Application of a Lipopolysaccharide (LPS)-Stimulated Mitogenesis Assay in Smallmouth Bass (Micropterus dolomieu) to Augment Wild Fish Health Studies
Abstract
1. Introduction
2. Materials and Methods
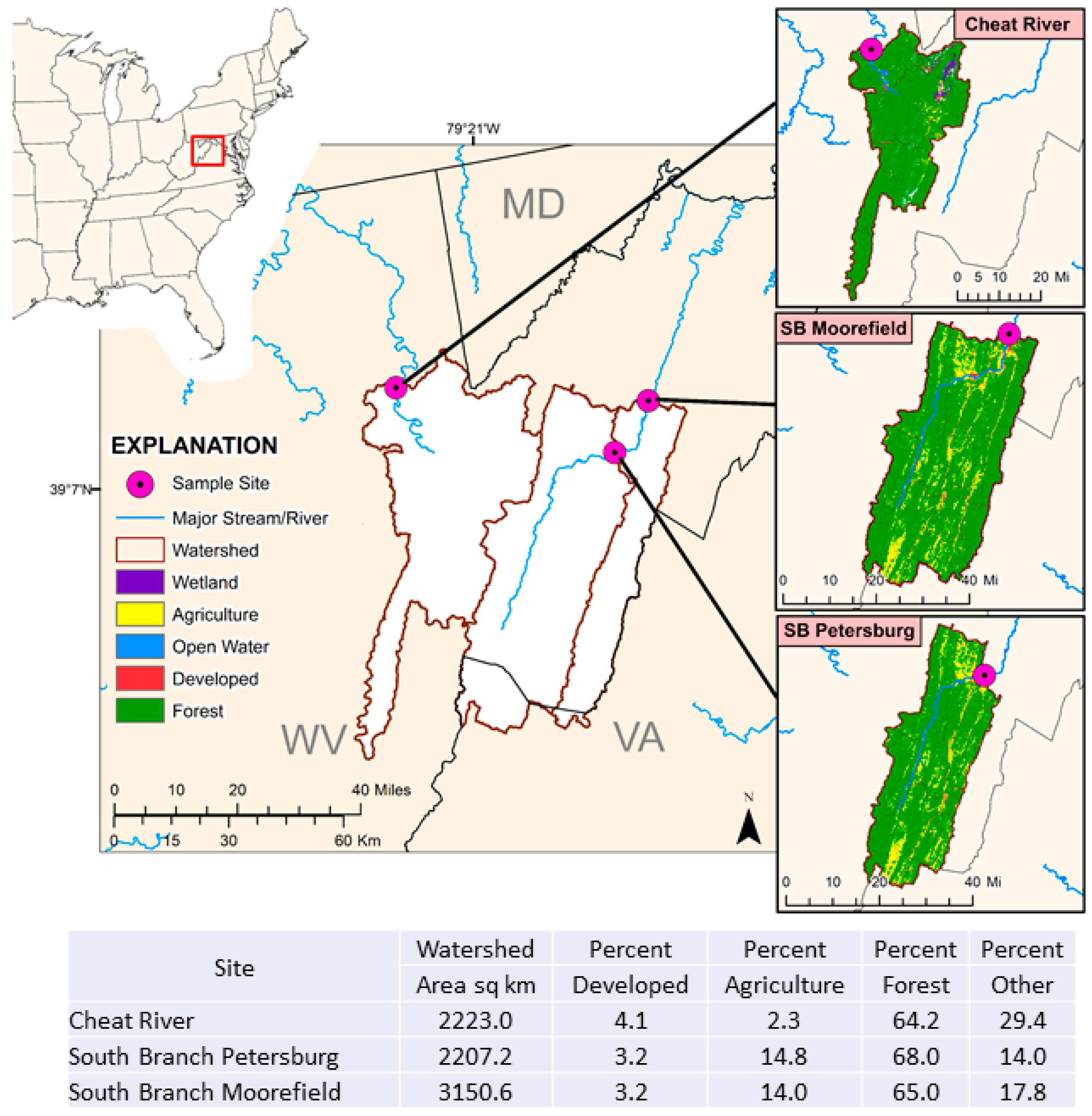
2.1. Field Sampling
2.2. Laboratory Analyses
2.2.1. Estimating Disease Indicators with Histology
2.2.2. Leukocyte Isolations
2.2.3. Mitogenesis
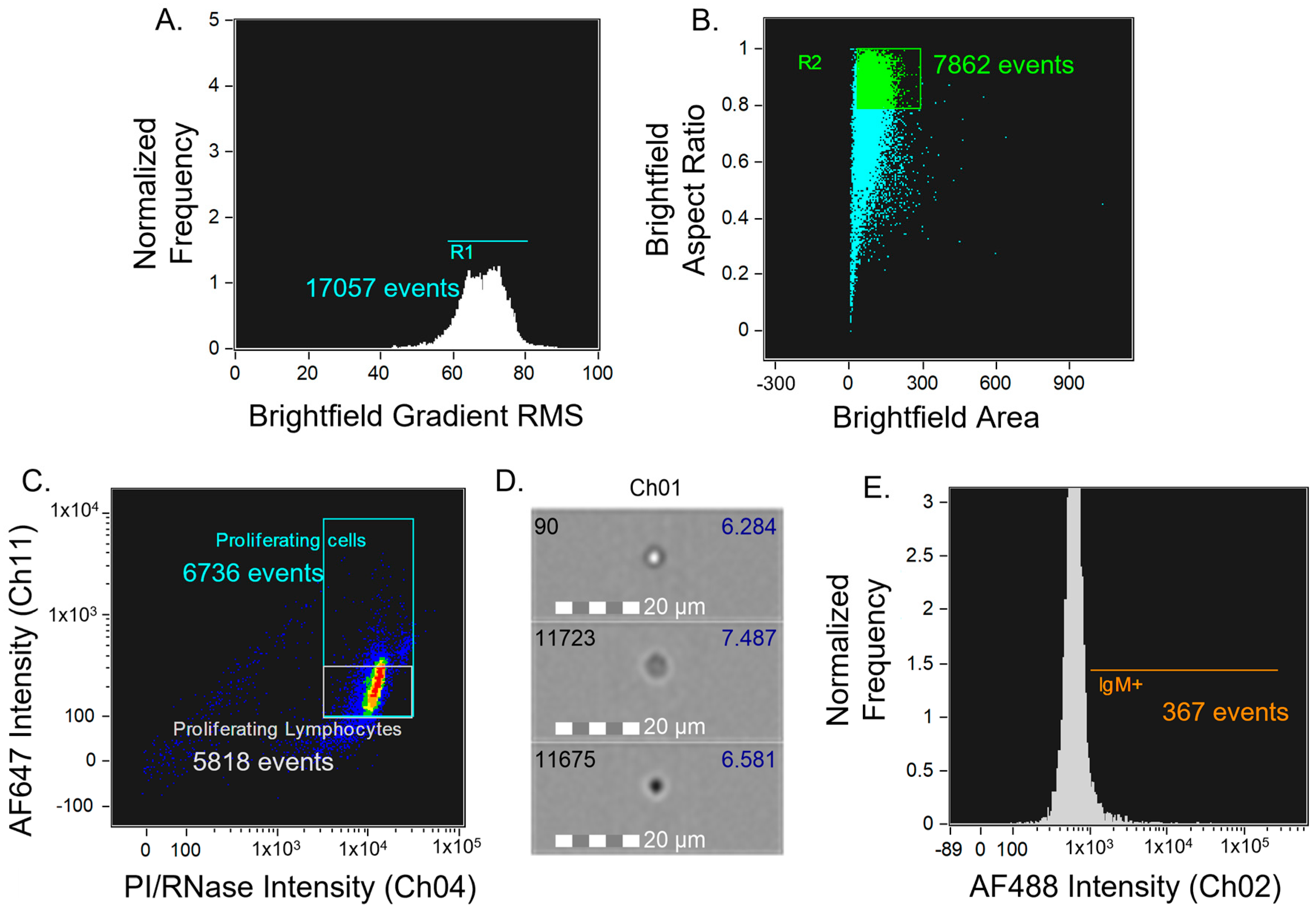
2.2.4. Detection of Surface IgM
2.2.5. Click Reaction
2.2.6. Imaging and Data Analyses
3. Results
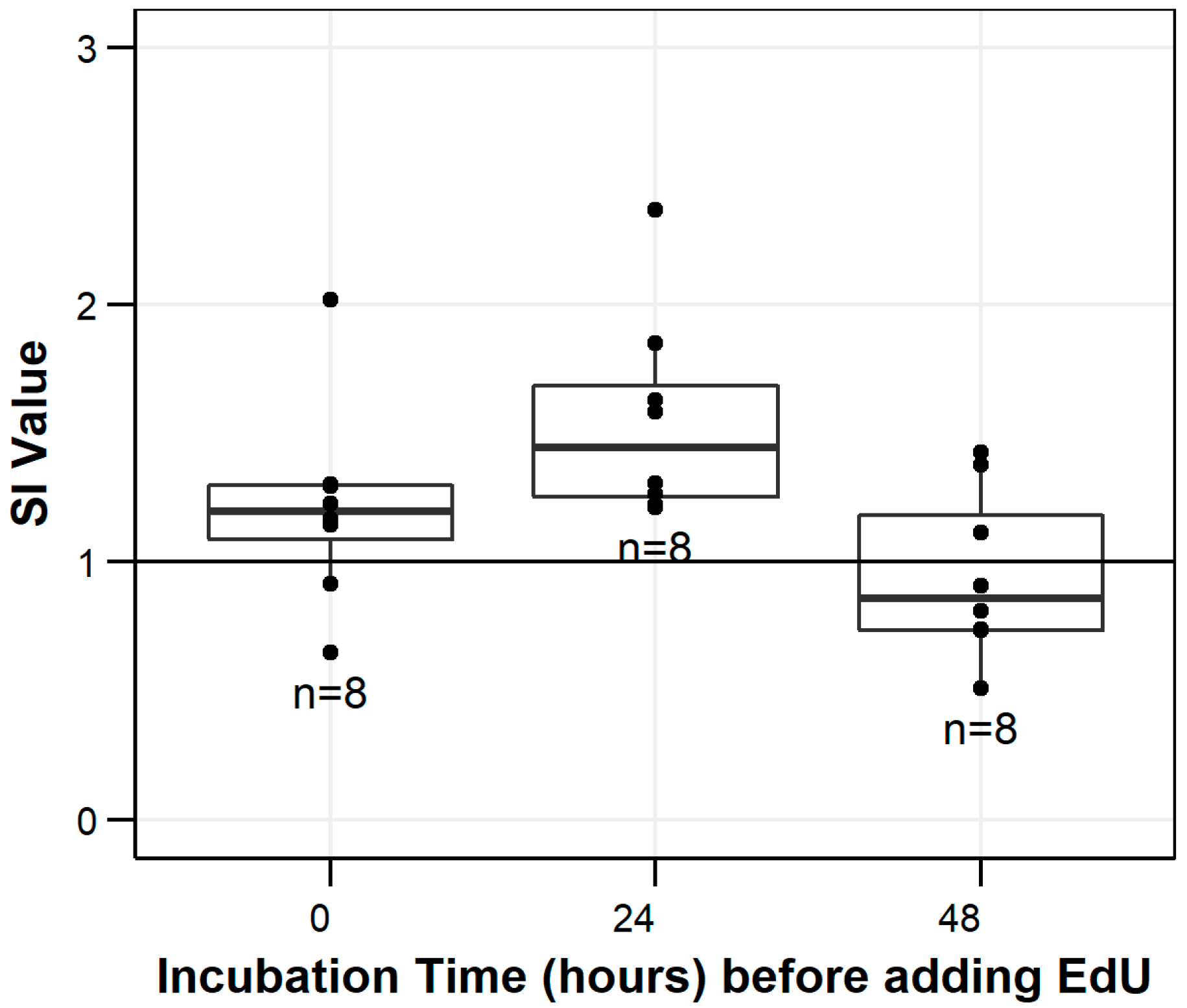
3.1. Mitogenesis Stimulation Indices
3.2. Background Cell Proliferation
3.3. Age and Morphometric Characteristics
3.4. Age and Mitogenesis
3.5. Sex and Mitogenesis
3.6. Tissue Parasites and Mitogenesis
3.7. Macrophage Aggregates and Mitogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, B.; Hedrick, J.; Blazer, V.S. Temporal Trends in Macroscopic Indicators of Fish Health in the South Branch Potomac River. N. Am. J. Fish. Manag. 2022, 42, 277–294. [Google Scholar] [CrossRef]
- Starliper, C.; Blazer, V.; Iwanowicz, L.; Walsh, H. Microbial isolates in diseased fishes, primarily Smallmouth Bass (Micropterus dolomieu), within the Chesapeake Bay drainage in 2009–2011. In Proceedings of the West Virginia Academy of Science, Fairmont, WV, USA, 6 April 2013; pp. 18–32. [Google Scholar] [CrossRef]
- Walsh, H.L.; Blazer, V.S.; Iwanowicz, L.R.; Smith, G. A redescription of Myxobolus inornatus from young-of-the-year Smallmouth Bass (Micropterus dolomieu). J. Parasitol. 2012, 98, 1236–1242. [Google Scholar] [CrossRef]
- Walsh, H.L.; Blazer, V.S.; Mazik, P.M. Identification of Aphanomyces invadans, the cause of epizootic ulcerative syndrome, in smallmouth bass (Micropterus dolomieu) from the Cheat River, West Virginia, USA. J. Fish Dis. 2021, 44, 1639–1641. [Google Scholar] [CrossRef]
- Snieszko, S.F. The effects of environmental stress on outbreaks of infectious diseases of fishes. J. FIsh Biol. 1974, 6, 197–208. [Google Scholar] [CrossRef]
