Microencapsulation of Bacillus subtilis E20 Probiotic, a Promising Approach for the Enrichment of Intestinal Microbiome in White Shrimp, Penaeus vannamei
Abstract
1. Introduction
2. Materials and Methods
2.1. Shrimp Husbandry and Culture Conditions
2.2. Probiotic Encapsulation
2.3. Experimental Population and Treatments
2.4. Intestinal Microbiota Analysis Using Next-Generation Sequencing (NGS)
2.5. Biodiversity and Abundances of Intestinal Microbiota
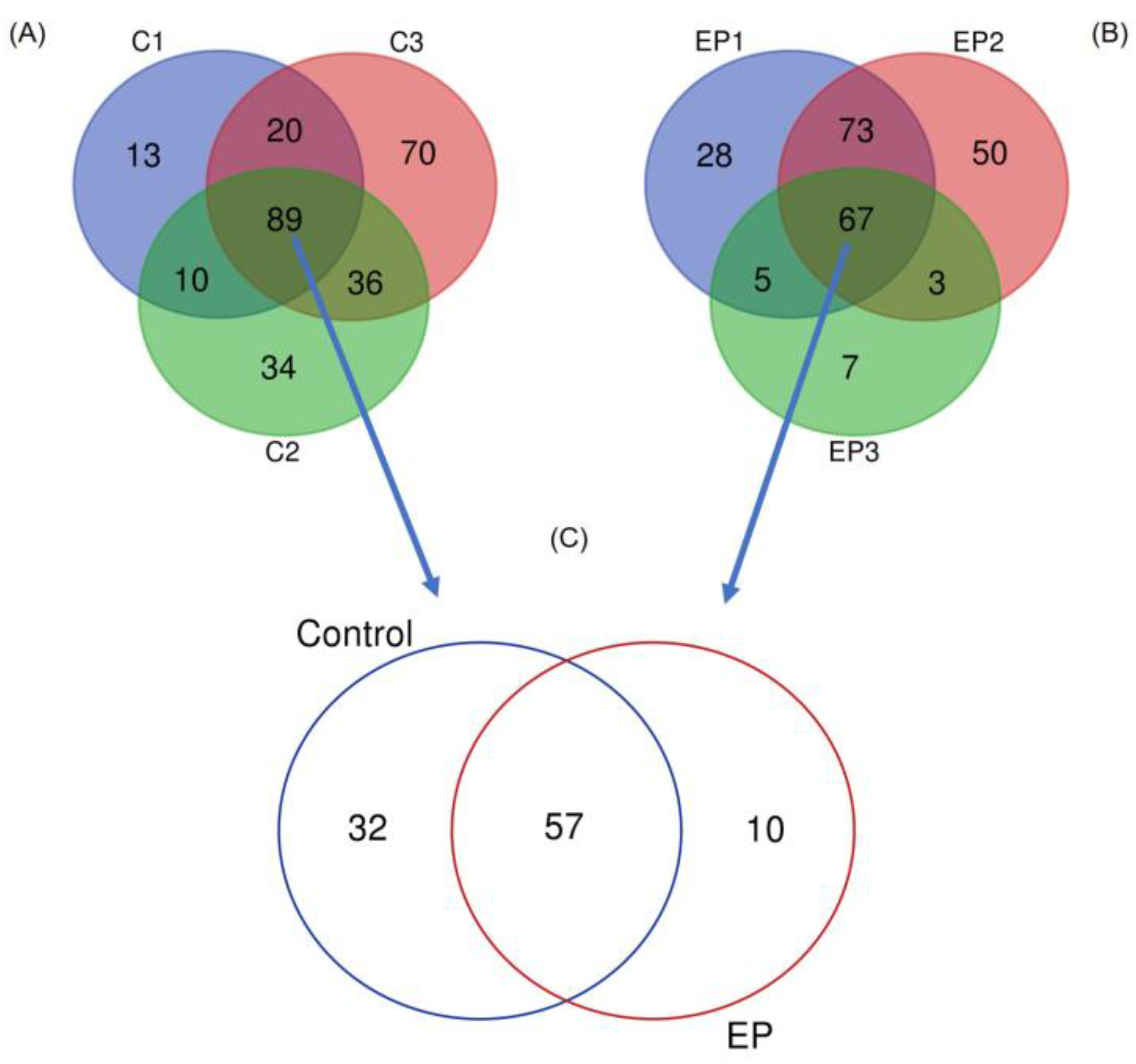
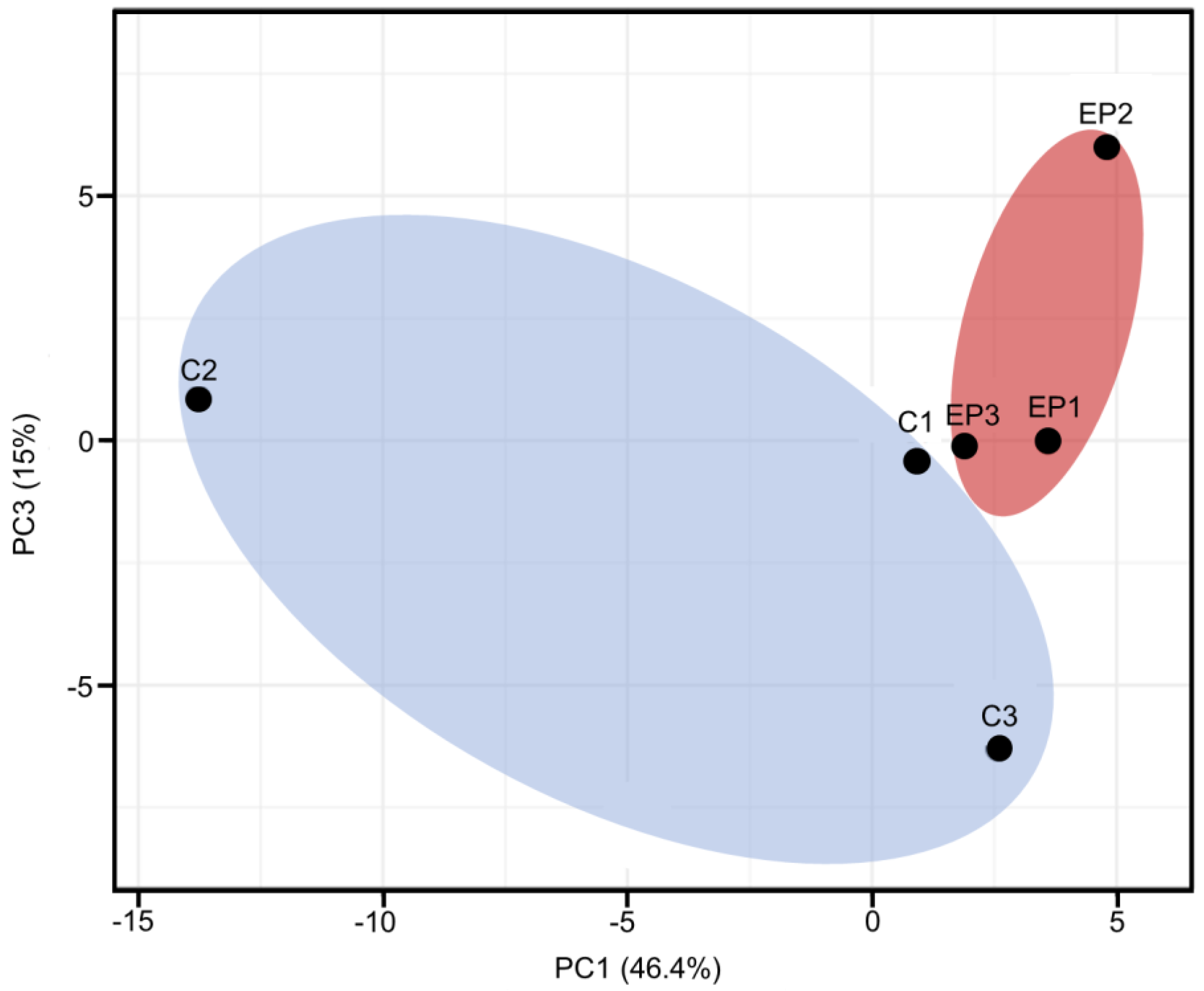
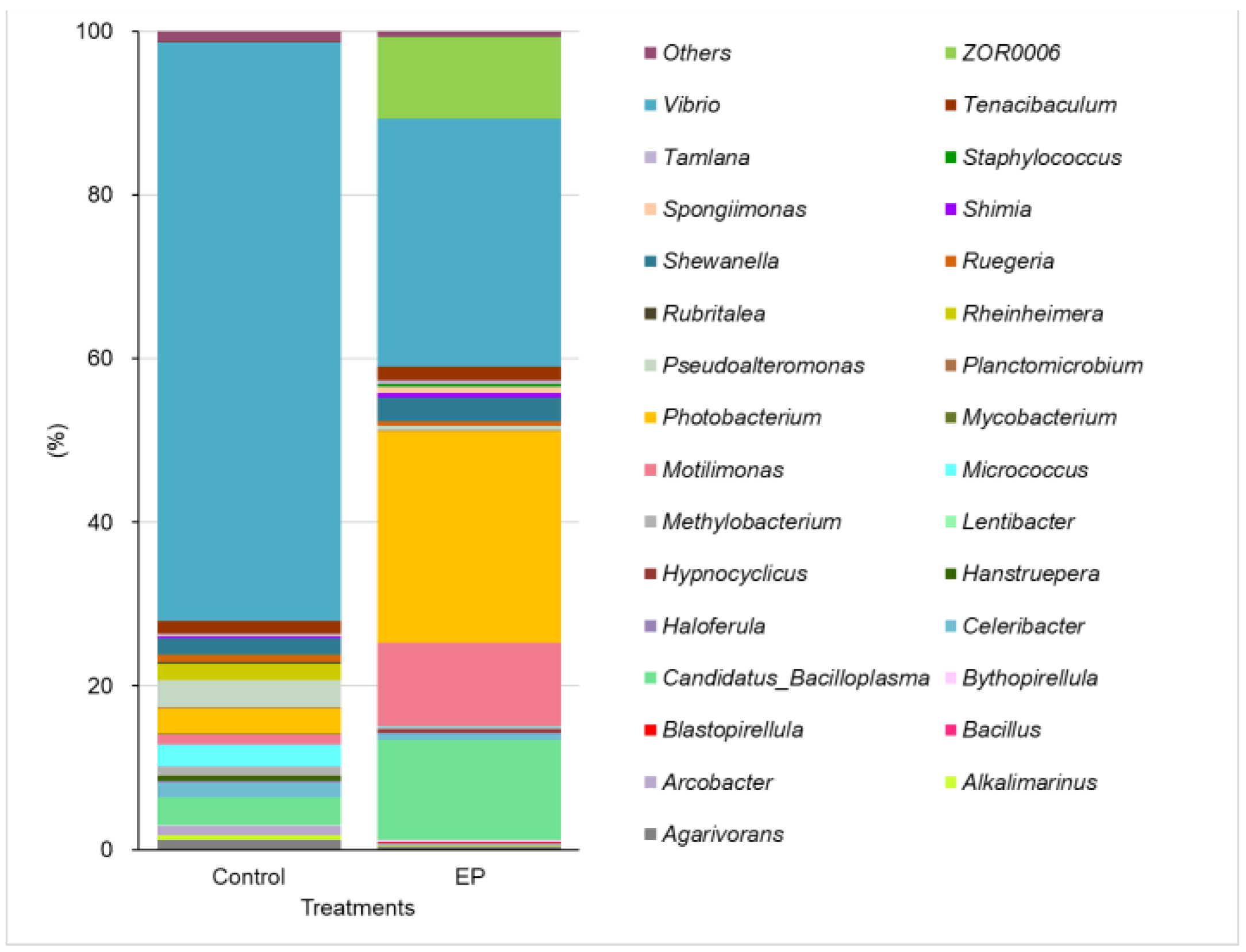
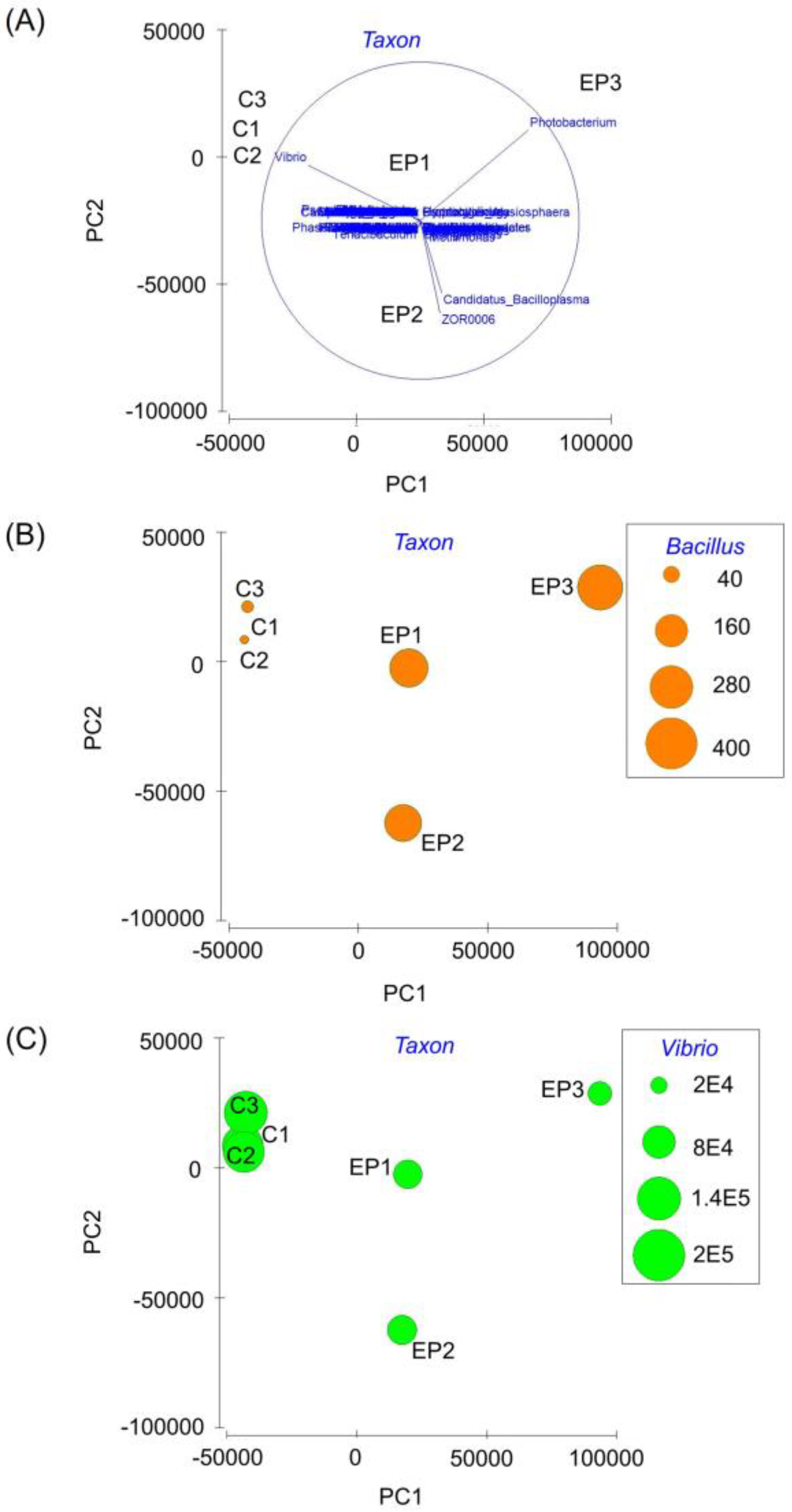
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tzuc, J.T.; Escalante, D.R.; Rojas Herrera, R.; Gaxiola Cortés, G.; Ortiz, M.L.A. Microbiota from Litopenaeus vannamei: Digestive tract microbial community of Pacific white shrimp (Litopenaeus vannamei). SpringerPlus 2014, 3, 280. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Yu, W.; Zhang, J.; Zhu, J.; Tao, Z.; Xiong, J. The gut eukaryotic microbiota influences the growth performance among cohabitating shrimp. Appl. Microbiol. Biotechnol. 2017, 101, 6447–6457. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Albores, F.; Porchas-Cornejo, M.A.; Martínez-Porchas, M.; Villalpando-Canchola, E.; Gollas-Galván, T.; Martínez-Córdova, L.R. Bacterial biota of shrimp intestine is significantly modified by the use of a probiotic mixture: A high throughput sequencing approach. Helgol. Mar. Res. 2017, 71, 5. [Google Scholar] [CrossRef]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Song, S.K.; Beck, B.R.; Kim, D.; Park, J.; Kim, J.; Kim, H.D.; Ringø, E. Prebiotics as immunostimulants in aquaculture: A review. Fish Shellfish Immunol. 2014, 40, 40–48. [Google Scholar] [CrossRef]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China—A review of the past decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef]
