Age and Growth of European Sardine (Sardina pilchardus) in the Central Mediterranean Sea: Implication for Stock Assessment
Abstract
1. Introduction
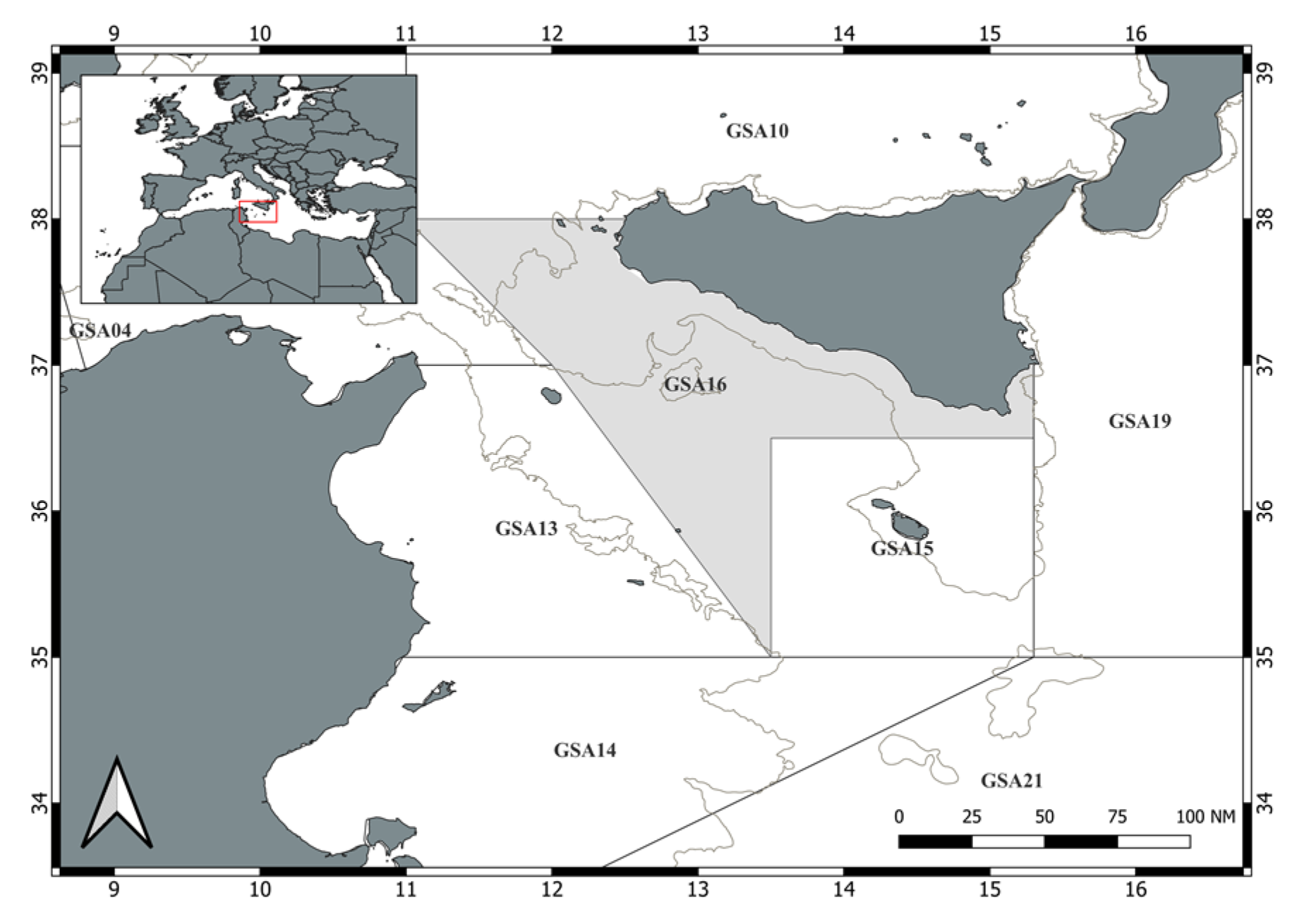
2. Material and Methods
2.1. Age Estimation
2.2. Growth and Mortality
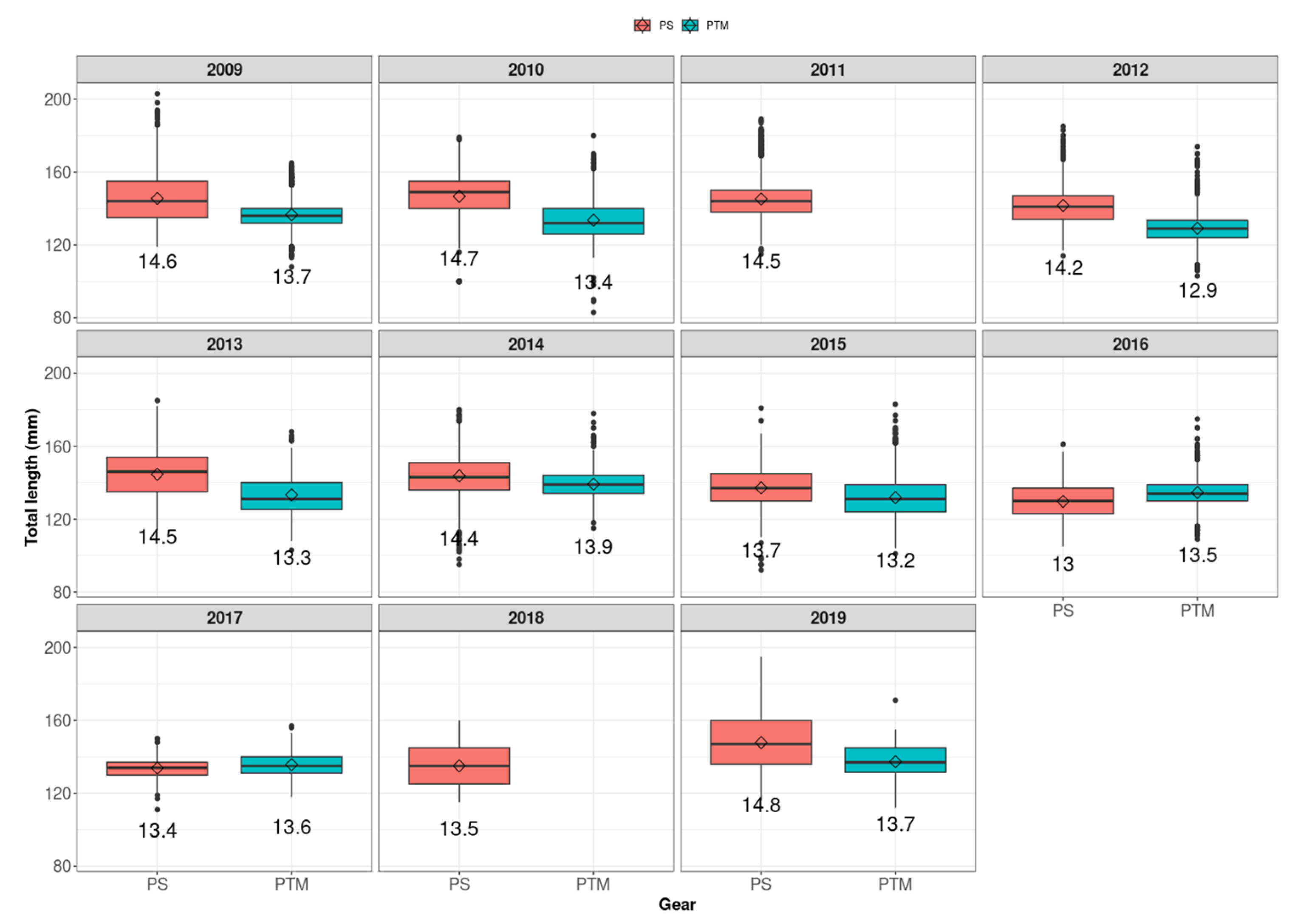
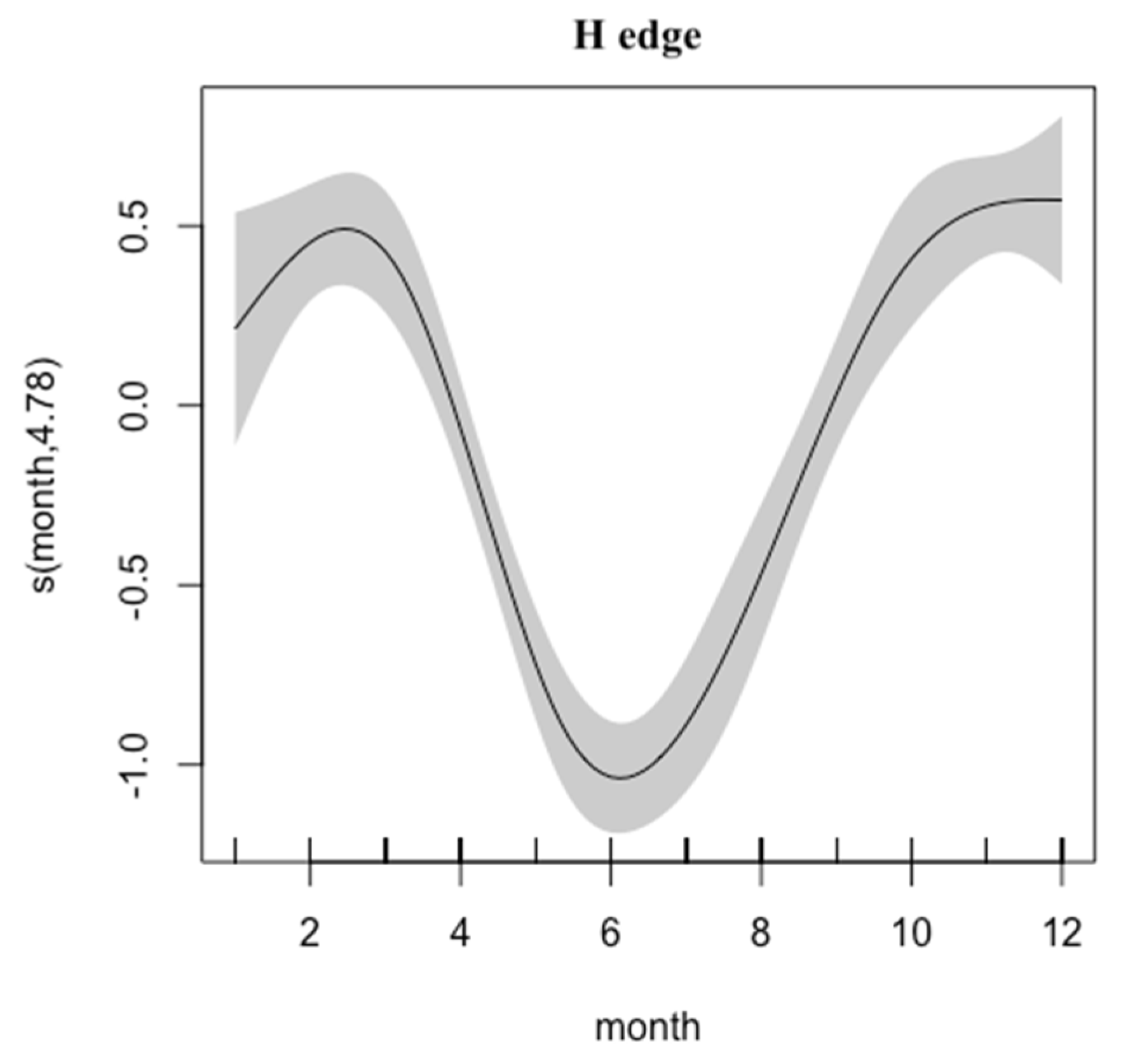
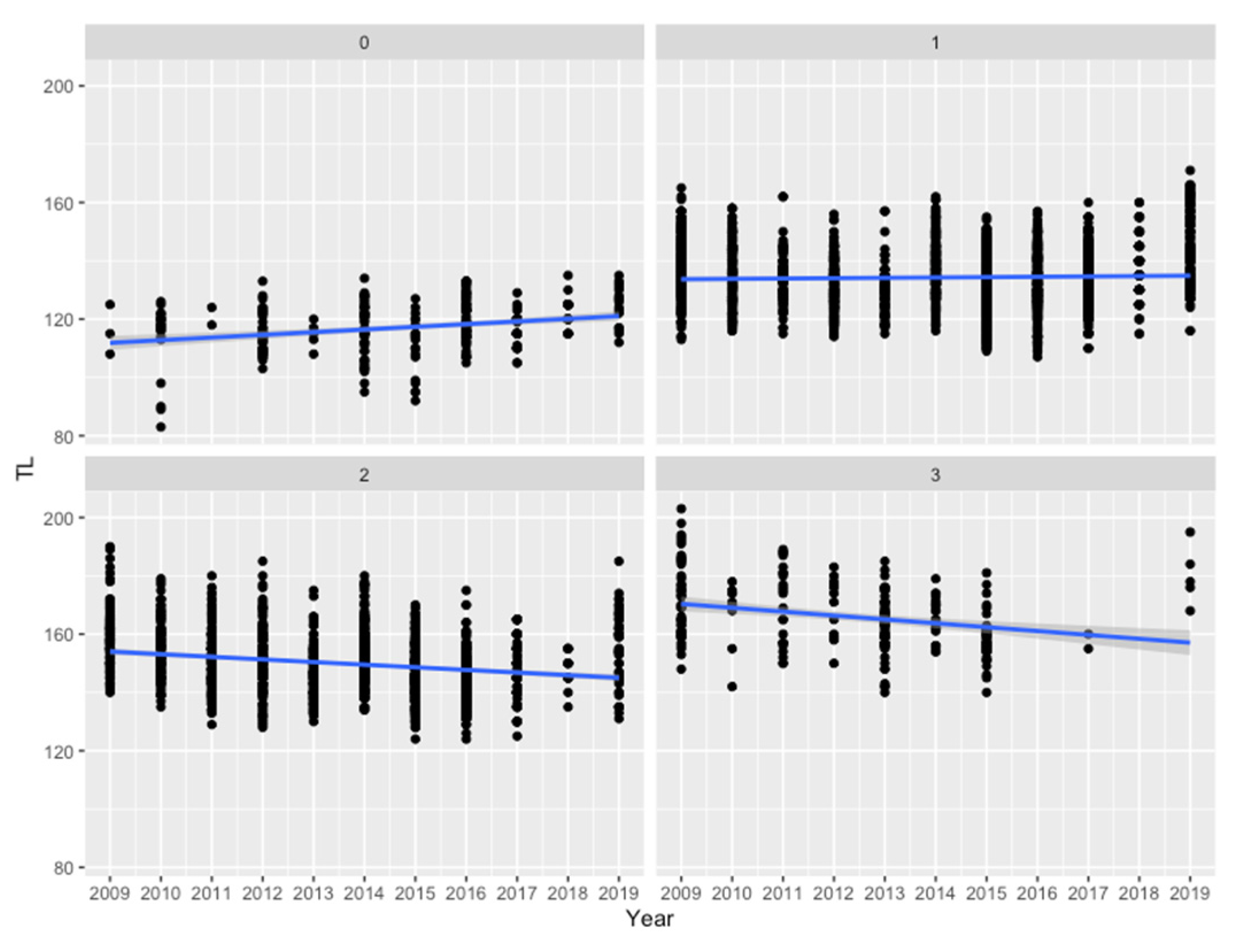
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganias, K. Biology and Ecology of Sardines and Anchovies; CRC Press Tayole&Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lleonart, J.; Maynou, F. Fish Stock Assessments in the Mediterranean: State of the Art. Sci. Mar. 2003, 67, 37–49. [Google Scholar] [CrossRef]
- Bakun, A. Fronts and Eddies as Key Structures in the Habitat of Marine Fish Larvae: Opportunity, Adaptive Response and Competitive Advantage. Sci. Mar. 2006, 70, 105–122. [Google Scholar] [CrossRef]
- Parrish, R.H.; Serra, R.; Grant, W.S. The Monotypic Sardines, Sardina and Sardinops: Their Taxonomy, Distribution, Stock Structure, and Zoogeography. Can. J. Fish. Aquat. Sci. 1989, 46, 2019–2036. [Google Scholar] [CrossRef]
- D’Elia, M.; Patti, B.; Bonanno, A.; Fontana, I.; Giacalone, G.; Basilone, G.; Fernandes, P.G. Analysis of Backscatter Properties and Application of Classification Procedures for the Identification of Small Pelagic Fish Species in the Central Mediterranean. Fish. Res. 2014, 149, 33–42. [Google Scholar] [CrossRef]
- Iglesias, M. Spatio-Temporal Patterns and Morphological Characterisation of Multispecies Pelagic Fish Schools in the North-Western Mediterranean Sea. Aquat. Living Resour. 2003, 16, 541–548. [Google Scholar] [CrossRef]
- FAO. The State of Mediterranean and Black Sea Fisheries 2022; General Fisheries Commission for the Mediterranean; FAO: Rome, Italy, 2022; ISBN 978-92-5-137370-5. [Google Scholar]