- Hedrick, R.P. Relationships of the host, pathogen, and environment: Implications for diseases of cultured and wild fish populations. J. Aquat. Anim. Health 1998, 10, 107–111. [Google Scholar] [CrossRef]
- Carey, C. Hypothesis concerning the causes of the disappearance of boreal toads from the mountains of Colorado. Conserv. Biol. 1993, 7, 355–362. [Google Scholar] [CrossRef]
- Rachowicz, L.J.; Hero, J.M.; Alford, R.A.; Taylor, J.W.; Morgan, J.A.T.; Vredenburg, V.T.; Collins, J.P.; Briggs, C.J. The Novel and Endemic Pathogen Hypotheses: Competing Explanations for the Origin of Emerging Infectious Diseases of Wildlife. Conserv Biol 2005, 19, 1441–1448. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Kuris, A.M. How environmental stress affects the impacts of parasites. Limnol. Oceanogr. 1999, 44, 925–931. [Google Scholar] [CrossRef]
- Segner, H.; Moller, A.M.; Wenger, M.; Casanova-Nakayama, A. Fish Immunotoxicology: Research at the Crossroads of Immunology, Ecology and Toxicology. In Interdisciplinary Studies on Environmental Chemistry; Kawaguchi, M., Misaki, K., Sato, H., Yokokawa, T., Itai, T., Nguyen, T.M., Ono, J., Tanabe, S., Eds.; Terra Scientific Publishing Company: Tokyo, Japan, 2012; Volume 6, pp. 1–12. [Google Scholar]
- Rehberger, K.; Werner, I.; Hitzfeld, B.; Segner, H.; Baumann, L. 20 years of fish immunotoxicology–what we know and where we are. Crit. Rev. Toxicol. 2017, 47, 516–542. [Google Scholar] [CrossRef]
- Basiji, D.A.; Ortyn, W.E.; Liang, L.; Venkatachalam, V.; Morrissey, P. Cellular image analysis and imaging by flow cytometry. Clin. Lab. Med. 2007, 27, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Zuba-Surma, E.K.; Ratajczak, M.Z. Analytical Capabilities of the ImageStream Cytometer. Recent Adv. Flow Cytom. Part A 2011, 102, 207–230. [Google Scholar]
- Basiji, D.A. Principles of Amnis Imaging Flow Cytometry. In Imaging Flow Cytometry; Humana Press: New York, NY, USA, 2016; pp. 13–21. [Google Scholar]
