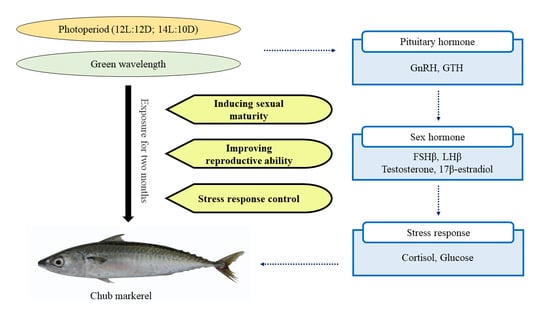
Physiological Effect of Extended Photoperiod and Green Wavelength on the Pituitary Hormone, Sex Hormone and Stress Response in Chub Mackerel, Scomber japonicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Experimental Design and Sampling
2.3. Pituitary Hormone
2.4. Pituitary Gonadotropins
2.5. Sex Hormone
2.6. Stress Hormone and Glucose
2.7. Statistical Analysis
3. Results
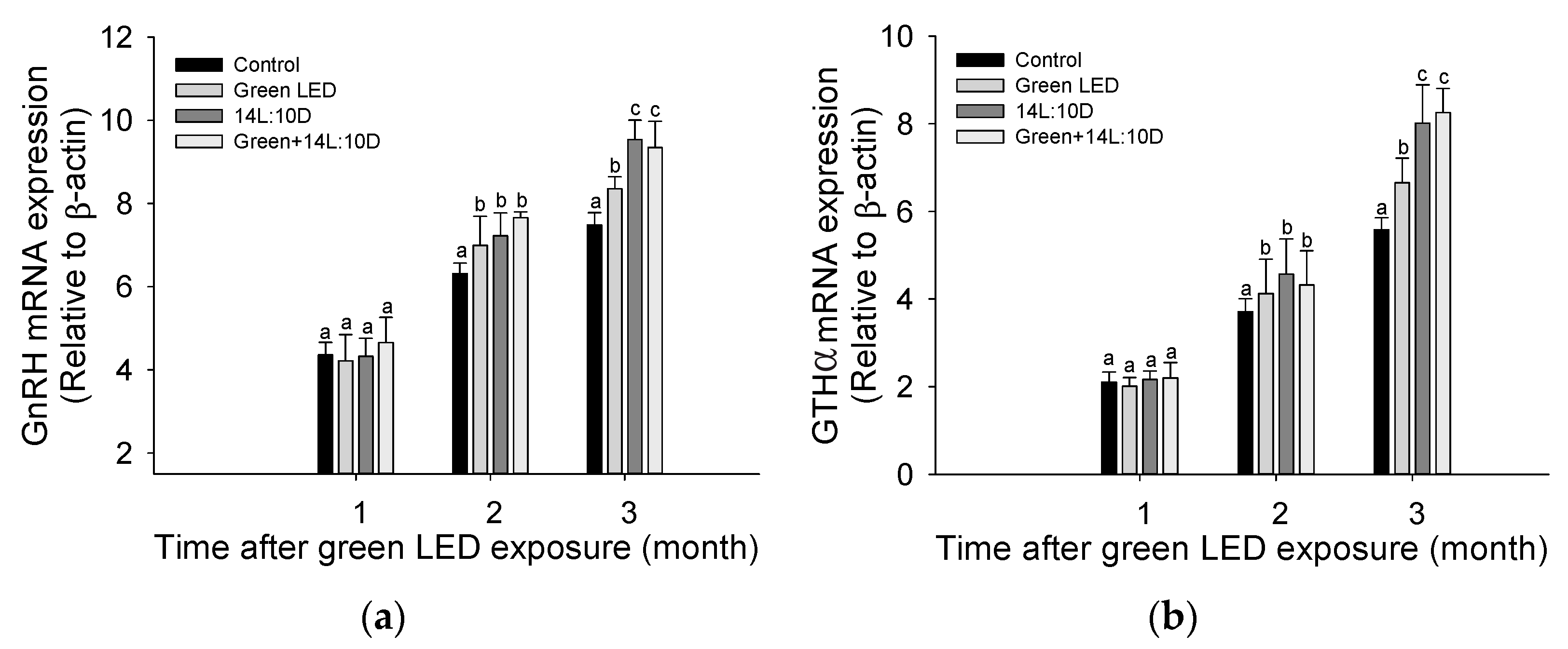
3.1. Pituitary Hormone
3.2. Pituitary Gonadotropins
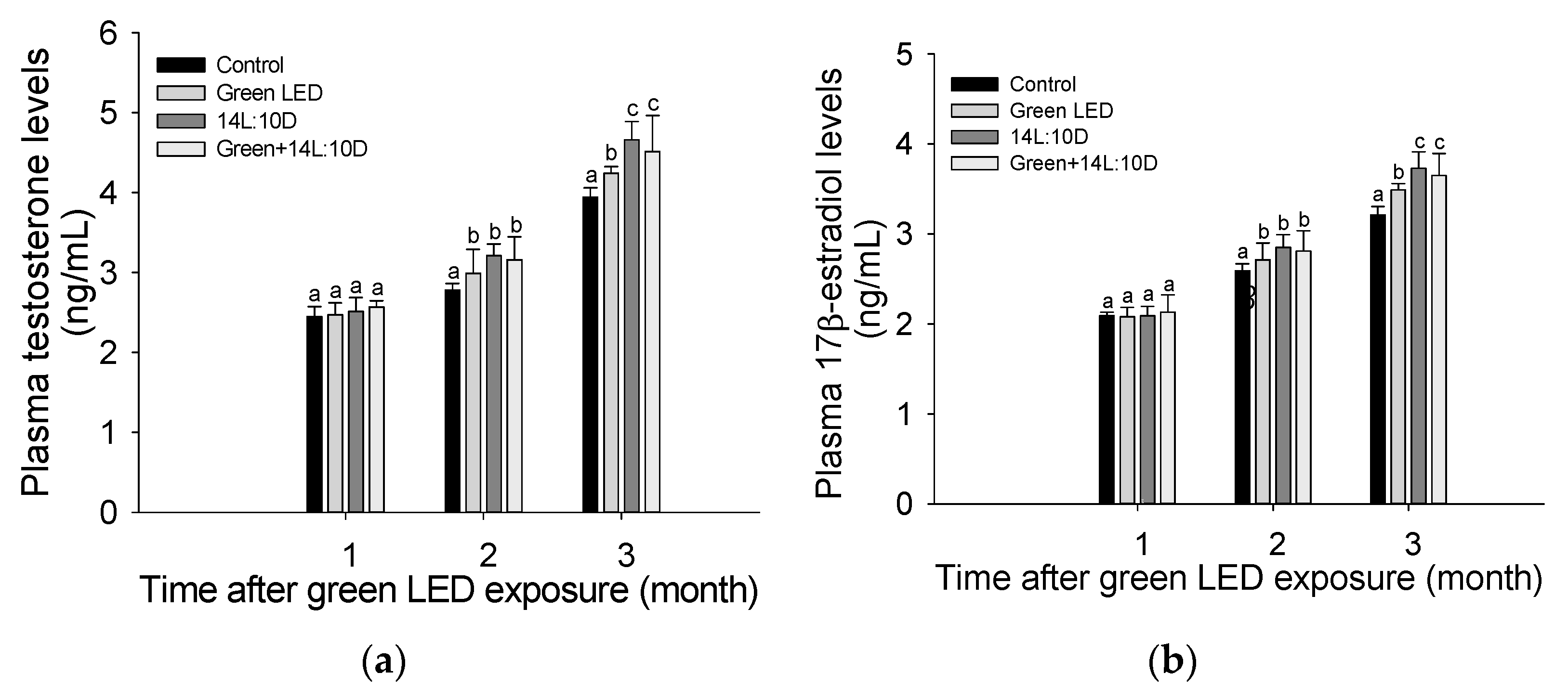
3.3. Sex Hormone
3.4. Stress Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Debes, P.V.; Piavchenko, N.; Ruokolainen, A.; Ovaskainen, O.; Moustakas-Verho, J.E.; Parre, N.; Primmer, C.R. Polygenic and major-locus contributions to sexual maturation timing in Atlantic salmon. Mol. Ecol. 2021, 30, 4505–4519. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.D.; Gunnarsson, S.; Thorarensen, H. Impact of environmental factors on the growth and maturation of farmed Arctic charr. Rev. Aquac. 2020, 12, 1689–1707. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, Y.; Fu, L.; Simco, B.A.; Goudie, C.A. Brood-stock management and natural spawning of American shad (Alosa sapidissima) in a recirculating aquaculture system. Aquaculture 2021, 532, 735952. [Google Scholar] [CrossRef]
- Veras, G.C.; Murgas, L.D.S.; Rosa, P.V.; Zangeronimo, M.G.; Ferreira, M.S.D.S.; Leon, J.A.S.D. Effect of photoperiod on locomotor activity, growth, feed efficiency and gonadal development of Nile tilapia. Rev. Bras. Zootec. 2013, 42, 844–849. [Google Scholar] [CrossRef]
- Czarnecka, M.; Kakareko, T.; Jermacz, Ł.; Pawlak, R.; Kobak, J. Combined effects of nocturnal exposure to artificial light and habitat complexity on fish foraging. Sci. Total Environ. 2019, 684, 14–22. [Google Scholar] [CrossRef]
- Ruchin, A.B. Environmental colour impact on the life of lower aquatic vertebrates: Development, growth, physiological and biochemical processes. Rev. Aquac. 2020, 12, 310–327. [Google Scholar] [CrossRef]
- Ruchin, A.B. Effect of illumination on fish and amphibian: Development, growth, physiological and biochemical processes. Rev. Aquac. 2021, 13, 567–600. [Google Scholar] [CrossRef]
- Schligler, J.; Cortese, D.; Beldade, R.; Swearer, S.E.; Mills, S.C. Long-term exposure to artificial light at night in the wild decreases survival and growth of a coral reef fish. Proc. Biol. Sci. 2021, 288, 20210454. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Rajesh, M.; Kamalam, B.S.; Ciji, A. Effect of photoperiod and temperature on indicators of immunity and wellbeing of endangered golden mahseer (Tor putitora) broodstock. J. Therm. Biol. 2020, 93, 102694. [Google Scholar] [CrossRef]
