Characterization of Red Sea Bream (Pagrus major) Interferon Regulatory Factor 5 and 6 Genes and Their Expression in Response to RSIV Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Virus
2.3. Sequence and Phylogenetic Analysis of PmIRF5 and PmIRF6 Genes
2.4. Viral Challenge, Nucleic Acid Extraction, and cDNA Synthesis
2.5. Quantitative PCR Analysis
2.6. Statistical Analysis
3. Results
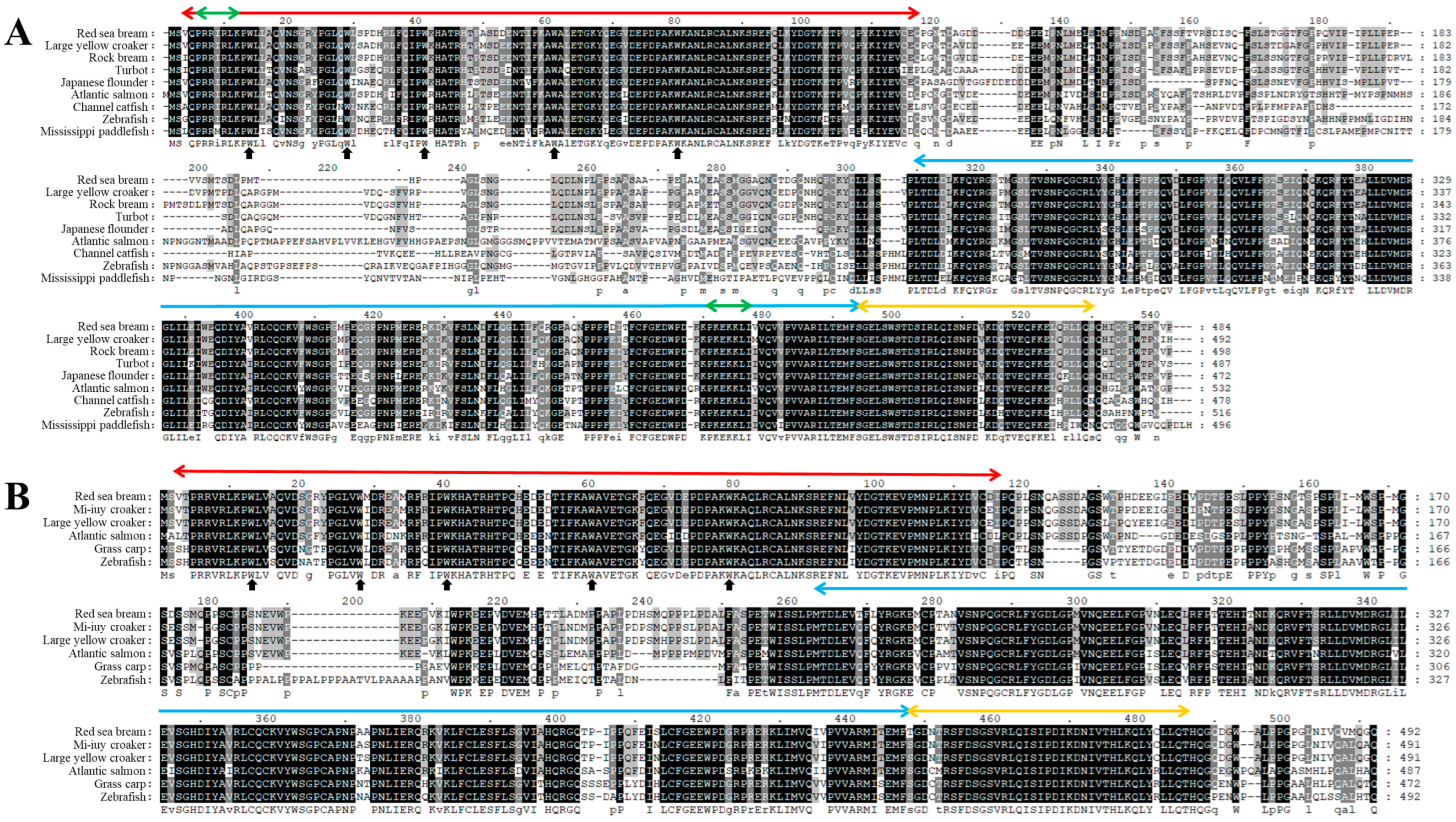
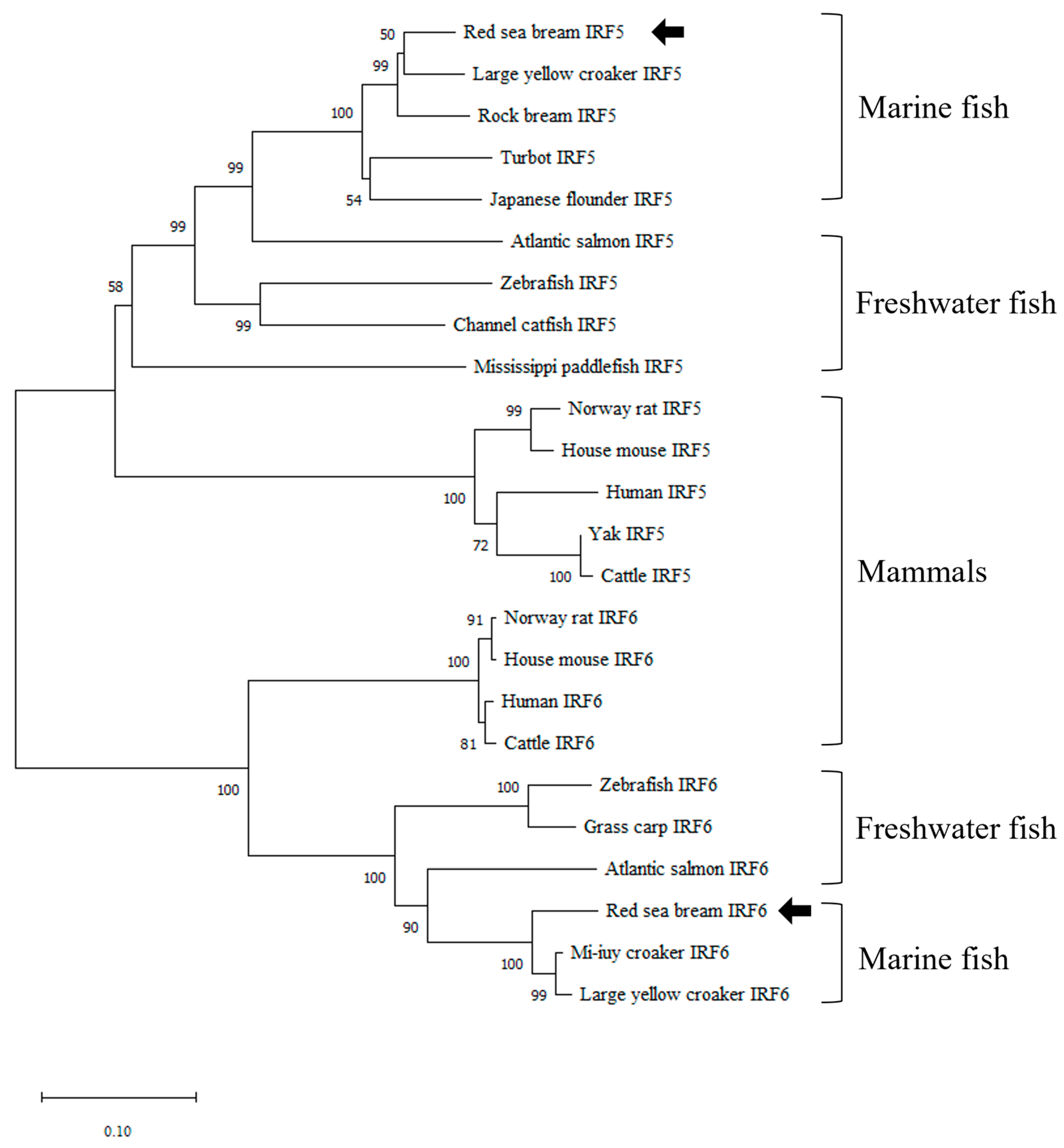
3.1. Identification and Characterization of PmIRF5 and PmIRF6 Sequence
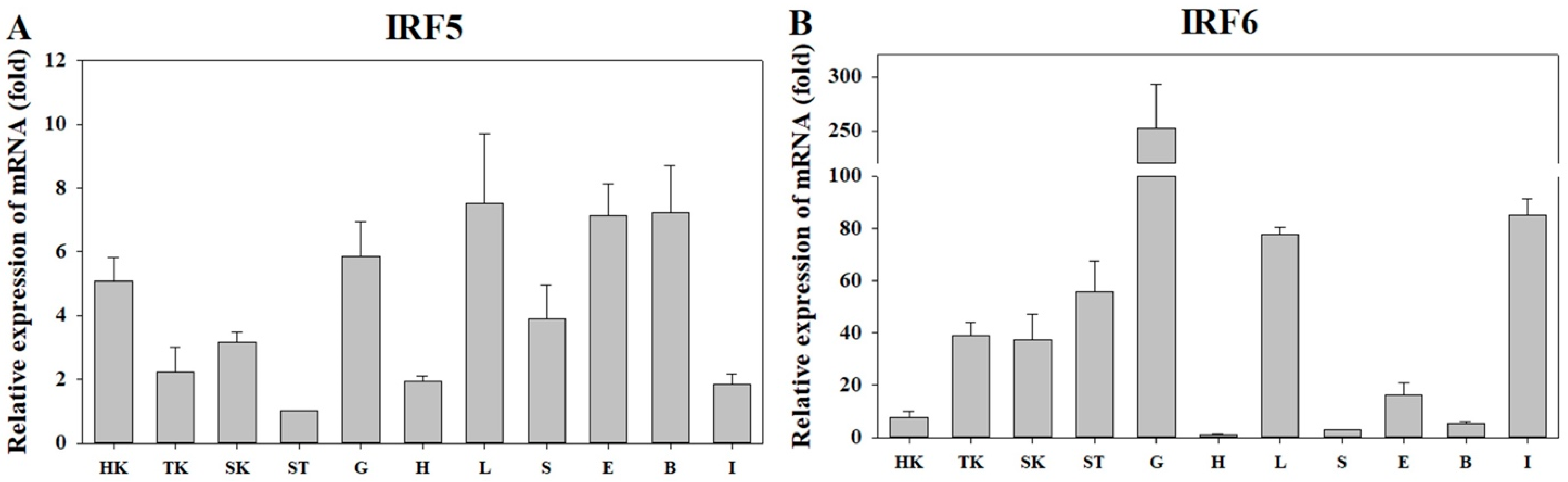
3.2. Expression of PmIRF5 and PmIRF6 mRNA in Various Organs
3.3. Expression of PmIRF5 and PmIRF6 mRNA after RSIV Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signaling by toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Kellum, M.J.; Field, A.E.; Pitha, P.M. Multiple regulatory domains of IRF-5 control activation, cellular localization, and induction of chemokines that mediate recruitment of T lymphocytes. Mol. Cell. Biol. 2002, 22, 5721–5740. [Google Scholar] [CrossRef]
- Yanai, H.; Negishi, H.; Taniguchi, T. The IRF family of transcription factors: Inception, impact and implications in oncogenesis. Oncoimmunology 2012, 1, 1376–1386. [Google Scholar] [CrossRef]