- Vorobjev, I.A.; Barteneva, N.S. Quantitative functional morphology by imaging flow cytometry. In Imaging Flow Cytometry; Humana Press: New York, NY, USA, 2016; pp. 3–11. [Google Scholar]
- Blasi, T.; Hennig, H.; Summers, H.D.; Theis, F.J.; Cerveira, J.; Patterson, J.O.; Davies, D.; Filby, A.; Carpenter, A.E.; Rees, P. Label-free cell cycle analysis for high-throughput imaging flow cytometry. Nat. Commun. 2016, 7, 10256. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Lillico, D.M.; Hall, B.; Rieger, A.M.; Stafford, J.L. Connected component masking accurately identifies the ratio of phagocytosed and cell-bound particles in individual cells by imaging flow cytometry. Cytom. Part A 2017, 91, 372–381. [Google Scholar] [CrossRef]
- Zwozdesky, M.A.; Fei, C.; Lillico, D.M.E.; Stafford, J.L. Imaging flow cytometry and GST pulldown assays provide new insights into channel catfish leukocyte immune-type receptor-mediated phagocytic pathways. Dev. Comp. Immunol. 2017, 67, 126–138. [Google Scholar] [CrossRef]
- Jones, L.O.; Stafford, J.L. Imaging flow cytometry and confocal microscopy-based examination of F-actin and phosphoinositide dynamics during leukocyte immune-type receptor-mediated phagocytic events. Dev. Comp. Immunol. 2019, 92, 199–211. [Google Scholar] [CrossRef]
- Park, Y.; Abihssira-García, I.S.; Thalmann, S.; Wiegertjes, G.F.; Barreda, D.R.; Olsvik, P.A.; Kiron, V. Imaging Flow Cytometry Protocols for Examining Phagocytosis of Microplastics and Bioparticles by Immune Cells of Aquatic Animals. Front. Immunol. 2020, 11, 203. [Google Scholar] [CrossRef]
- Ottinger, C.A.; Smith, C.R.; Blazer, V.S. In vitro immune function in laboratory-reared age-0 smallmouth bass (Micropterus dolomieu) relative to diet. Fish Shellfish Immunol. 2019, 95, 1–10. [Google Scholar] [CrossRef]
