Fish Biozonation in the Balkan Peninsula, Especially in Bulgaria: A Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Data Analysis
- Best available (=near-reference) sites: Fish index values as calculated according to Belkinova et al. [22] preferably higher than 0.85 for the last sampling years.
- Historical data: species community, mainly presence/absence (partly dominance).
- -
- total referent abundance = 10,000 ind./ha as accepted by the national fish index
- -
- relative proportion of dominant species >75%
- -
- relative proportion accompanying species 20–75%
- -
- relative proportion of rare species 5–20%
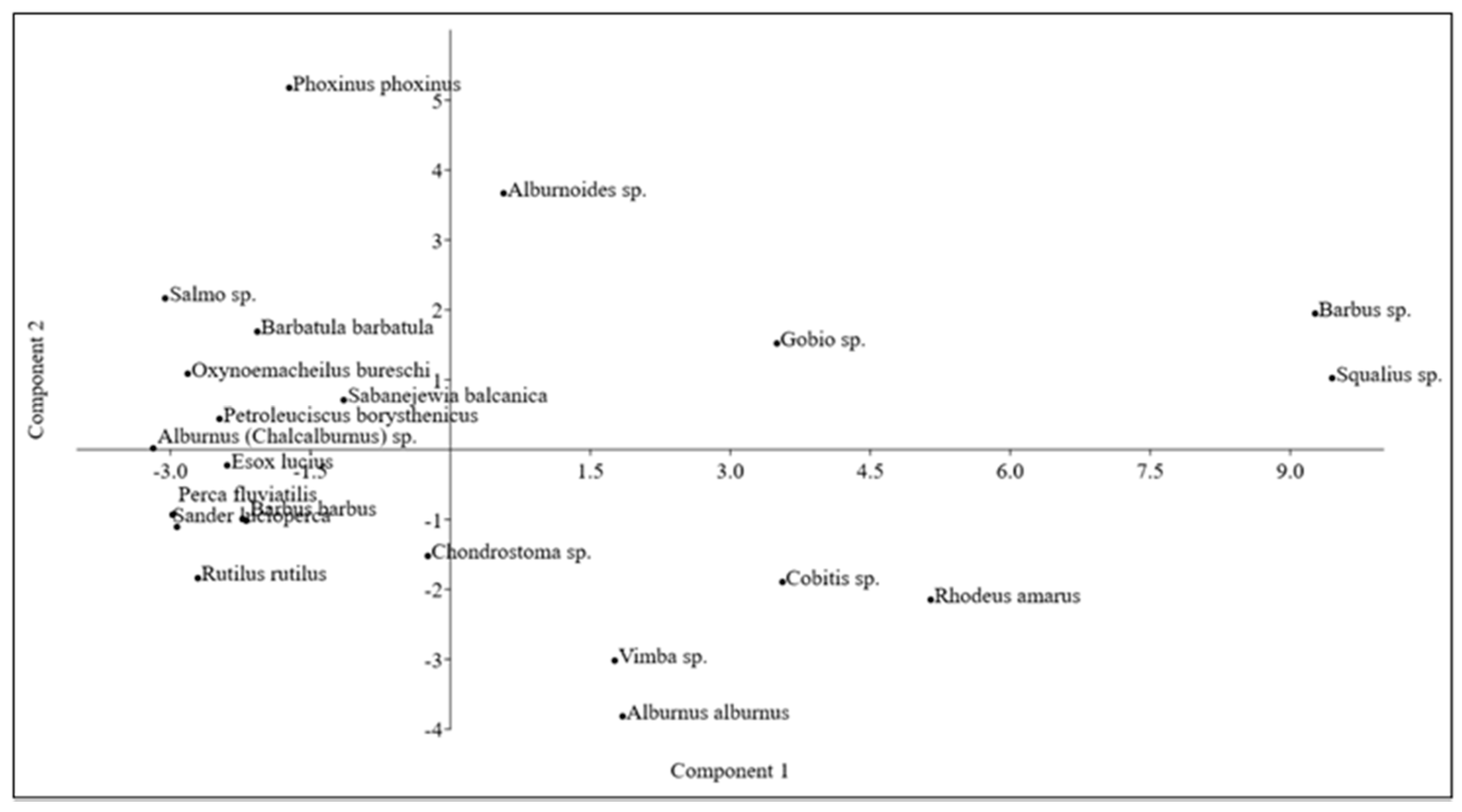
3. Results
- Low-altitude lakes, where cyprinid fish communities together with percid predators, as well as Silurus glanis Linnaeus 1758, are common.