- Alheit, J.; Licandro, P.; Coombs, S.; Garcia, A.; Giráldez, A.; Santamaría, M.T.G.; Slotte, A.; Tsikliras, A.C. Reprint of “Atlantic Multidecadal Oscillation (AMO) Modulates Dynamics of Small Pelagic Fishes and Ecosystem Regime Shifts in the Eastern North and Central Atlantic”. J. Mar. Syst. 2014, 133, 88–102. [Google Scholar] [CrossRef]
- Bonanno, A.; Barra, M.; Basilone, G.; Genovese, S.; Rumolo, P.; Goncharov, S.; Popov, S.; Buongiorno Nardelli, B.; Iudicone, D.; Procaccini, G.; et al. Environmental Processes Driving Anchovy and Sardine Distribution in a Highly Variable Environment: The Role of the Coastal Structure and Riverine Input. Fish. Oceanogr. 2016, 25, 471–490. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Licandro, P.; Pardalou, A.; McQuinn, I.H.; Gröger, J.P.; Alheit, J. Synchronization of Mediterranean Pelagic Fish Populations with the North Atlantic Climate Variability. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 159, 143–151. [Google Scholar] [CrossRef]
- Begg, G.A.; Campana, S.E.; Fowler, A.J.; Suthers, I.M. Otolith Research and Application: Current Directions in Innovation and Implementation. Mar. Freshw. Res. 2005, 56, 477. [Google Scholar] [CrossRef]
- Sinovčić, G.; Keč, V.Č.; Zorica, B. Population Structure, Size at Maturity and Condition of Sardine, Sardina Pilchardus (Walb., 1792), in the Nursery Ground of the Eastern Adriatic Sea (Krka River Estuary, Croatia). Estuar. Coast. Shelf Sci. 2008, 76, 739–744. [Google Scholar] [CrossRef]
- Campana, S.E.; Thorrold, S.R. Otoliths, Increments, and Elements: Keys to a Comprehensive Understanding of Fish Populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, M.A. Stock-Specific Assessment of Precise Age and Growth in the Long-Whiskered Catfish Sperata Aor from the Ganges River. Mar. Freshw. Res. 2020, 71, 1693. [Google Scholar] [CrossRef]
- Abaunza, P.; Gordo, L.; Karlou-Riga, C.; Murta, A.; Zimmermann, C.; Hammer, C.; Lucio, P.; Iversen, S.A.; Molloy, J.; Gallo, E. Growth and Reproduction of Horse Mackerel, Trachurus Trachurus (Carangidae). Rev. Fish Biol. Fish. 2003, 13, 27–61. [Google Scholar] [CrossRef]