- Xie, J.J.; Liu, Q.Q.; Liao, S.; Fang, H.H.; Yin, P.; Xie, S.W.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary mixed probiotics on growth, non-specific immunity, intestinal morphology and microbiota of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 90, 456–465. [Google Scholar] [CrossRef]
- Imaizumi, K.; Tinwongger, S.; Kondo, H.; Hirono, I. Analysis of microbiota in the stomach and midgut of two penaeid shrimps during probiotic feeding. Sci. Rep. 2021, 11, 9936. [Google Scholar]
- Jantarathin, S.; Borompichaichartkul, C.; Sanguandeekul, R. Microencapsulation of probiotic and prebiotic in alginate-chitosan capsules and its effect on viability under heat process in shrimp feeding. Mater. Today Proc. 2017, 4, 6166–6172. [Google Scholar] [CrossRef]
- Bauer-Estrada, K.; Sandoval-Cuellar, C.; Rojas-Muñoz, Y.; Quintanilla-Carvajal, M.X. The modulatory effect of encapsulated bioactives and probiotics on gut microbiota: Improving health status through functional food. Food Funct. 2022, 14, 32–55. [Google Scholar] [CrossRef]
- Willer, D.F.; Aldridge, D.C. Microencapsulated diets to improve growth and survivorship in juvenile European flat oysters (Ostrea edulis). Aquaculture 2019, 505, 256–262. [Google Scholar] [CrossRef]
- Willer, D.F.; Furse, S.; Aldridge, D.C. Microencapsulated algal feeds as a sustainable replacement diet for broodstock in commercial bivalve aquaculture. Sci. Rep. 2020, 10, 12577. [Google Scholar] [CrossRef]
- Amir, I.; Zuberi, A.; Kamran, M.; Imran, M.; Murtaza, M.U.H. Evaluation of commercial application of dietary encapsulated probiotic (Geotrichum candidum QAUGC01): Effect on growth and immunological indices of Rohu (Labeo rohita, Hamilton 1822) in semi-intensive culture system. Fish Shellfish Immunol. 2019, 95, 464–472. [Google Scholar] [CrossRef]