- High-altitude lakes, which are inhabited by primarily salmonid fish communities.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU. Common Implementation Strategy for the Water Framework Directive 2000/60/EC. Guidance Document No. 10, River and Lakes-Typology, Reference Conditions and Classification Systems; Office for Official Publications of the European Communities: Luxembourg, 2003; pp. 1–87. [Google Scholar]
- Zogaris, S.; Economou, A.; Dimopoulos, P. Ecoregions in the southern Balkans: Should they be revised? Environ. Manag. 2009, 43, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Vavalidis, T.; Zogaris, S.; Economou, A.; Kallimanis, A.; Bobori, D. Changes in Fish Taxonomy Affect Freshwater Biogeographical Regionalisations: Insights from Greece. Water 2019, 11, 1743. [Google Scholar] [CrossRef]
- Aarts, B.; Nienhuis, P. Fish zonations and guilds as the basis for assessment of ecological integrity of large rivers. Hydrobiologia 2003, 500, 157–178. [Google Scholar] [CrossRef]
- Wolter, C.; Borcherding, J.; Ferreira, M.; Freyhof, J.; Gessner, J.; Górski, K.; Năstase, A.; Schomaker, C.; Eros, T. Characterization of European lampreys and fishes by their longitudinal and lateral distribution traits. Ecol. Indic. 2021, 123, 107350. [Google Scholar] [CrossRef]
- Stefanov, T. Fauna and Distribution of Fishes in Bulgaria. In Biogeography and Ecology of Bulgaria, 1st ed.; Fet, V., Popov, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 109–140. [Google Scholar]
- Cheshmedjiev, S.; Marinov, M. Typology of water ecosystems in Bulgaria (implementation of Water Framework Directive 2000/60/EC). In Proceedings of the Anniversary Scientific Conference of Ecology, Plovdiv, Bulgaria, 1 November 2008; Velcheva, I., Tsekov, A., Eds.; pp. 371–383. [Google Scholar]
- Kohout, J.; Šedivá, A.; Apostolou, A.; Stefanov, T.; Marić, S.; Gaffaroğlu, M.; Šlechta, V. Genetic diversity and phylogenetic origin of brown trout Salmo trutta populations in eastern Balkans. Biologia 2013, 68, 1229–1237. [Google Scholar] [CrossRef]
- Illies, J. Versuch einer allgemeinen biozönotischen Gliederung der Fließgewässer Internationale. Rev. Der Gesamten Hydrobiol. Und Hydrogr. 1961, 46, 205–213. [Google Scholar] [CrossRef]
- Apostolou, A.; Koutrakis, M.; Pehlivanov, L.; Vassilev, M.; Stefanov, T.; Velkov, B. Notes on the Fish Fauna Composition of Mesta (Nestos) River in Regard to Management and Conservation. Acta Zool. Bulg. 2010, 62, 271–276. [Google Scholar]
- Huet, M. Profiles and Biology of Western European Streams as Related to Fish Management. Trans. Am. Fish. Soc. 1959, 88, 155–163. [Google Scholar] [CrossRef]
- Uzunova, E.; Studenkov, S.; Dashinov, D. First records of largemouth bass Micropterus salmoides (Lacépède, 1802) from Bulgaria (Balkan Peninsula). BioInvasions Rec. 2019, 8, 427–436. [Google Scholar] [CrossRef]
- Oikonomou, A.; Leprieur, F.; Leonardos, I. Biogeography of freshwater fishes of the Balkan Peninsula. Hydrobiologia 2014, 738, 205–220. [Google Scholar] [CrossRef]
- Cheshmedjiev, S.; Karagiozova, T.; Michailov, M.; Valev, V. Revision of River & Lake Typology in Bulgaria within Ecoregion 12 (Pontic Province) and Ecoregion 7 (Eastern Balkans) According to the Water Framework Directive. Ecol. Balk. 2010, 2, 75–96. [Google Scholar]
- MOEW. River Basin Management Plans 2016–2021. (In Bulgarian). Available online: https://www.moew.government.bg/bg/vodi/planove-za-upravlenie/planove-za-upravlenie-na-rechnite-basejni-purb/ (accessed on 21 January 2023).