- Nehyba, J.; Hrdlicková, R.; Burnside, J.; Bose, H.R., Jr. A novel interferon regulatory factor (IRF), IRF-10, has a unique role in immune defense and is induced by the v-Rel oncoprotein. Mol. Cell. Biol. 2002, 22, 3942–3957. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.S.; Zhu, M.H.; Chen, S.; Wang, Z.X.; Liang, Y.; Huang, B.; Nie, P. Molecular cloning and expression analysis of a fish specific interferon regulatory factor, IRF11, in orange spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2017, 60, 368–379. [Google Scholar] [CrossRef]
- Huang, B.; Qi, Z.T.; Xu, Z.; Nie, P. Global characterization of interferon regulatory factor (IRF) genes in vertebrates: Glimpse of the diversification in evolution. BMC Immunol. 2010, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Yanai, H.; Savitsky, D.; Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 2008, 26, 535–584. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ogasawara, K.; Takaoka, A.; Tanaka, N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 2001, 19, 623–655. [Google Scholar] [CrossRef]
- Veals, S.A.; Schindler, C.; Leonard, D.; Fu, X.Y.; Aebersold, R.; Darnell, J.E.; Levy, D.E. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol. Cell. Biol. 1992, 12, 3315–3324. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef]
- Panne, D.; Maniatis, T.; Harrison, S.C. An atomic model of the interferon-beta enhanceosome. Cell 2007, 129, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Royer, W.E., Jr. Structural insights into interferon regulatory factor activation. Cell. Signal. 