- Kumaree, K.K.; Akbar, A.; Anal, A.K. Bioencapsulation and application of Lactobacillus plantarum isolated from catfish gut as an antimicrobial agent and additive in fish feed pellets. Ann. Microbiol. 2015, 65, 1439–1445. [Google Scholar] [CrossRef]
- Annan, N.T.; Borza, A.D.; Hansen, L.T. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res. Int. 2008, 41, 184–193. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending viability of Bifidobacterium longumin chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef]
- Adilah, R.N.; Chiu, S.T.; Hu, S.Y.; Ballantyne, R.; Happy, N.; Cheng, A.C.; Liu, C.H. Improvement in the probiotic efficacy of Bacillus subtilis E20-stimulates growth and health status of white shrimp, Litopenaeus vannamei via encapsulation in alginate and coated with chitosan. Fish Shellfish Immunol. 2022, 125, 74–83. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Hardy, R.W. Protein and lipid sources affect cholesterol concentrations of juvenile Pacific white shrimp, Litopenaeus vannamei (Boone). J. Anim. Sci. 2004, 82, 1136–1145. [Google Scholar] [CrossRef]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; van der Giezen, M. Understanding the Role of the shrimp gut microbiome in health and disease. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Wang, C.; Zhang, L.; Tu, K.; Zheng, Z. Insights into the intestinal microbiota of several aquatic organisms and association with the surrounding environment. Aquaculture 2019, 507, 196–202. [Google Scholar] [CrossRef]
- Riaz, Q.U.A.; Masud, T. Recent Trends and Applications of Encapsulating Materials for probiotic stability. Crit. Rev. Food Sci. Nutr. 2013, 53, 231–244. [Google Scholar] [CrossRef]