- Checkley, D.M., Jr.; Alheit, J.; Oozeki, Y.; Roy, C. Climate Change and Small Pelagic Fish; Cambridge University Press: Cambridge, UK, 2009; ISBN 978-0-521-88482-2. [Google Scholar]
- Silva, A.; Santos, M.B.; Caneco, B.; Pestana, G.; Porteiro, C.; Carrera, P.; Stratoudakis, Y. Temporal and Geographic Variability of Sardine Maturity at Length in the Northeastern Atlantic and the Western Mediterranean. ICES J. Mar. Sci. 2006, 63, 663–676. [Google Scholar] [CrossRef]
- Ganias, K. Determining the Indeterminate: Evolving Concepts and Methods on the Assessment of the Fecundity Pattern of Fishes. Fish. Res. 2013, 138, 23–30. [Google Scholar] [CrossRef]
- Ganias, K.; Lowerre-Barbieri, S.K.; Cooper, W. Understanding the Determinate–Indeterminate Fecundity Dichotomy in Fish Populations Using a Temperature Dependent Oocyte Growth Model. J. Sea Res. 2015, 96, 1–10. [Google Scholar] [CrossRef]
- Gordo, L.S.; Costa, A.; Abaunza, P.; Lucio, P.; Eltink, A.T.G.W.; Figueiredo, I. Determinate versus Indeterminate Fecundity in Horse Mackerel. Fish. Res. 2008, 89, 181–185. [Google Scholar] [CrossRef]
- Kjesbu, O.S.; Witthames, P.R. Evolutionary Pressure on Reproductive Strategies in Flatfish and Groundfish: Relevant Concepts and Methodological Advancements. J. Sea Res. 2007, 58, 23–34. [Google Scholar] [CrossRef]
- McBride, R.S.; Wuenschel, M.J.; Nitschke, P.; Thornton, G.; King, J.R. Latitudinal and Stock-Specific Variation in Size- and Age-at-Maturity of Female Winter Flounder, Pseudopleuronectes Americanus, as Determined with Gonad Histology. J. Sea Res. 2013, 75, 41–51. [Google Scholar] [CrossRef]
- GFCM. Working Group on Stock Assessment of Small Pelagic Species (WGSASP), Online Meeting, 17–22 January 2022. p. 65. Available online: https://www.fao.org/gfcm/technical-meetings/detail/en/c/1542569/ (accessed on 28 February 2023).
- GFCM. Establishment of Geographical Sub-Areas in the GFCM Area Amending the Resolution GFCM/31/2007/2; 23–27 March 2009; FAO: Rome, Italy, 2009. [Google Scholar]