2010, 22, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Antonczyk, A.; Krist, B.; Sajek, M.; Michalska, A.; Piaszyk-Borychowska, A.; Plens-Galaska, M.; Wesoly, J.; Bluyssen, H.A.R. Direct inhibition of IRF-dependent transcriptional regulatory mechanisms associated with disease. Front. Immunol. 2019, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Chen, X.; Gong, Q.; Liu, Q.; Zhang, S.; Dong, X. Structural and expression studies of interferon regulatory factor 8 in Japanese flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2013, 35, 1016–1024. [Google Scholar] [CrossRef]
- Schoenemeyer, A.; Barnes, B.J.; Mancl, M.E.; Latz, E.; Goutagny, N.; Pitha, P.M.; Fitzgerald, K.A.; Golenbock, D.T. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 2005, 280, 17005–17012. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Li, Q.J.; Sumen, C.; Huppa, J.B.; Huse, M.; Davis, M.M. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature 2005, 434, 238–243. [Google Scholar] [CrossRef]
- Hu, G.; Mancl, M.E.; Barnes, B.J. Signaling through IFN regulatory factor-5 sensitizes p53-deficient tumors to DNA damage-induced apoptosis and cell death. Cancer Res. 2005, 65, 7403–7412. [Google Scholar] [CrossRef]
- Takaoka, A.; Yanai, H.; Kondo, S.; Duncan, G.; Negishi, H.; Mizutani, T.; Kano, S.; Honda, K.; Ohba, Y.; Mak, T.W.; et al. Integral role of IRF-5 in the gene induction programme activated by toll-like receptors. Nature 2005, 434, 243–249. [Google Scholar] [CrossRef]