- Smith, C.R.; Ottinger, C.A.; Walsh, H.L.; Blazer, V.S. Development of a Suite of Functional Immune Assays and Initial Assessment of Their Utility in Wild Smallmouth Bass Health Assessments; U.S. Geological Survey Open-File Report 2020-1077; USGS: Reston, VA, USA, 2020; p. 23. [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Starliper, C.E.; Iwanowicz, D.D.; Barbash, P.; Hedrick, J.D.; Reeser, S.J.; Mullican, J.E.; Zaugg, S.D.; Burkhardt, M.R.; et al. Mortality of centrarchid fishes in the Potomac drainage: Survey results and overview of potential contributing factors. J. Aquat. Anim. Health 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Henderson, H.; Mazik, P.M.; Jenkins, J.A.; Alvarez, D.A.; Young, J.A. Reproductive endocrine disruption in smallmouth bass (Micropterus dolomieu) in the Potomac River basin: Spatial and temporal comparisons of biological effects. Environ. Monit. Assess. 2012, 184, 4309–4334. [Google Scholar] [CrossRef]
- Blazer, V.S.; Walsh, H.L.; Braham, R.P.; Smith, C.R. Necropsy-based wild fish health assessment. J. Vis. Exp. 2018, e57946. [Google Scholar] [CrossRef]
- Luna, L.G. Histopathological Methods and Color Atlas of Special Stains and Tissue Artifacts; American Histolabs, Inc.: Gaithersburg, MD, USA, 1992; p. 767. [Google Scholar]
- Sharp, G.J.E.; Pike, A.W.; Secombes, C.J. Leucocyte migration in rainbow trout (Oncorhynchus mykiss [Walbaum]): Optimization of migration conditions and responses to host and pathogen (Diphyllobothrium dendriticum [Nitzsch]) derived chemoattractants. Dev. Comp. Immunol. 1991, 15, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Iwanowicz, L.R.; Blazer, V.S.; Hitt, N.P.; McCormick, S.D.; DeVault, D.S.; Ottinger, C.A. Histologic, immunologic and endocrine biomarkers indicate contaminant effects in fishes of the Ashtabula River. Ecotoxicology 2012, 21, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Ottinger, C.A.; Smith, C.R.; Blazer, V.; Iwanowicz, L.R.; Vogelbein, M.A.; Kaattari, S.L. Production and characterization of a mouse monoclonal antibody against smallmouth bass (Micropterus dolomieu) IgM. Fish Shellfish Immunol. 2021, 113, 20–23. [Google Scholar] [CrossRef]