- SIBM. Linee Guida per la Raccolta dei dati Biologici Sullo Stato delle Risorse da Pesca. Data Collection Framework, Commissione Europea; SIBM: Genova, Italy, 2010; p. 70. [Google Scholar]
- Basilone, G.; Barra, M.; Ferreri, R.; Mangano, S.; Pulizzi, M.; Giacalone, G.; Fontana, I.; Aronica, S.; Gargano, A.; Rumolo, P.; et al. First Annulus Formation in the European Anchovy; a Two-Stage Approach for Robust Validation. Sci. Rep. 2020, 10, 1079. [Google Scholar] [CrossRef]
- Basilone, G.; Mangano, S.; Pulizzi, M.; Fontana, I.; Giacalone, G.; Ferreri, R.; Gargano, A.; Aronica, S.; Barra, M.; Genovese, S.; et al. European Anchovy (Engraulis Encrasicolus) Age Structure and Growth Rate in Two Contrasted Areas of the Mediterranean Sea: The Paradox of Faster Growth in Oligotrophic Seas. Mediterr. Mar. Sci. 2017, 18/3, 504–516. [Google Scholar] [CrossRef]
- ICES. Report of the Workshop on Age Estimation of European Anchovy (Engraulis Encrasicolus); WKARA2 2016 Report 28 November–2 December 2016. Pasaia, Spain. ICES CM 2016/SSGIEOM:17. 2017, p. 223. Available online: https://ices-library.figshare.com/articles/report/Report_of_the_Workshop_on_Age_estimation_of_European_anchovy_Engraulis_encrasicolus_/18615329 (accessed on 28 February 2023).
- Villamor, B.; Uriarte, A.; Basilone, G. Report of Otolith Exchange Analysis for Anchovy. ICES CM 2018/EOSG:07. 2018, p. 95. Available online: https://smartdots.ices.dk/sampleImages/2018/81/Anchovy%20Exchange%202018%20report_FINAL_20032019.pdf (accessed on 28 February 2023).
- ICES. Report of the Working Group on Acoustic and Egg Surveys for Sardine and Anchovy in ICES Areas 7, 8, and 9. WGACEGG Report 2016 Capo, Granitola, Sicily, Italy, 14–18 November 2016. ICES CM 2016/SSGIEOM:31. 2017, p. 326. Available online: https://ices-library.figshare.com/articles/report/Report_of_the_Working_Group_on_Acoustic_and_Egg_Surveys_for_Sardine_and_Anchovy_in_ICES_Areas_7_8_and_9_WGACEGG_/18615275 (accessed on 28 February 2023).