- CEN-EN 14011; Water Quality-Sampling of Fish with Electricity. European Committee for Standardization. European Committee for Standardization: Brussels, Belgium, 2003; pp. 1–16.
- Kelly, L.; Connor, L.; Matson, R.; Feeney, R.; Morrissey, E.; Coyne, J.; Rocks, K. Sampling Fish for the Water Framework Directive-Summary Report 2014. Inland Fisheries Ireland, Citywest Business, Campus, Dublin 24, Ireland. Available online: [wfdfish.ie/wp-content/uploads/2010/05/WFD_Report_2014_FINAL.pdf] (accessed on 21 January 2023).
- CEN-EN 14757; Water Quality. Water Quality-Sampling of Fish with Multi-Mesh Gillnets. European Committee for Standardization, Brussels, Belgium, 2005; pp. 1–31.
- Apostolou, A.; Pehlivanov, L. Pilot underwater visual census study in a Bulgarian Sub-Mediterranean reservoir. Thalass. Salentina 2018, 40, 21–26. [Google Scholar]
- Apostolou, A.; Pehlivanov, L.; Schabuss, M.; Zornig, H.; Wolfram, G.; Konecny, R.; Todorov, E.; Todorov, D.; Pont, D. Report on fitting a new classification method to the results of the completed intercalibration of the Fish Cross GIG (Danubian Group). Bulgaria, Vs. 6.2 (21 June 2016). Ministry of Environment and Water of Bulgaria, Consortium DICON–UBA, Sofia-Vienna. 2016, pp. 1–28. Available online: [https://circabc.europa.eu/sd/a/09fcff1a-5f80-432e-a6b8-f37443c0a0e5/BG_FISH_DanubGroup_Rivers_Fish_BG_fit_in%20Draft%2016-06-21%20vs.%206.2_final.pdf] (accessed on 21 January 2023).
- Vassilev, M.; Pehlivanov, L. Checklist of Bulgarian freshwater fishes. Acta Zool. Bulg. 2005, 57, 161–190. [Google Scholar]
- Belkinova, D.; Gecheva, G.; Cheshmedjiev, S.; Dimitrova-Dyulgerova, I.; Mladenov, R.; Marinov, M.; Teneva, I.; Stoyanov, P.; Mihov, S.; Pehlivanov, L.; et al. Biological Analysis and Ecological Status Assessment of Bulgarian Surface Water Ecosystems; University of Plovdiv, Publishing House: Plovdiv, Bulgaria, 2013; pp. 1–235. (In Bulgarian) [Google Scholar]
- Jepsen, N.; Pont, D.; Intercalibration of Fish-Based Methods to Evaluate River Ecological Quality. JRC Scientific and Technical Reports. European Communities, Luxembourg. 2007, pp. 1–194. Available online: https://www.unisdr.org/files/2775_EUR22878EN.pdf (accessed on 21 January 2023).
- Vassilev, I.; Georgiev, B. River runoff changes and recent climatic fluctuations in Bulgaria. GeoJournal 1996, 40, 379–385. [Google Scholar] [CrossRef]
- MOEW. Ordinance № H-4 from 14.09.2012 for characterization of surface waters. Issued by the Minister of Environment and Water, promulgated, SG, no. 22 of 05.03.2013, in force from March 5, 2013, amended and add., no. 79 of 23.09.2014, in force since 23.09.2014, amended and ext. DV. issue 85 of October 2 2020. Sofia, pp.1–119. (In Bulgarian). Available online: [https://www.moew.government.bg/static/media/ups/tiny/filebase/Water/Legislation/Naredbi/vodi/Naredba%20H-4.pdf] (accessed on 21 January 2023).
- Lasne, E.; Bergerot, B.; Lek, S.; Laffaille, P. Fish zonation and indicator species for the evaluation of the ecological status of rivers: Example of the Loire Basin (France). River Res. Appl. 2007, 23, 877–890. [Google Scholar] [CrossRef]
- Weiss, S.; Apostolou, A.; Đug, S.; Marčić, Z.; Oikonomou, A.; Schumka, S.; Simonović, P.; Zabric, D. Endangered fish species in Balkan rivers: Their distributions and threats from hydropower development. RiverWatch & EuroNatur. 2018, pp. 1–161. Available online: [https://balkanrivers.net/sites/default/files/Fish_Study_web.pdf] (accessed on 21 January 2023).