- Paun, A.; Reinert, J.T.; Jiang, Z.; Medin, C.; Balkhi, M.Y.; Fitzgerald, K.A.; Pitha, P.M. Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J. Biol. Chem. 2008, 283, 14295–14308. [Google Scholar] [CrossRef]
- Ingraham, C.R.; Kinoshita, A.; Kondo, S.; Yang, B.; Sajan, S.; Trout, K.J.; Malik, M.I.; Dunnwald, M.; Goudy, S.L.; Lovett, M.; et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6). Nat. Genet. 2006, 38, 1335–1340. [Google Scholar] [CrossRef]
- Richardson, R.J.; Dixon, J.; Malhotra, S.; Hardman, M.J.; Knowles, L.; Boot-Handford, R.P.; Shore, P.; Whitmarsh, A.; Dixon, M.J. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat. Genet. 2006, 38, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.J.; Standley, J.; Compton, J.; Bale, S.; Schutte, B.C.; Murray, J.C. Comparative analysis of IRF6 variants in families with Van der Woude syndrome and popliteal pterygium syndrome using public whole-exome databases. Genet. Med. 2013, 15, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, L.F.; Wang, Z.X.; Chen, D.D.; Zhang, Y.A. Fish IRF6 is a positive regulator of IFN expression and involved in both of the MyD88 and TBK1 pathways. Fish Shellfish Immunol. 2016, 57, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ben, J.; Jabs, E.W.; Chong, S.S. Genomic, cDNA and embryonic expression analysis of zebrafish IRF6, the gene mutated in the human oral clefting disorders van der Woude and popliteal pterygium syndromes. Gene Expr. Patterns 2005, 5, 629–638. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.X.; Hu, Y.H. Molecular characterization and expression analysis of eleven interferon regulatory factors in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol. 2015, 44, 272–282. [Google Scholar] [CrossRef]