- Campana, S.E. Accuracy, Precision and Quality Control in Age Determination, Including a Review of the Use and Abuse of Age Validation Methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Catalán, I.A.; Alós, J.; Díaz-Gil, C.; Pérez-Mayol, S.; Basterretxea, G.; Morales-Nin, B.; Palmer, M. Potential Fishing-Related Effects on Fish Life History Revealed by Otolith Microchemistry. Fish. Res. 2018, 199, 186–195. [Google Scholar] [CrossRef]
- Gislason, H.; Daan, N.; Rice, J.C.; Pope, J.G. Size, Growth, Temperature and the Natural Mortality of Marine Fish: Natural Mortality and Size. Fish Fish. 2010, 11, 149–158. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Erdogan, Z.; Torcu-Koç, H.; Gicili, S.; Ulunehir, G. Age, Growth and Mortality of European Pilchard, Sardina Pilchardus in Edremit Bay (Northern Aegean Sea, Turkey). Cybium 2010, 34, 185–193. [Google Scholar]
- Alemany, F.; Álvarez, F. Growth Differences among Sardine (Sardina Pilchardus Walb.) Populations in Western Mediterranean. Sci. Mar. 1993, 57, 229–234. [Google Scholar]
- Gaamour, A.; Khemiri, S. Evaluation of Growth Differences in Populations of Sardina Pilchardus (Walbum, 1792) from Two Regions along the Tunisian Costs. Cah. Biol. Mar. 2010, 51, 101–108. [Google Scholar] [CrossRef]
- Silva, A.; Carrera, P.; Massé, J.; Uriarte, A.; Santos, M.B.; Oliveira, P.B.; Soares, E.; Porteiro, C.; Stratoudakis, Y. Geographic Variability of Sardine Growth across the Northeastern Atlantic and the Mediterranean Sea. Fish. Res. 2008, 90, 56–69. [Google Scholar] [CrossRef]
- ICES. Workshop on Age Validation Studies of Small Pelagic Species (WKVALPEL). Sci. Rep. 2020, 2, 76pp. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Koutrakis, E.T. Growth and Reproduction of European Sardine, Sardina Pilchardus (Pisces: Clupeidae), in Northeastern Mediterranean. Cah. Biol. Mar. 2013, 54, 365–374. [Google Scholar]
- Basilone, G.; Guisande, C.; Patti, B.; Mazzola, S.; Cuttitta, A.; Bonanno, A.; Kallianiotis, A. Linking Habitat Conditions and Growth in the European Anchovy (Engraulis Encrasicolus). Fish. Res. 2004, 68, 9–19. [Google Scholar] [CrossRef]
- Antonakakis, K.; Giannoulaki, M.; Machias, A.; Somarakis, S.; Sanchez, S.; Ibaibarriaga, L.; Uriarte, A. Assessment of the Sardine (Sardina Pilchardus Walbaum, 1792) Fishery in the Eastern Mediterranean Basin (North Aegean Sea). Mediterr. Mar. Sci. 2011, 12, 333. [Google Scholar] [CrossRef]