- Cheng, A.C.; Yeh, S.P.; Hu, S.Y.; Lin, H.L.; Liu, C.H. Intestinal microbiota of white shrimp, Litopenaeus vannamei, fed diets containing Bacillus subtilis E20-fermented soybean meal (FSBM) or an antimicrobial peptide derived from B. subtilis E20-FSBM. Aquac. Res. 2020, 51, 41–50. [Google Scholar] [CrossRef]
- Landsman, A.; St-Pierre, B.; Rosales-Leija, M.; Brown, M.; Gibbons, W. Impact of aquaculture practices on intestinal bacterial profiles of Pacific whiteleg shrimp Litopenaeus vannamei. Microorganisms 2019, 7, 93. [Google Scholar] [CrossRef]
- Li, J.; Ni, J.; Li, J.; Wang, C.; Li, X.; Wu, S.; Zhang, T.; Yu, Y.; Yan, Q. Comparative study on gastrointestinal microbiota of eight fish species with different feeding habits. J. Appl. Microbiol. 2014, 117, 1750–1760. [Google Scholar] [CrossRef]
- Kuang, T.; He, A.; Lin, Y.; Huang, X.; Liu, L.; Zhou, L. Comparative analysis of microbial communities associated with the gill, gut, and habitat of two filter-feeding fish. Aquac. Rep. 2020, 18, 100501. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.M.; Zhou, Y.L.; Almeida, A.; Finn, R.D.; Danchin, A.; He, L.S. Phylogenomics of expanding uncultured environmental Tenericutes provides insights into their pathogenicity and evolutionary relationship with Bacilli. BMC Genom. 2020, 21, 408. [Google Scholar] [CrossRef]
- Huang, L.; Guo, H.; Chen, C.; Huang, X.; Chen, W.; Bao, F.; Liu, W.; Wang, S.; Zhang, D. The bacteria from large-sized bioflocs are more associated with the shrimp gut microbiota in culture system. Aquaculture 2020, 523, 735159. [Google Scholar] [CrossRef]
- Van Kessel, M.A.H.J.; Dutilh, B.E.; Neveling, K.; Kwint, M.P.; Veltman, J.A.; Flik, G.; Jetten, M.S.; Klaren, P.H.; den Camp, H.J.M.O. Pyrosequencing of 16S rRNA gene amplicons to study the microbiota in the gastrointestinal tract of carp (Cyprinus carpio L.). AMB Express 2011, 1, 41. [Google Scholar] [CrossRef]