- Team, R.C. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; Francois, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: ‘ggplot2’ based publication ready plots (R package version 0.4.0). 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 12 January 2022).
- Wilke, C.O. cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’ (R package version 1.1.1). 2020. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 12 January 2022).
- Wickham, H. Reshaping data with the reshape package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Grothendieck, G. gsubfn: Utilities for Strings and Function Arguments (R package version 0.7). 2018. Available online: https://CRAN.R-project.org/package=gsubfn (accessed on 12 January 2022).
- Aphalo, P.J. ggpmisc: Miscellaneous Extensions of ‘ggplot2’ (Version 0.3.8-1). 2021. Available online: https://CRAN.R-project.org/package=ggpmisc (accessed on 12 January 2022).
- Arnold, J.B. ggthemes: Extra themes, Scales and Geoms for ‘ggplot2’ (R package version 4.2.4). 2021. Available online: https://CRAN.R-project.org/package=ggthemes (accessed on 12 January 2022).
- Sarkar, D. Lattice: Multivariate Data Visualization with R.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Lemon, J. Plotrix: A package in the red light district of R. R-News 2006, 6, 8–12. [Google Scholar]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis (R package version 0.8.32). 2021. Available online: https://CRAN.R-project.org/package=FSA (accessed on 12 January 2022).
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Iwanowicz, L.R.; Blazer, V.S.; McCormick, S.D.; VanVeld, P.A.; Ottinger, C.A. Aroclor 1248 exposure leads to immunomodulation, decreased disease resistance and endocrine disruption in the brown bullhead, Ameiurus nebulosus. Aquat. Toxicol. 2009, 93, 70–82. [Google Scholar] [CrossRef]
- Daly, J.G.; Moore, A.R.; Olivier, G. A colorimetric assay for the quantification of brook trout (Salvelinus fontinalis) lymphocyte mitogenesis. Fish Shellfish Immunol. 1995, 5, 265–273. [Google Scholar] [CrossRef]