- Baekelandt, S.; Milla, S.; Cornet, V.; Flamion, E.; Ledoré, Y.; Redivo, B.; Kestemont, P. Seasonal simulated photoperiods influence melatonin release and immune markers of pike perch Sander lucioperca. Sci. Rep. 2020, 10, 2650. [Google Scholar] [CrossRef]
- Garcia, I.D.; Plaul, S.E.; Torres, D.; Del Fresno, P.S.; Miranda, L.A.; Colautti, D.C. Effect of photoperiod on ovarian maturation in Cheirodon interruptus (Teleostei: Characidae). Braz. J. Biol. 2018, 79, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Freitas, E.; Carvalho, T.B.; Oliveira, R.F. Photoperiod modulation of aggressive behavior is independent of androgens in a tropical cichlid fish. Gen. Comp. Endocrinol. 2014, 207, 41–49. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.J.; Fobert, E.K.; Besson, M.; Jacob, H.; Lecchini, D. Live fast, die young: Behavioural and physiological impacts of light pollution on a marine fish during larval recruitment. Mar. Pollut. Bull. 2019, 146, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Kissil, G.W.; Lupatsch, I.; Elizur, A.; Zohar, Y. Long photoperiod delayed spawning and increased somatic growth in gilthead seabream (Sparus aurata). Aquaculture 2001, 200, 363–379. [Google Scholar] [CrossRef]
- Arambam, K.; Singh, S.K.; Biswas, P.; Patel, A.B.; Jena, A.K.; Pandey, P.K. Influence of light intensity and photoperiod on embryonic development, survival and growth of threatened catfish Ompok bimaculatus early larvae. J. Fish Biol. 2020, 97, 740–752. [Google Scholar] [CrossRef]
- Sánchez-Vázquez, F.J.; López-Olmeda, J.F.; Vera, L.M.; Migaud, H.; López-Patiño, M.A.; Míguez, J.M. Environmental cycles, melatonin, and circadian control of stress response in fish. Front. Endocrinol. 2019, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hur, S.W.; Kim, B.H.; Soyano, K.; Lee, Y.D. Induced maturation and fertilized egg production of the red-spotted grouper, Epinephelus akaara, using adaptive physiology of photoperiod and water temperature. Aquac. Res. 2020, 51, 2084–2090. [Google Scholar] [CrossRef]
- Murugananthkumar, R.; Sudhakumari, C.C. Understanding the impact of stress on teleostean reproduction. Aquac Fish. 2022, 7, 553–561. [Google Scholar] [CrossRef]