- de Moraes, A.V.; Owatari, M.S.; da Silva, E.; de Oliveira Pereira, M.; Piola, M.; Ramos, C.; Farias, D.R.; Schleder, D.D.; Jesus, G.F.A.; Jatobá, A. Effects of microencapsulated probiotics-supplemented diet on growth, non-specific immunity, intestinal health and resistance of juvenile Nile tilapia challenged with Aeromonas hydrophila. Anim. Feed Sci. Technol. 2022, 287, 115286. [Google Scholar] [CrossRef]
- Garibay-Valdez, E.; Martínez-Córdova, L.R.; López-Torres, M.A.; Almendariz-Tapia, F.J.; Martínez-Porchas, M.; Calderón, K. The implication of metabolically active Vibrio spp. in the digestive tract of Litopenaeus vannamei for its post-larval development. Sci. Rep. 2020, 10, 11428. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Wang, L.; Shao, Z. Changes in the intestinal bacterial community during the growth of white shrimp, Litopenaeus vannamei. Aquac. Res. 2016, 47, 1737–1746. [Google Scholar] [CrossRef]
- Gainza, O.; Ramírez, C.; Ramos, A.S.; Romero, J. Intestinal microbiota of white shrimp Penaeus vannamei under intensive cultivation conditions in Ecuador. Microb. Ecol. 2018, 75, 562–568. [Google Scholar] [CrossRef]
- Jiao, L.F.; Dai, T.M.; Zhong, S.Q.; Jin, M.; Sun, P.; Zhou, Q.C. Vibrio parahaemolyticus infection impaired intestinal barrier function and nutrient absorption in Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 99, 184–189. [Google Scholar] [CrossRef]
- Adel, M.; Yeganeh, S.; Dawood, M.A.O.; Safari, R.; Radhakrishnan, S. Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2017, 23, 1401–1409. [Google Scholar] [CrossRef]