- Laghari, Z.A.; Li, L.; Chen, S.N.; Huo, H.J.; Huang, B.; Zhou, Y.; Nie, P. Composition and transcription of all interferon regulatory factors (IRFs), IRF1–11 in a perciform fish, the mandarin fish, Siniperca chuatsi. Dev. Comp. Immunol. 2018, 81, 127–140. [Google Scholar] [CrossRef]
- Inkpen, S.M.; Solbakken, M.H.; Jentoft, S.; Eslamloo, K.; Rise, M.L. Full characterization and transcript expression profiling of the interferon regulatory factor (IRF) gene family in Atlantic cod (Gadus morhua). Dev. Comp. Immunol. 2019, 98, 166–180. [Google Scholar] [CrossRef]
- Zhan, F.B.; Jakovlić, I.; Wang, W.M. Identification, characterization and expression in response to Aeromonas hydrophila challenge of five interferon regulatory factors in Megalobrama amblycephala. Fish Shellfish Immunol. 2019, 86, 204–212. [Google Scholar] [CrossRef]
- Inouye, K.; Yamano, K.; Maeno, Y.; Nakajima, K.; Matsuoka, M.; Wada, Y.; Sorimachi, M. Iridovirus infection of cultured red sea bream, Pagrus major. Fish Pathol. 1992, 27, 19–27. [Google Scholar] [CrossRef]
- WOAH (World Organisation for Animal Health). Red Sea bream iridoviral disease. In Manual of Diagnostic Tests for Aquatic Animals; WOAH: Paris, France, 2012; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/2.3.07_RSIVD.pdf (accessed on 29 March 2022).