- Giordano, M. Genetic causes of isolated and combined pituitary hormone deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; He, J.; Zhu, J.H.; Tao, Y.F.; Bao, J.W.; Yan, Y.; Zhu, X. Optimal combination of temperature and photoperiod for sex steroid hormone secretion and egg development of Oreochromis niloticus as determined by response surface methodology. J. Therm. Biol. 2021, 97, 102889. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Navarro-Martín, L.; Trudeau, V.L. Mechanisms of crosstalk between endocrine systems: Regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen. Comp. Endocrinol. 2014, 203, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Sudo, R.; Yada, T. Anguillid Eels as a Model Species for Understanding Endocrinological Influences on the Onset of Spawning Migration of Fishes. Biology 2022, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Pfaff, D.W.; Parhar, I.S. Fish as a model in social neuroscience: Conservation and diversity in the social brain network. Biol. Rev. 2021, 96, 999–1020. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Al-Emran, M.; Islam, S.M.; Baten, S.A.; Rashid, H.; Haque, M.M. Prolonged photoperiod inhibits growth and reproductive functions of rohu Labeo rohita. Aquac. Rep. 2020, 16, 100272. [Google Scholar] [CrossRef]
- Song, J.A.; Choi, C.Y. Effects of blue light spectra on retinal stress and damage in goldfish (Carassius auratus). Fish Physiol. Biochem. 2019, 45, 391–400. [Google Scholar] [CrossRef]
- Aragón-Flores, E.A.; Martínez-Cárdenas, L.; Hernández-González, C.; Barba-Quintero, G.; Zavala-Leal, O.I.; Ruiz-Velazco, J.M.; Juárez-López, P. Effect of light intensity and photoperiod on growth and survival of the Mexican cichlid, Cichlasoma beani in culture conditions. Lat. Am. J. Aquat. Res. 2017, 45, 293–301. [Google Scholar] [CrossRef]
- Dara, M.; Dioguardi, M.; Vazzana, M.; Vazzana, I.; Accardi, D.; Carbonara, P.; Cammarata, M. Effects of Social Hierarchy Establishment on Stress Response and Cell Phagocytosis in Gilt-Head Sea Bream (Sparus aurata). Fishes 2022, 7, 75. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.Z.; Chen, J.Y.; Chen, D.Z.; Deng, S.G.; Xu, B. Effect of cold plasma on maintaining the quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. J. Food Sci. 2019, 99, 39–46. [Google Scholar] [CrossRef]
- Choi, C.Y.; Choi, J.Y.; Choi, Y.J.; Yoo, J.H. Physiological effects of various light spectra on oxidative stress by starvation in olive flounder, Paralichthys olivaceus. Mol. Cell Toxicol. 2018, 14, 399–408. [Google Scholar] [CrossRef]
- Takahashi, A.; Kasagi, S.; Murakami, N.; Furufuji, S.; Kikuchi, S.; Mizusawa, K.; Andoh, T. Effects of different green light intensities on the growth performance and endocrine properties of barfin flounder Verasper moseri. Gen. Comp. Endocrinol. 2018, 257, 203–210. [Google Scholar] [CrossRef]