- Pohlenz, C.; Buentello, A.; Mwangi, W.; Gatlin, D.M. Arginine and glutamine supplementation to culture media improves the performance of various channel catfish immune cells. Fish Shellfish Immunol. 2012, 32, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Sitjà-Bobadilla, A. Living off a fish: A trade-off between parasites and the immune system. Fish Shellfish Immunol. 2008, 25, 358–372. [Google Scholar] [CrossRef]
- Secombes, C.J.; Chappell, L.H. Fish immune responses to experimental and natural infection with helminth parasites. Annu. Rev. Fish Dis. 1996, 6, 167–177. [Google Scholar] [CrossRef]
- Souza, D.C.M.; dos Santos, M.C.; Chagas, E.C. Immune response of teleost fish to helminth parasite infection. Braz. J. Vet. Parasitol. 2019, 28, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, T.M.; Valtonen, E.T.; Jokinen, E.I. Humoral response of roach (Rutilus rutilus) to digenean Rhipidocotyle fennica infection. Parasitology 1997, 114, 285–291. [Google Scholar] [CrossRef]
- Polinski, M.; Shirakashi, S.; Bridle, A.; Nowak, B. Transcriptional immune response of cage-cultured Pacific bluefin tuna during infection by two Cardicola blood fluke species. Fish Shellfish Immunol. 2014, 36, 61–67. [Google Scholar] [CrossRef]
- Wolke, R.E.; Murchelano, R.A.; Dickstein, C.D.; George, C.J. Preliminary evaluation of the use of macrophage aggregates (MA) as fish health monitors. Bull. Environ. Contam. Toxicol. 1985, 35, 222–227. [Google Scholar] [CrossRef]
- Fournie, J.W.; Summers, J.K.; Courtney, L.A.; Engle, V.D.; Blazer, V.S. Utility of splenic macrophage aggregates as an indicator of fish exposure to degraded environments. J. Aquat. Anim. Health 2001, 13, 105–116. [Google Scholar] [CrossRef]