- Kotori, L.; Vavalidis, T.; Zogaris, D.; Šanda, R.; Vukić, J.; Geci, D.; Ibrahimi, H.; Bilalli, A.; Zogaris, S. Fish distribution patterns in the White Drin (Drini i Bardhë) river, Kosovo. Knowl. Manag. Aquat. Ecosyst. 2021, 421, 29. [Google Scholar]
- Moore, J.; Olden, J. Response diversity, nonnative species, and disassembly rules buffer freshwater ecosystem processes from anthropogenic change. Glob. Chang. Biol. 2017, 23, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- MOEW. Ordinance № 37 from 10.11.2008 for the use of reservoirs-state property, in terms of fisheries and the rules for commercial, recreational fishing, and aquaculture in the sites-state property under Art. 3, para. 1 of the Fisheries and Aquaculture Law. 2018; pp. 1–24, (In Bulgarian). Available online: [https://www.mzh.government.bg/media/filer_public/2020/12/22/naredba__37_ot_2008_g.pdf] (accessed on 21 January 2023).
- Holling, C.S. Understanding the complexity of economic, ecological, and social systems. Ecosystems 2001, 4, 390–405. [Google Scholar] [CrossRef]
- Angermeier, P.L.; Winston, M.R. Characterizing fish community diversity across Virginia landscapes: Prerequisite for conservation. Ecol. Appl. 1999, 9, 335–349. [Google Scholar] [CrossRef]

| Water Body Type (L = lake) | Count | Water Body Type (R = river) | Count | Freshwater Basin | Count |
|---|---|---|---|---|---|
| L01 | 5 | R01 | 9 | Black Sea | 82 |
| L02 | 5 | R02 | 27 | Danube River | 173 |
| L03 | 6 | R03 | 14 | East Aegean | 93 |
| L04 | 4 | R04 | 58 | West Aegean | 41 |
| L05 | 3 | R05 | 12 | ||
| L06 | 3 | R06 | 15 | ||
| L07 | 3 | R07 | 32 | ||
| L08 | 5 | R08 | 35 | ||
| L09 | 8 | R09 | 7 | ||
| L10 | 4 | R10 | 9 | ||
| L11 | 5 | R11 | 9 | ||
| L12 | 5 | R12 | 13 | ||
| L13 | 5 | R13 | 12 | ||
| L14 | 4 | R14 | 40 | ||
| L15 | 5 | R15 | 8 | ||
| L16 | 5 | R16 | 9 | ||
| L17 | 5 | Total | 389 |
| Zone | Species | |||||
|---|---|---|---|---|---|---|
| epirythral | Salmo sp. | Phoxinus sp. | ||||
| metarhythral | Barbus sp. | |||||
| hyporhythral | Barbus sp. | Squalius sp. | ||||
| metapotamal | Barbus sp. | Squalius sp. | Rhodeus amarus | Cobitis sp. | Vimba sp. | Alburnus sp. |
| epipotamal | Barbus sp. | Squalius sp. | Rhodeus amarus | Cobitis sp. | ||
| Transitional | all brackish Alburnus sp. (without A. alburnus) | Petroleuciscus borysthenicus | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolou, A.; Pehlivanov, L.; Schabuss, M.; Zornig, H.; Wolfram, G. Fish Biozonation in the Balkan Peninsula, Especially in Bulgaria: A Challenge. Fishes 2023, 8, 91. https://doi.org/10.3390/fishes8020091
Apostolou A, Pehlivanov L, Schabuss M, Zornig H, Wolfram G. Fish Biozonation in the Balkan Peninsula, Especially in Bulgaria: A Challenge. Fishes. 2023; 8(2):91. https://doi.org/10.3390/fishes8020091
Chicago/Turabian StyleApostolou, Apostolos, Luchezar Pehlivanov, Michael Schabuss, Horst Zornig, and Georg Wolfram. 2023. "Fish Biozonation in the Balkan Peninsula, Especially in Bulgaria: A Challenge" Fishes 8, no. 2: 91. https://doi.org/10.3390/fishes8020091
APA StyleApostolou, A., Pehlivanov, L., Schabuss, M., Zornig, H., & Wolfram, G. (2023). Fish Biozonation in the Balkan Peninsula, Especially in Bulgaria: A Challenge. Fishes, 8(2), 91. https://doi.org/10.3390/fishes8020091