- Brosset, P.; Fromentin, J.-M.; Van Beveren, E.; Lloret, J.; Marques, V.; Basilone, G.; Bonanno, A.; Carpi, P.; Donato, F.; Čikeš Keč, V.; et al. Spatio-Temporal Patterns and Environmental Controls of Small Pelagic Fish Body Condition from Contrasted Mediterranean Areas. Prog. Oceanogr. 2017, 151, 149–162. [Google Scholar] [CrossRef]
- Hattab, T.; Gucu, A.; Ventero, A.; De Felice, A.; Machias, A.; Saraux, C.; Gašparević, D.; Basilone, G.; Costantini, I.; Leonori, I.; et al. Temperature Strongly Correlates with Regional Patterns of Body Size Variation in Mediterranean Small Pelagic Fish Species. Mediterr. Mar. Sci. 2021, 22, 800. [Google Scholar] [CrossRef]
- Menu, C.; Pecquerie, L.; Bacher, C.; Doray, M.; Hattab, T.; van der Kooij, J.; Huret, M. Testing the Bottom-up Hypothesis for the Decline in Size of Anchovy and Sardine across European Waters through a Bioenergetic Modeling Approach. Prog. Oceanogr. 2023, 210, 102943. [Google Scholar] [CrossRef]
- Saraux, C.; Van Beveren, E.; Brosset, P.; Queiros, Q.; Bourdeix, J.-H.; Dutto, G.; Gasset, E.; Jac, C.; Bonhommeau, S.; Fromentin, J.-M. Small Pelagic Fish Dynamics: A Review of Mechanisms in the Gulf of Lions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 159, 52–61. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Tsikliras, A.C. Linking Growth Patterns to Sea Temperature and Oxygen Levels across European Sardine (Sardina Pilchardus) Populations. Environ. Biol. Fishes 2022, 105, 1335–1345. [Google Scholar] [CrossRef]
- Quinn, T.J.; Deriso, R.B. Quantitative Fish Dynamics; Oxford University Press: Oxford, UK, 1999; ISBN 0-19-536040-0. [Google Scholar]
- Kenchington, T.J. Natural Mortality Estimators for Information-Limited Fisheries. Fish Fish. 2014, 15, 533–562. [Google Scholar] [CrossRef]
- Executive Agency for Small and Medium sized Enterprises. SPELMED, Evaluation of the Population Status and Specific Management Alternatives for the Small Pelagic Fish Stocks in the Northwestern Mediterranean Sea; Publications Office of the European Union: Luxembourg, 2020; Volume 89. [CrossRef]
- Pertierra, J.P.; Perrotta, R.G. On the Population Dynamics of Sardine, Sardina Pilchardus, from the Catalan Sea (North-Western Mediterranean Sea). Sci. Mar. 1993, 57, 235–241. [Google Scholar]
- Silva, A.; Garrido, S.; Ibaibarriaga, L.; Pawlowski, L.; Riveiro, I.; Marques, V.; Ramos, F.; Duhamel, E.; Iglesias, M.; Bryère, P.; et al. Adult-Mediated Connectivity and Spatial Population Structure of Sardine in the Bay of Biscay and Iberian Coast. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 159, 62–74. [Google Scholar] [CrossRef]