- Aribah, D.; Widanarni; Wahyudi, A.T. The effectiveness of marine bacterial microcapsules in controlling vibriosis disease caused by the infection of Vibrio parahaemolyticus in white shrimp Litopenaeus vannamei. Aquaculture 2022, 549, 737795. [Google Scholar] [CrossRef]
- Kongnum, K.; Hongpattarakere, T. Effect of Lactobacillus plantarum isolated from digestive tract of wild shrimp on growth and survival of white shrimp (Litopenaeus vannamei) challenged with Vibrio harveyi. Fish Shellfish Immunol. 2012, 32, 170–177. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Rojas-Luna, T. Inhibitory activity of probiotic Bacillus subtilis UTM 126 against Vibrio Species confers protection against vibriosis in juvenile shrimp (Litopenaeus vannamei). Curr. Microbiol. 2007, 55, 409–412. [Google Scholar] [CrossRef]
- Mohamed, N.M.; Saito, K.; Tal, Y.; Hill, R.T. Diversity of aerobic and anaerobic ammonia-oxidizing bacteria in marine sponges. ISME J. 2009, 4, 38–48. [Google Scholar] [CrossRef]
- Lawler, S.N.; Kellogg, C.A.; France, S.C.; Clostio, R.W.; Brooke, S.D.; Ross, S.W. Coral-associated bacterial diversity is conserved across two deep-sea Anthothela Species. Front. Microbiol. 2016, 7, 458. [Google Scholar] [CrossRef]
- Fuerst, J.A.; Sambhi, S.K.; Paynter, J.L.; Hawkins, J.A.; Atherton, J.G. Isolation of a bacterium resembling Pirellula species from primary tissue culture of the giant tiger prawn (Penaeus monodon). Appl. Environ. Microbiol. 1991, 57, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, M.; Zhou, M.; Wu, L.; Yang, H.; Huang, L.; Chen, C. Isolation and Genomic characterization of five novel strains of Erysipelotrichaceae from commercial pigs. BMC Microbiol. 