- Facey, D.E.; Blazer, V.S.; Gasper, M.M.; Turcotte, C.L. Using fish biomarkers to monitor improvements in environmental quality. J. Aquat. Anim. Health 2005, 17, 263–266. [Google Scholar] [CrossRef]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Fournie, J.W.; Weeks-Perkins, B.A. Macrophage Aggregates: Biomarker for Immune Function in Fishes. In Environmental Toxicology and Risk Assessment: Modeling and Risk Assessment Sixth Volume; Dwyer, F., Doane, T., Hinman, M., Eds.; ASTM International: West Conshohocken, PA, USA, 1997; pp. 360–375. [Google Scholar]
- Steinel, N.C.; Bolnick, D.I. Melanomacrophage Centers As a Histological Indicator of Immune Function in Fish and Other Poikilotherms. Front. Immunol. 2017, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Segner, H.; Rehberger, K.; Bailey, C.; Bo, J. Assessing Fish Immunotoxicity by Means of In Vitro Assays: Are We There Yet? Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Rehberger, K.; Escher, B.I.; Scheidegger, A.; Werner, I.; Segner, H. Evaluation of an in vitro assay to screen for the immunotoxic potential of chemicals to fish. Sci. Rep. 2021, 11, 3167. [Google Scholar] [CrossRef]

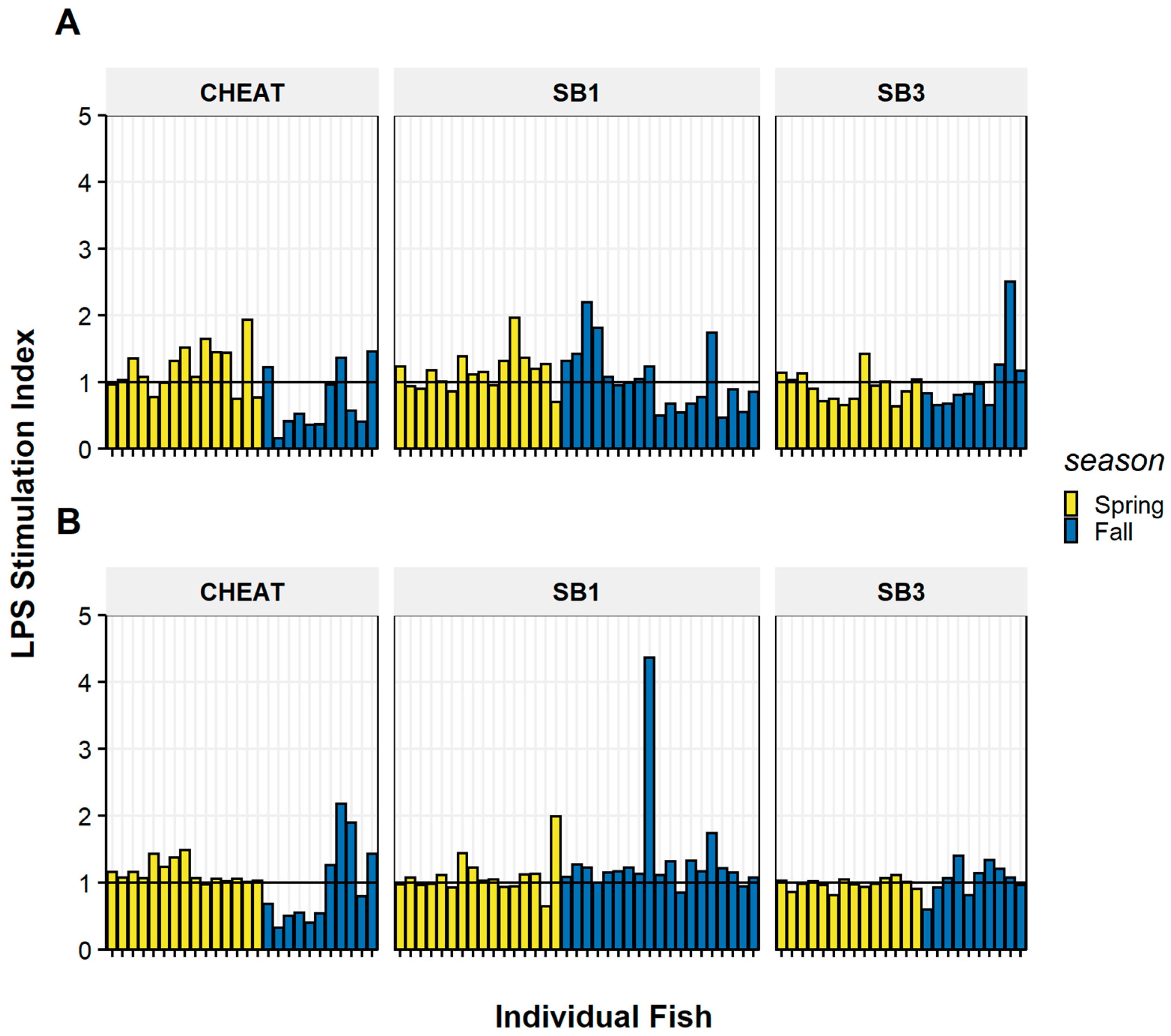
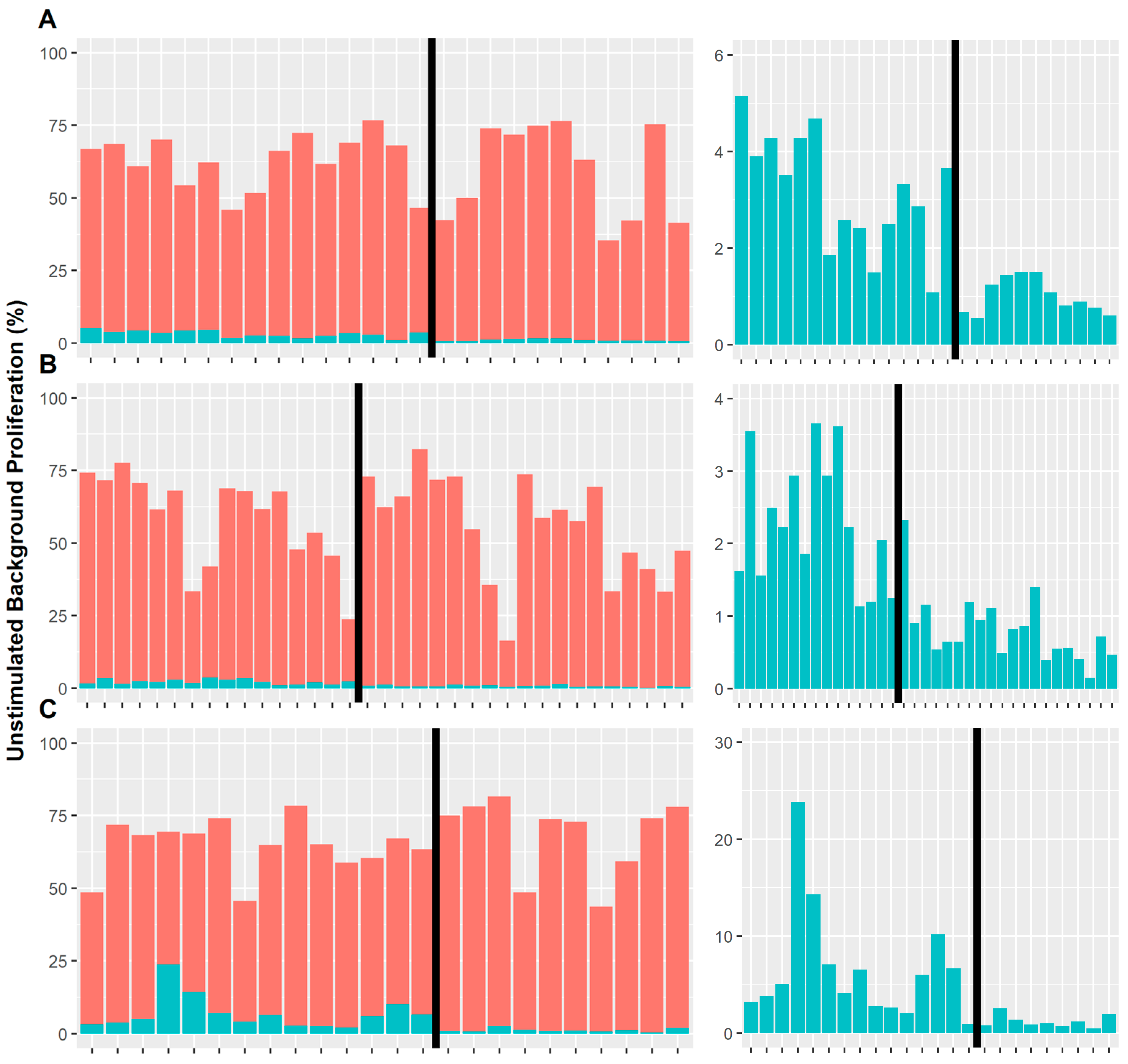


| Treatment | Cell Type | Season | Site | r | p Value |
|---|---|---|---|---|---|
| LPS | IgM+ | Spring | CH | −0.68 | 0.006 |
| LPS | IgM+ | Spring | SB1 | −0.55 | 0.026 |
| LPS | IgM− | Spring | CH | −0.67 | 0.007 |
| LPS | IgM− | Spring | SB1 | −0.69 | 0.003 |
| LPS | IgM− | Spring | SB3 | −0.64 | 0.015 |
| LPS | IgM− | Fall | CH | −0.69 | 0.019 |
| LPS | IgM− | Fall | SB1 | −0.58 | 0.009 |
| LPS | IgM− | Fall | SB3 | −0.75 | 0.012 |
| Site | Females (n) | Length (mm) | Weight (gm) | Age (yr) | Males (n) | Length (mm) | Weight (gm) | Age (yr) |
|---|---|---|---|---|---|---|---|---|
| Spring | ||||||||
| CH | 2 | 274 ± 8 | 229 ± 34 | 7.0 ± 1.0 a | 18 | 333 ± 11 | 477 ± 51 | 8.1 ± 0.4 a |
| SB1 | 7 | 281 ± 15 | 317 ± 53 | 3.1 ± 0.4 b | 13 | 309 ± 13 | 438 ± 62 | 3.4 ± 0.4 b |
| SB3 | 14 | 318 ± 17 | 493 ± 84 | 3.9 ± 0.4 b | 6 | 358 ± 37 | 627 ± 153 | 3.8 ± 0.8 b |
| Fall | ||||||||