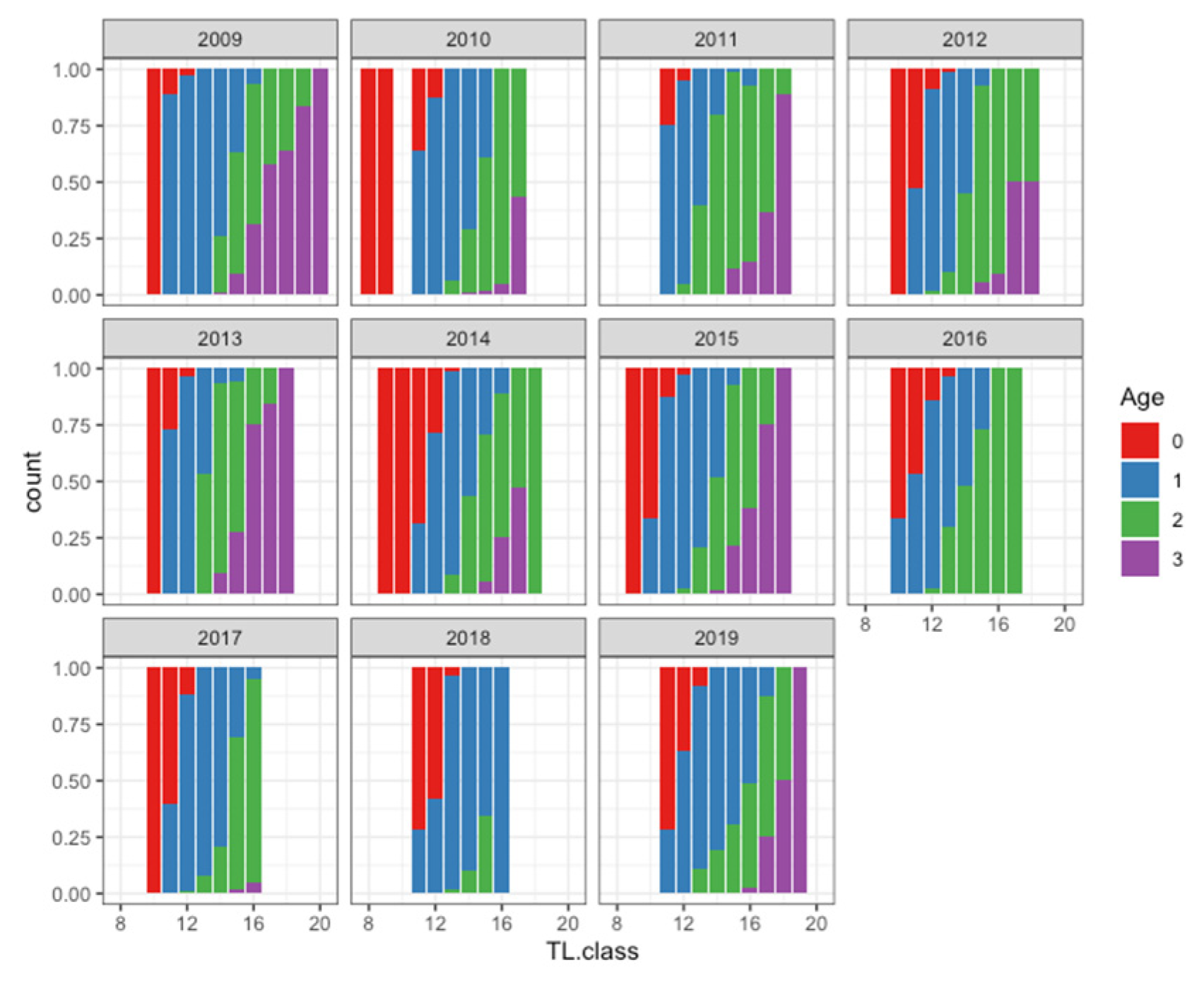
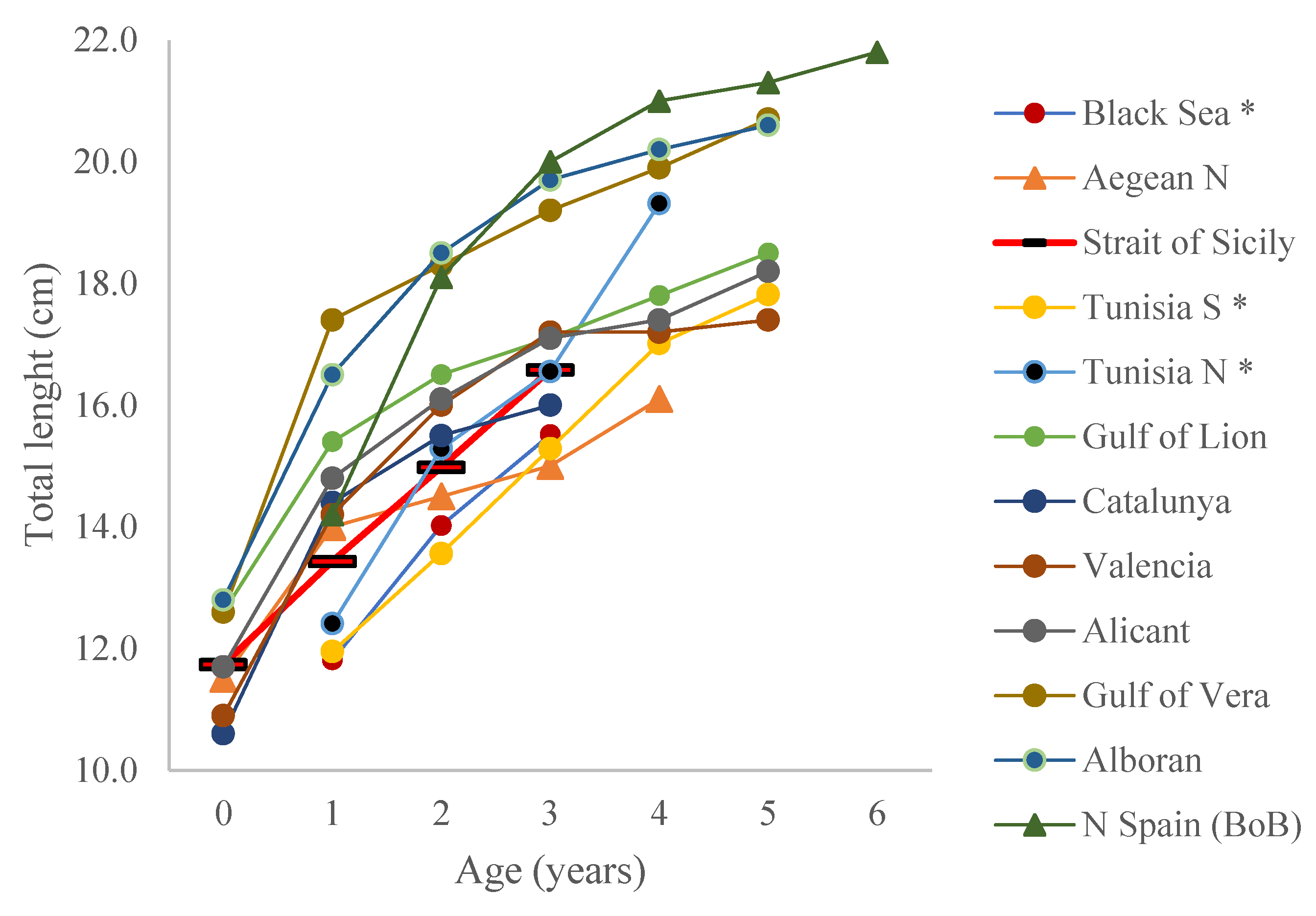
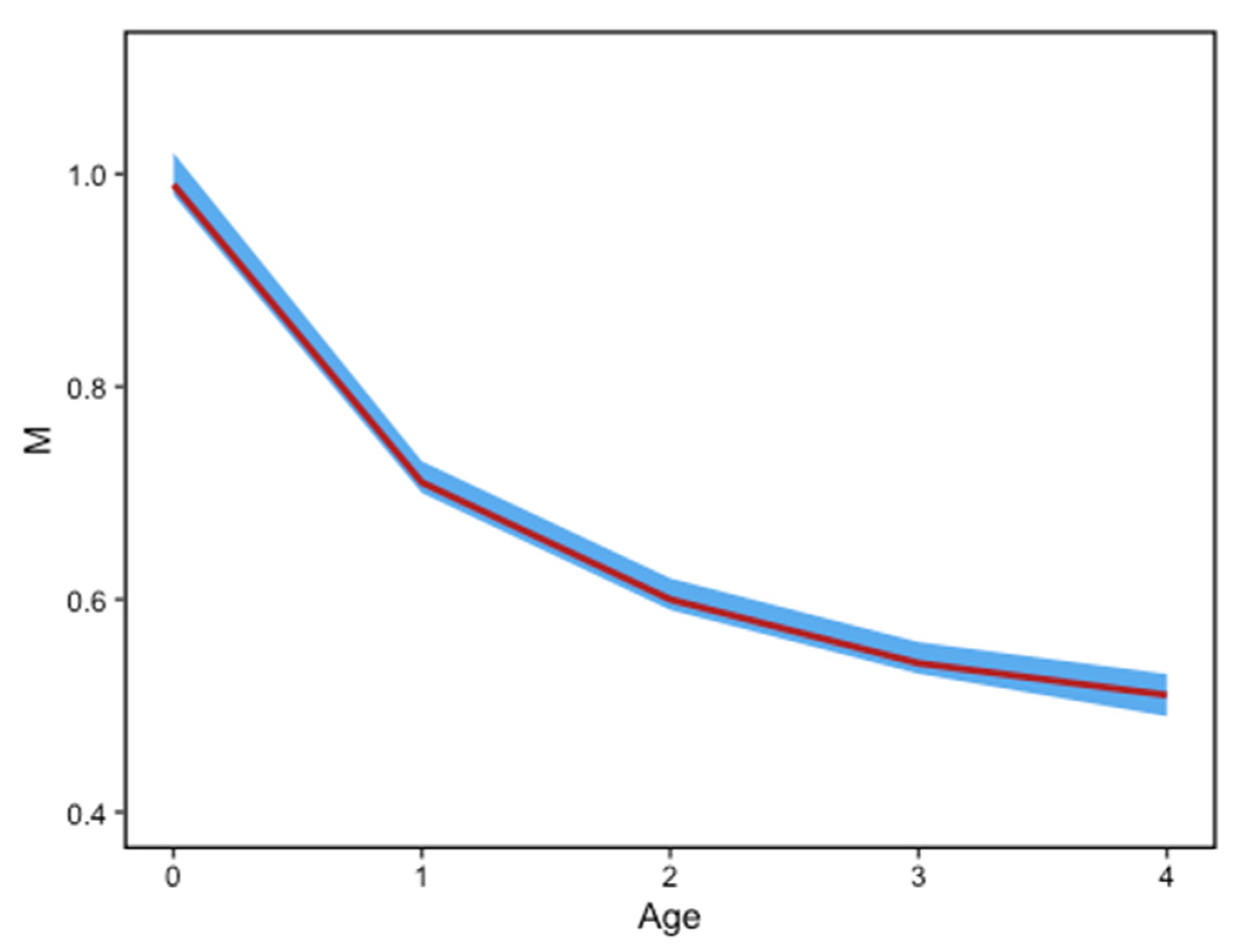
| Month | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | 44 | 198 | 242 | |||||||||
| February | 50 | 45 | 58 | 49 | 49 | 251 | ||||||
| March | 37 | 79 | 32 | 66 | 27 | 58 | 77 | 64 | 56 | 39 | 535 | |
| April | 44 | 35 | 31 | 27 | 53 | 52 | 30 | 272 | ||||
| May | 37 | 52 | 31 | 112 | 64 | 107 | 30 | 433 | ||||
| June | 35 | 41 | 28 | 18 | 49 | 61 | 62 | 30 | 324 | |||
| July | 44 | 31 | 53 | 119 | 247 | |||||||
| August | 85 | 68 | 63 | 64 | 280 | |||||||
| September | 90 | 43 | 96 | 34 | 76 | 76 | 60 | 65 | 30 | 570 | ||
| October | 53 | 40 | 44 | 35 | 163 | 40 | 375 | |||||
| November | 45 | 64 | 33 | 43 | 46 | 89 | 82 | 234 | 70 | 706 | ||
| December | 40 | 98 | 77 | 76 | 135 | 39 | 465 | |||||
| Total | 354 | 339 | 264 | 454 | 261 | 525 | 797 | 689 | 574 | 205 | 238 | 4700 |
| Quarter | Gear | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | PS | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 1 | |||
| II | 2 | 2 | 3 | 1 | 1 | 3 | 2 | 2 | ||||
| III | 2 | 5 | 2 | 3 | 1 | 1 | 1 | |||||
| IV | 4 | 1 | 2 | 3 | 3 | 5 | 3 | 3 | 1 | |||
| I | PTM | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | |||
| II | 4 | 4 | 3 | 3 | 3 | 2 | 1 | |||||
| III | 4 | 5 | 3 | 4 | 4 | 3 | 4 | |||||
| IV | 3 | 1 | 6 | 1 |
| Age Class | Coefficient | Estimate | Std. Error | t Value | Pr (>|t|) | F Value | d.f | p-Value |
|---|---|---|---|---|---|---|---|---|
| 0 | (Intercept) | −1729.61 | 370.56 | −4.67 | 5 × 10−6 | 24.84 | 1, 288 | 1.1 × 10−6 |
| Year | 0.92 | 0.18 | 4.98 | 1 × 10−6 | ||||
| 1 | (Intercept) | −106.31 | 134.32 | −0.79 | 4 × 10−1 | 3.21 | 1, 2770 | 0.07331 |
| Year | 0.12 | 0.07 | 1.79 | 7 × 10−2 | ||||
| 2 | (Intercept) | 1957.48 | 209.91 | 9.33 | <2 × 10-16 | 74.16 | 1, 1410 | <2 × 10−16 |
| Year | −0.90 | 0.10 | −8.61 | <2 × 10-16 | ||||
| 3 | (Intercept) | 2842.05 | 627.15 | 4.53 | 9 × 10−6 | 18.21 | 1, 226 | 2.91 × 10−5 |
| Year | −1.33 | 0.31 | −4.27 | 3 × 10−5 |
| Age | ||||
|---|---|---|---|---|
| Year | 0 | 1 | 2 | 3 |
| 2009 | 1 | 57 | 29 | 13 |
| 2010 | 6 | 65 | 26 | 3 |
| 2011 | 1 | 27 | 63 | 9 |
| 2012 | 9 | 60 | 29 | 3 |
| 2013 | 2 | 27 | 49 | 22 |
| 2014 | 8 | 52 | 34 | 5 |
| 2015 | 3 | 63 | 30 | 5 |
| 2016 | 9 | 60 | 31 | 0 |
| 2017 | 7 | 75 | 17 | 0 |
| 2018 | 18 | 74 | 8 | 0 |
| 2019 | 7 | 68 | 23 | 2 |
| Average | 6 | 59 | 30 | 5 |
| Year | Linf (mm) | t0 (Years) | K (Years−1) | Linf itv | t0 itv | k itv | Mean itv |