- Lee, Y.H.; Du, J.L.; Yen, F.P.; Lee, C.Y.; Dufour, S.; Huang, J.D.; Chang, C.F. Regulation of plasma gonadotropin II secretion by sex steroids, aromatase inhibitors, and antiestrogens in the protandrous black porgy, Acanthopagrus schlegeli Bleeker. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 399–406. [Google Scholar] [CrossRef]
- Moussavi, M.; Wlasichuk, M.; Chang, J.P.; Habibi, H.R. Seasonal effect of GnIH on gonadotrope functions in the pituitary of goldfish. Mol. Cell. Endocrinol. 2012, 350, 53–60. [Google Scholar] [CrossRef]
- Ogawa, S.; Parhar, I.S. Single-cell gene profiling reveals social status-dependent modulation of nuclear hormone receptors in GnRH neurons in a male cichlid fish. Int. J. Mol. Sci. 2020, 21, 2724. [Google Scholar] [CrossRef]
- Fukaya, K.; Amano, M.; Ueda, H. Diurnal changes in salmon GnRH secretion in the brain of masu salmon (Oncorhynchus masou). Gen. Comp. Endocrinol. 2013, 192, 77–80. [Google Scholar] [CrossRef]
- Shin, H.S.; Song, J.A.; Choi, J.Y.; Kim, N.N.; Choi, Y.J.; Sung, S.N.; Choi, C.Y. Effects of various photoperiods on Kisspeptin and reproductive hormones in the goldfish, Carassius auratus. Anim. Cells Syst. 2014, 18, 109–118. [Google Scholar] [CrossRef]
- Shao, Y.T.; Roufidou, C.; Chung, P.C.; Borg, B. Changes in kisspeptin, GnRH, and gonadotropin mRNA levels in male Threespine stickleback (Gasterosteus aculeatus) during photoperiod-induced sexual maturation. Evol. Ecol. Res. 2019, 20, 317–329. [Google Scholar]
- Klausen, C.; Chang, J.P.; Habibi, H.R. The effect of gonadotropin-releasing hormone on growth hormone and gonadotropin subunit gene expression in the pituitary of goldfish, Carassius auratus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 511–516. [Google Scholar] [CrossRef]
- Molés, G.; Hausken, K.; Carrillo, M.; Zanuy, S.; Levavi-Sivan, B.; Gómez, A. Generation and use of recombinant gonadotropins in fish. Gen. Comp. Endocrinol. 2020, 299, 113555. [Google Scholar] [CrossRef]
- Nyuji, M.; Hamada, K.; Kazeto, Y.; Mekuchi, M.; Gen, K.; Soyano, K.; Okuzawa, K. Photoperiodic regulation of plasma gonadotropin levels in previtellogenic greater amberjack (Seriola dumerili). Gen. Comp. Endocrinol. 2018, 269, 149–155. [Google Scholar] [CrossRef]
- Shao, Y.T.; Tseng, Y.C.; Chang, C.H.; Yan, H.Y.; Hwang, P.P.; Borg, B. GnRH mRNA levels in male three-spined sticklebacks, Gasterosteus aculeatus, under different reproductive conditions. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 180, 6–17. [Google Scholar] [CrossRef]