2021, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Granados, F.; Lopez-Zavala, A.A.; Gallardo-Becerra, L.; Mendoza-Vargas, A.; Sánchez, F.; Vichido, R.; Brieba, L.G.; Viana, M.T.; Sotelo-Mundo, R.R.; Ochoa-Leyva, A. Microbiome of Pacific whiteleg shrimp reveals differential bacterial community composition between wild, aquaculture and AHPND/EMS Outbreak Conditions. Sci. Rep. 2017, 7, 11783. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, H.; Hori, K.; Muiswinkel, V. Establishing a percutaneous infection model using zebrafish and a salmon pathogen. Biology 2021, 10, 166. [Google Scholar] [CrossRef]
- Mondal, H.K.; Maji, U.J.; Mohanty, S.; Sahoo, P.K.; Maiti, N.K. Alteration of gut microbiota composition and function of Indian major carp, Rohu (Labeo rohita) infected with Argulus siamensis. Microb. Pathog. 2022, 164, 105420. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiang, P.W.; Rogozin, D.Y.; Degermendzhy, A.G.; Chiu, H.H.; Tang, S.L. Salvaging high-quality genomes of microbial species from a meromictic lake using a hybrid sequencing approach. Commun. Biol. 2021, 4, 996. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Xie, S.W.; Li, Y.T.; Zhou, W.W.; Tian, L.X.; Li, Y.M.; Zeng, S.L.; Liu, Y.J. Effect of γ-aminobutyric acid supplementation on growth performance, endocrine hormone and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei, fed low fishmeal diet. Aquacult. Nutr. 2017, 23, 54–62. [Google Scholar] [CrossRef]
- Chen, W.Y.; Ng, T.H.; Wu, J.H.; Chen, J.W.; Wang, H.C. Microbiome dynamics in a shrimp grow-out pond with possible outbreak of acute hepatopancreatic necrosis disease. Sci. Rep. 2017, 7, 9395. [Google Scholar] [CrossRef]


| Ingredients | Experimental Diets (g kg⁻¹) | |
|---|---|---|
| Control | EP | |
| Fish meal | 410 | 410 |
| Soybean meal | 300 | 300 |
| Squid meal | 50 | 50 |
| Fish oil | 29 | 29 |
| α-Starch | 149.6 | 149.6 |
| Vitamin Premix * | 20 | 20 |
| Mineral premix * | 40 | 40 |
