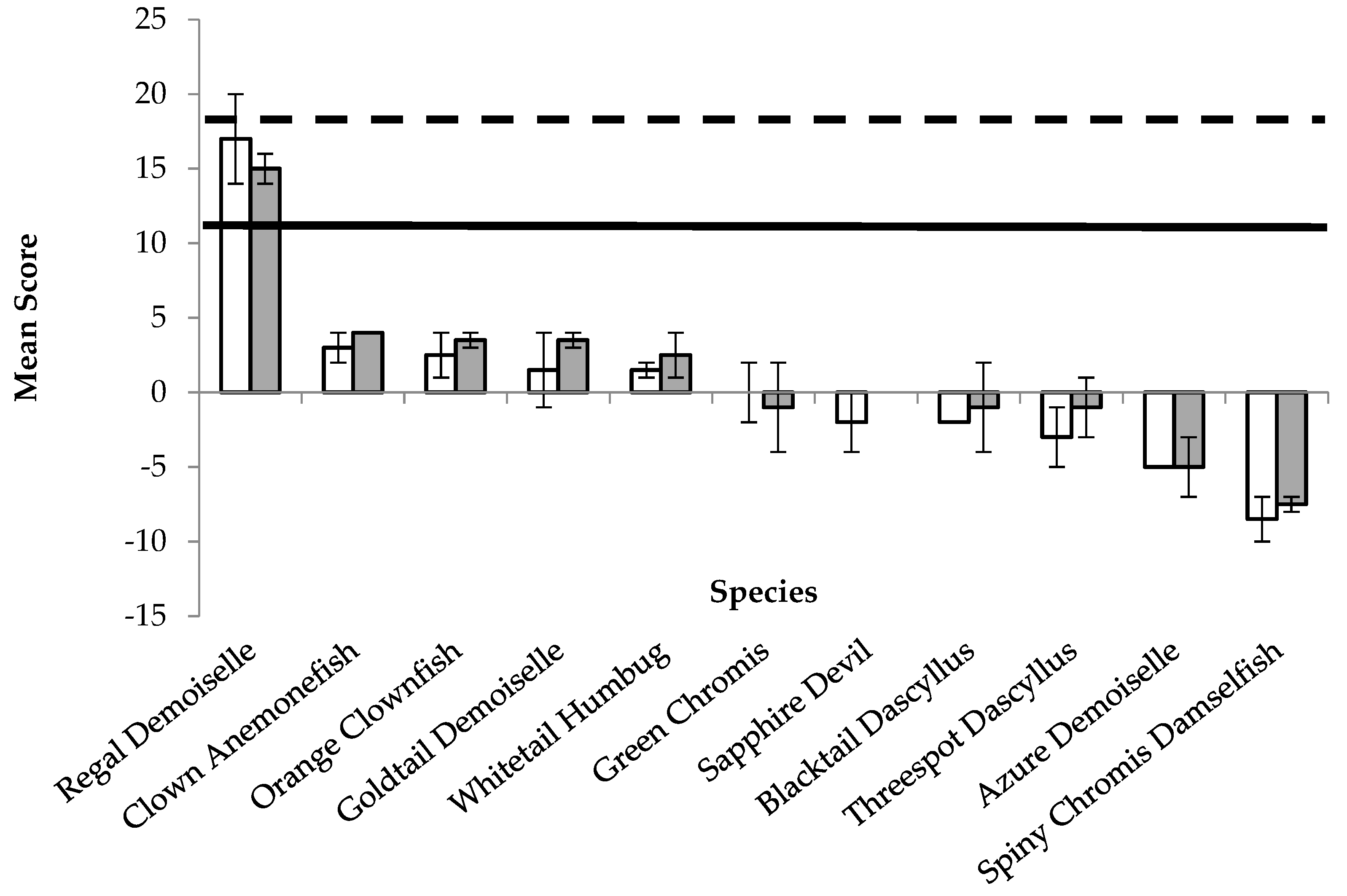
| Species | Mean BRA | Δ BRA | CFBRA | Risk Category (BRA) | Mean BRA+CCA | Δ BRA+CCA | CFCCA | CFBRA+CCA | Risk Category (BRA+CCA) |
|---|---|---|---|---|---|---|---|---|---|
| Regal Demoiselle | 17 | 6 | 0.69 | High | 15 | 2 | 0.56 | 0.68 | Medium |
| Clown Anemonefish | 3 | 2 | 0.65 | Medium | 4 | 0 | 0.56 | 0.64 | Medium |
| Orange Clownfish | 2.5 | 3 | 0.66 | Medium | 3.5 | 1 | 0.58 | 0.65 | Medium |
| Goldtail Demoiselle | 1.5 | 5 | 0.71 | Medium | 3.5 | 1 | 0.60 | 0.70 | Medium |
| Whitetail Humbug | 1.5 | 1 | 0.76 | Medium | 2.5 | 3 | 0.42 | 0.72 | Medium |
| Green Chromis | 0 | 4 | 0.75 | Low | −1 | 6 | 0.52 | 0.73 | Low |
| Sapphire Devil | −2 | 4 | 0.63 | Low | 0 | 0 | 0.44 | 0.61 | Low |
| Blacktail Dascyllus | −2 | 0 | 0.73 | Low | −1 | 6 | 0.40 | 0.69 | Low |
| Threespot Dascyllus | −3 | 4 | 0.67 | Low | −1 | 4 | 0.35 | 0.63 | Low |
| Azure Demoiselle | −5 | 0 | 0.65 | Low | −5 | 4 | 0.31 | 0.61 | Low |
| Spiny Chromis Damselfish | −8.5 | 3 | 0.68 | Low | −7.5 | 1 | 0.44 | 0.65 | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, J.E.; Durland Donahou, A.; Wooley, E.S.; Lapham, L.N.; Tuckett, Q.M. Narrowing the Horizon: Using Known Invasives and Propagule Pressure to Focus Risk Screening Efforts on Potential Invasives. Fishes 2023, 8, 266. https://doi.org/10.3390/fishes8050266
Hill JE, Durland Donahou A, Wooley ES, Lapham LN, Tuckett QM. Narrowing the Horizon: Using Known Invasives and Propagule Pressure to Focus Risk Screening Efforts on Potential Invasives. Fishes. 2023; 8(5):266. https://doi.org/10.3390/fishes8050266
Chicago/Turabian StyleHill, Jeffrey E., Allison Durland Donahou, Emily S. Wooley, Lauren N. Lapham, and Quenton M. Tuckett. 2023. "Narrowing the Horizon: Using Known Invasives and Propagule Pressure to Focus Risk Screening Efforts on Potential Invasives" Fishes 8, no. 5: 266. https://doi.org/10.3390/fishes8050266
APA StyleHill, J. E., Durland Donahou, A., Wooley, E. S., Lapham, L. N., & Tuckett, Q. M. (2023). Narrowing the Horizon: Using Known Invasives and Propagule Pressure to Focus Risk Screening Efforts on Potential Invasives. Fishes, 8(5), 266. https://doi.org/10.3390/fishes8050266