- Mateos, J.; Mananos, E.; Carrillo, M.; Zanuy, S. Regulation of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) gene expression by gonadotropin-releasing hormone (GnRH) and sexual steroids in the Mediterranean Sea bass. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dickey, J.T.; Swanson, P. Effects of salmon gonadotropin-releasing hormone on follicle stimulating hormone secretion and subunit gene expression in coho salmon (Oncorhynchus kisutch). Gen. Comp. Endocrinol. 2000, 118, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.L. Neuroendocrine control of reproduction in teleost fish: Concepts and controversies. Annu. Rev. Anim. Biosci. 2022, 10, 107–130. [Google Scholar] [CrossRef]
- Bayarri, M.J.; Rodrıguez, L.; Zanuy, S.; Madrid, J.A.; Sanchez-Vazquez, F.J.; Kagawa, H.; Carrillo, M. Effect of photoperiod manipulation on the daily rhythms of melatonin and reproductive hormones in caged European sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol. 2004, 136, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Fiszbein, A.; Cánepa, M.; Vázquez, G.R.; Maggese, C.; Pandolfi, M. Photoperiodic modulation of reproductive physiology and behaviour in the cichlid fish Cichlasoma dimerus. Physiol. Behav. 2010, 99, 425–432. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Li, J.; Luo, Z.; Li, Z.; Chai, M.; Liu, S. Effects of sex hormone rescue on gametogenesis in allotriploid crucian carp. Aquaculture 2022, 561, 738645. [Google Scholar] [CrossRef]
- Gabilondo, A.R.; Pérez, L.H.; Rodríguez, R.M. Hormonal and neuroendocrine control of reproductive function in teleost fish. Bionatura 2022, 6, 2122. [Google Scholar] [CrossRef]
- Taranger, G.L.; Haux, C.; Stefansson, S.O.; Björnsson, B.T.; Walther, B.T.; Hansen, T. Abrupt changes in photoperiod affect age at maturity, timing of ovulation and plasma testosterone and oestradiol-17β profiles in Atlantic salmon, Salmo salar. Aquaculture 1998, 162, 85–98. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, N.N.; Choi, Y.J.; Habibi, H.R.; Kim, J.W.; Choi, C.Y. Light-emitting diode spectral sensitivity relationship with reproductive parameters and ovarian maturation in yellowtail damselfish, Chrysiptera parasema. J. Photochem. Photobiol. B 2013, 127, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Bani, A.; Tabarsa, M.; Falahatkar, B.; Banan, A. Effects of different photoperiods on growth, stress and haematological parameters in juvenile great sturgeon Huso huso. Aquac. Res. 2009, 40, 1899–1907. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Maternal stress and fish reproduction: The role of cortisol revisited. Fish Fish. 2018, 19, 1016–1030. [Google Scholar] [CrossRef]
- Fagundes, M.; Urbinati, E.C. Stress in pintado (Pseudoplatystoma corruscans) during farming procedures. Aquaculture 2008, 276, 112–119. [Google Scholar] [CrossRef]