|---|---|---|---|---|---|---|---|
| 2009 | 197 (188; 205) | −1.81(−1.99; −1.49) | 0.43 (0.38; 0.53) | 8.63 | 27.62 | 34.18 | 23.48 |
| 2010 | 185 (176; 196) | −1.9 (−2; −1.71) | 0.46 (0.39; 0.54) | 10.81 | 15.26 | 31.44 | 19.17 |
| 2011 | 188 (176; 199) | −1.89 (−2; −1.61) | 0.43 (0.36; 0.54) | 12.23 | 20.63 | 41.26 | 24.71 |
| 2012 | 180 (172; 186) | −1.96 (−2; −1.85) | 0.46 (0.42; 0.52) | 8.33 | 7.65 | 22.29 | 12.76 |
| 2013 | 185 (176; 193) | −1.9 (−2; −1.68) | 0.42 (0.37; 0.48) | 9.19 | 16.84 | 26.82 | 17.62 |
| 2014 | 173 (168; 178) | −1.93 (−2; −1.8) | 0.56 (0.51; 0.62) | 5.78 | 10.36 | 19.78 | 11.97 |
| 2015 | 173 (168; 180) | −1.94 (−2; −1.79) | 0.48 (0.43; 0.54) | 6.94 | 10.82 | 22.89 | 13.55 |
| 2016 | 151 (150; 154) | −1.96 (−2; −1.85) | 0.73 (0.69; 0.78) | 2.65 | 7.65 | 12.3 | 7.53 |
| 2017 | 162 (158; 168) | −1.94 (−2; −1.81) | 0.6 (0.53; 0.67) | 6.17 | 9.79 | 22.98 | 12.98 |
| 2018 | 154 (150; 180) | −1.88 (−2; −1.63) | 0.81 (0.49; 0.96) | 19.48 | 19.68 | 57.46 | 32.21 |
| 2019 | 169 (161; 175) | −1.87(−1.98; −1.59) | 0.68 (0.56; 0.83) | 10.65 | 20.86 | 44.74 | 25.42 |
| Parameter | Mean | sd | LCI (2.5%) | UCI (97.5%) |
|---|---|---|---|---|
| Linf cm | 18 | 1.15 | 17.8 | 18.2 |
| t0 year | −1.99 | 0.018 | −2 | −1.95 |
| K year−1 | 0.459 | 0.008 | 0.448 | 0.474 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basilone, G.; Ferreri, R.; Bonanno, A.; Genovese, S.; Barra, M.; Aronica, S. Age and Growth of European Sardine (Sardina pilchardus) in the Central Mediterranean Sea: Implication for Stock Assessment. Fishes 2023, 8, 202. https://doi.org/10.3390/fishes8040202
Basilone G, Ferreri R, Bonanno A, Genovese S, Barra M, Aronica S. Age and Growth of European Sardine (Sardina pilchardus) in the Central Mediterranean Sea: Implication for Stock Assessment. Fishes. 2023; 8(4):202. https://doi.org/10.3390/fishes8040202
Chicago/Turabian StyleBasilone, Gualtiero, Rosalia Ferreri, Angelo Bonanno, Simona Genovese, Marco Barra, and Salvatore Aronica. 2023. "Age and Growth of European Sardine (Sardina pilchardus) in the Central Mediterranean Sea: Implication for Stock Assessment" Fishes 8, no. 4: 202. https://doi.org/10.3390/fishes8040202
APA StyleBasilone, G., Ferreri, R., Bonanno, A., Genovese, S., Barra, M., & Aronica, S. (2023). Age and Growth of European Sardine (Sardina pilchardus) in the Central Mediterranean Sea: Implication for Stock Assessment. Fishes, 8(4), 202. https://doi.org/10.3390/fishes8040202