| CH | 6 | 275 ± 25 | 287 ± 103 | 8.0 ± 1.4 a | 13 | 280 ± 13 | 283 ± 51 | 8.2 ± 0.7 a |
| SB1 | 11 | 290 ± 9 | 316 ± 30 | 4.2 ± 0.3 b | 9 | 289 ± 11 | 314 ± 36 | 4.7 ± 0.5 b |
| SB3 | 3 | 318 ± 81 | 548 ± 366 | 4.7 ± 2.2 a,b | 8 | 262 ± 23 | 308 ± 98 | 3.5 ± 0.7 b |
| Treatment | Tissue Parasites | Cell Type | Season | Site | r | p Value |
|---|---|---|---|---|---|---|
| Unstimulated | Spleen | IgM− | Fall | SB3 | −0.68 | 0.030 |
| LPS | Spleen | IgM+ | Spring | CH | −0.51 | 0.054 |
| LPS | Spleen | IgM+ | Fall | CH | 0.62 | 0.041 |
| Treatment | Cell Type | Season | Site | r | p Value |
|---|---|---|---|---|---|
| Unstimulated | IgM− | Fall | CH | −0.62 | 0.040 |
| LPS | IgM+ | Fall | CH | 0.89 | <0.001 |
| LPS | IgM− | Fall | CH | 0.62 | 0.043 |
| LPS | IgM− | Fall | SB3 | −0.72 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, C.R.; Ottinger, C.A.; Walsh, H.L.; Mazik, P.M.; Blazer, V.S. Application of a Lipopolysaccharide (LPS)-Stimulated Mitogenesis Assay in Smallmouth Bass (Micropterus dolomieu) to Augment Wild Fish Health Studies. Fishes 2023, 8, 159. https://doi.org/10.3390/fishes8030159
Smith CR, Ottinger CA, Walsh HL, Mazik PM, Blazer VS. Application of a Lipopolysaccharide (LPS)-Stimulated Mitogenesis Assay in Smallmouth Bass (Micropterus dolomieu) to Augment Wild Fish Health Studies. Fishes. 2023; 8(3):159. https://doi.org/10.3390/fishes8030159
Chicago/Turabian StyleSmith, Cheyenne R., Christopher A. Ottinger, Heather L. Walsh, Patricia M. Mazik, and Vicki S. Blazer. 2023. "Application of a Lipopolysaccharide (LPS)-Stimulated Mitogenesis Assay in Smallmouth Bass (Micropterus dolomieu) to Augment Wild Fish Health Studies" Fishes 8, no. 3: 159. https://doi.org/10.3390/fishes8030159
APA StyleSmith, C. R., Ottinger, C. A., Walsh, H. L., Mazik, P. M., & Blazer, V. S. (2023). Application of a Lipopolysaccharide (LPS)-Stimulated Mitogenesis Assay in Smallmouth Bass (Micropterus dolomieu) to Augment Wild Fish Health Studies. Fishes, 8(3), 159. https://doi.org/10.3390/fishes8030159