- Cerdá-Reverter, J.M.; Zanuy, S.; Carrillo, M.; Madrid, J.A. Time-course studies on plasma glucose, insulin, and cortisol in sea bass (Dicentrarchus labrax) held under different photoperiodic regimes. Physiol. Behav. 1998, 64, 245–250. [Google Scholar] [CrossRef]
- De Araújo, F.C.T.; Ribeiro, R.P.; Campos, E.C.; Todesco, H.; Tsujii, K.M.; Mantovani, L.S.C.; de Oliveira, C.A.L. Could serum glucose be a selection criterion in Nile tilapia breeding programs? Aquaculture 2022, 548, 737573. [Google Scholar] [CrossRef]
- Pavlidis, M.; Greenwood, L.; Paalavuo, M.; Mölsä, H.; Laitinen, J.T. The effect of photoperiod on diel rhythms in serum melatonin, cortisol, glucose, and electrolytes in the common dentex, Dentex dentex. Gen. Comp. Endocrinol. 1999, 113, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Seoka, M.; Ueno, K.; Yong, A.S.; Biswas, B.K.; Kim, Y.S.; Kumai, H. Growth performance and physiological responses in striped knifejaw, Oplegnathus fasciatus, held under different photoperiods. Aquaculture 2008, 279, 42–46. [Google Scholar] [CrossRef]
- Biswas, A.; Seoka, M.; Inagaki, H.; Takii, K. Reproduction, growth and stress response in adult red sea bream, Pagrus major (Temminck & Schlegel) exposed to different photoperiods at spawning season. Aquac. Res. 2010, 41, 519–527. [Google Scholar] [CrossRef]
- Biswas, A.K.; Maita, M.; Yoshizaki, G.; Takeuchi, T. Physiological responses in Nile tilapia exposed to different photoperiod regimes. J. Fish Biol. 2004, 65, 811–821. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Park, S.G.N.R.; Jo, A.-H.; Kim, J.-H. Physiological Effect of Extended Photoperiod and Green Wavelength on the Pituitary Hormone, Sex Hormone and Stress Response in Chub Mackerel, Scomber japonicus. Fishes 2023, 8, 77. https://doi.org/10.3390/fishes8020077
Choi YJ, Park SGNR, Jo A-H, Kim J-H. Physiological Effect of Extended Photoperiod and Green Wavelength on the Pituitary Hormone, Sex Hormone and Stress Response in Chub Mackerel, Scomber japonicus. Fishes. 2023; 8(2):77. https://doi.org/10.3390/fishes8020077
Chicago/Turabian StyleChoi, Young Jae, Seul Gi Na Ra Park, A-Hyun Jo, and Jun-Hwan Kim. 2023. "Physiological Effect of Extended Photoperiod and Green Wavelength on the Pituitary Hormone, Sex Hormone and Stress Response in Chub Mackerel, Scomber japonicus" Fishes 8, no. 2: 77. https://doi.org/10.3390/fishes8020077
APA StyleChoi, Y. J., Park, S. G. N. R., Jo, A.-H., & Kim, J.-H. (2023). Physiological Effect of Extended Photoperiod and Green Wavelength on the Pituitary Hormone, Sex Hormone and Stress Response in Chub Mackerel, Scomber japonicus. Fishes, 8(2), 77. https://doi.org/10.3390/fishes8020077