| E-probiotic (109 CFU g−1) | 0 | 0.01 |
| α-Cellulose | 1.4 | 1.39 |
| Probiotic level (CFU kg−1) | 0 | 2.5 × 10⁷ |
| Proximate analysis | ||
| Moisture (%) | 7.2 ± 0.2 | 7.2 ± 0.3 |
| Ash (%) | 14.1 ± 0.1 | 13.8 ± 0.2 |
| Crude protein (%) | 40.1 ± 0.5 | 40.5 ± 0.2 |
| Crude lipid (%) | 6.9 ± 0.6 | 6.5 ± 0.2 |
| Treatments | Genus | Margalef’s Species Richness (d) | Pielou’s Evenness (J’) | Shannon Index | Simpson Index |
|---|---|---|---|---|---|
| Control | 275 | 14.07 ± 2.39 | 0.32 ± 0.1 | 1.64 ± 0.52 | 0.55 ± 0.16 |
| EP | 236 | 12.12 ± 3.41 | 0.36 ± 0.05 | 1.79 ± 0.33 | 0.71 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, A.-C.; Ballantyne, R.; Chiu, S.-T.; Liu, C.-H. Microencapsulation of Bacillus subtilis E20 Probiotic, a Promising Approach for the Enrichment of Intestinal Microbiome in White Shrimp, Penaeus vannamei. Fishes 2023, 8, 264. https://doi.org/10.3390/fishes8050264
Cheng A-C, Ballantyne R, Chiu S-T, Liu C-H. Microencapsulation of Bacillus subtilis E20 Probiotic, a Promising Approach for the Enrichment of Intestinal Microbiome in White Shrimp, Penaeus vannamei. Fishes. 2023; 8(5):264. https://doi.org/10.3390/fishes8050264
Chicago/Turabian StyleCheng, Ann-Chang, Rolissa Ballantyne, Shieh-Tsung Chiu, and Chun-Hung Liu. 2023. "Microencapsulation of Bacillus subtilis E20 Probiotic, a Promising Approach for the Enrichment of Intestinal Microbiome in White Shrimp, Penaeus vannamei" Fishes 8, no. 5: 264. https://doi.org/10.3390/fishes8050264
APA StyleCheng, A.-C., Ballantyne, R., Chiu, S.-T., & Liu, C.-H. (2023). Microencapsulation of Bacillus subtilis E20 Probiotic, a Promising Approach for the Enrichment of Intestinal Microbiome in White Shrimp, Penaeus vannamei. Fishes, 8(5), 264. https://doi.org/10.3390/fishes8050264