- Sohn, S.G.; Choi, D.L.; Do, J.W.; Hwang, J.Y.; Park, J.W. Mass mortalities of cultured striped beakperch, Oplegnathus fasciatus by iridoviral infection. Fish Pathol. 2000, 13, 121–127. [Google Scholar]
- Kim, K.H.; Choi, K.M.; Kang, G.; Woo, W.S.; Sohn, M.Y.; Son, H.J.; Yun, D.; Kim, D.H.; Park, C.I. Development and validation of a quantitative polymerase chain reaction assay for the detection of red sea bream Iridovirus. Fishes 2022, 7, 236. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, K.M.; Joo, M.S.; Kang, G.; Woo, W.S.; Sohn, M.Y.; Son, H.J.; Kwon, M.G.; Kim, J.O.; Kim, D.H.; et al. Red sea bream Iridovirus (RSIV) kinetics in rock bream (Oplegnathus fasciatus) at various fish-rearing seawater temperatures. Animals 2022, 12, 1978. [Google Scholar] [CrossRef]
- Kim, K.I.; Hwang, S.D.; Cho, M.Y.; Jung, S.H.; Kim, Y.C.; Jeong, H.D. A natural infection by the red sea bream Iridovirus-type Megalocytivirus in the golden mandarin fish Siniperca scherzeri. J. Fish Dis. 2018, 41, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.J.; Yoon, M.J.; Jin, J.W.; Kim, K.I.; Kim, Y.C.; Hong, S.; Jeong, H.D. Development and characterization of megalocytivirus persistently-infected cell cultures for high yield of virus. Tissue and Cell 2020, 66, 101387. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Au, W.C.; Moore, P.A.; Lowther, W.; Juang, Y.T.; Pitha, P.M. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. USA 1995, 92, 11657–11661. [Google Scholar] [CrossRef] [PubMed]
- Escalante, C.R.; Nistal-Villán, E.; Shen, L.; García-Sastre, A.; Aggarwal, A.K. Structure of IRF-3 bound to the PRDIII-I regulatory element of the human interferon-beta enhancer. Mol. Cell 2007, 26, 703–716. [Google Scholar] [CrossRef]
- Xiang, Z.; Dong, C.; Qi, L.; Chen, W.; Huang, L.; Li, Z.; Xia, Q.; Liu, D.; Huang, M.; Weng, S.; et al. Characteristics of the interferon regulatory factor pairs zfIRF5/7 and their stimulation expression by ISKNV Infection in zebrafish (Danio rerio). Dev. Comp. Immunol. 2010, 34, 1263–1273. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Chang, M.X.; Xiao, F.S.; Huang, B.; Nie, P. The gene and virus-induced expression of IRF-5 in grass carp Ctenopharyngodon idella. Vet. Immunol. Immunopathol. 2010, 134, 269–278. [Google Scholar] [CrossRef]
- Xia, J.; Hu, G.B.; Dong, X.Z.; Liu, Q.M.; Zhang, S.C. Molecular characterization and expression analysis of interferon regulatory factor 5 (IRF-5) in turbot, Scophthalmus maximus. Fish Shellfish Immunol. 2012, 32, 211–218. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, R.; Zhang, J.; Chen, Y.; Shan, S.; Zhu, Y.; Yang, G.; Li, H. Negative regulation of interferon regulatory factor 6 (IRF6) in interferon and NF-κB signalling pathways of common carp (Cyprinus carpio L.). BMC Vet. Res. 2022, 18, 433. [Google Scholar] [CrossRef] [PubMed]
- Meraro, D.; Hashmueli, S.; Koren, B.; Azriel, A.; Oumard, A.; Kirchhoff, S.; Hauser, H.; Nagulapalli, S.; Atchison, M.L.; Levi, B.Z. Protein-protein and DNA-protein interactions affect the activity of lymphoid-specific IFN regulatory factors. J. Immunol. 1999, 163, 6468–6478. [Google Scholar] [CrossRef]
- Yoneyama, M.; Suhara, W.; Fukuhara, Y.; Fukuda, M.; Nishida, E.; Fujita, T. Direct triggering of the type I interferon system by virus infection: Activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998, 17, 1087–1095. [Google Scholar] [CrossRef]
- Yang, H.; Ma, G.; Lin, C.H.; Orr, M.; Wathelet, M.G. Mechanism for transcriptional synergy between interferon regulatory factor (IRF)-3 and IRF-7 in activation of the interferon-beta gene promoter. Eur. J. Biochem. 2004, 271, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Marié, I.; Smith, E.; Prakash, A.; Levy, D.E. Phosphorylation-induced dimerization of interferon regulatory factor 7 unmasks DNA binding and a bipartite transactivation domain. Mol. Cell. Biol. 2000, 20, 8803–8814. [Google Scholar] [CrossRef]
- Holland, J.W.; Bird, S.; Williamson, B.; Woudstra, C.; Mustafa, A.; Wang, T.; Zou, J.; Blaney, S.C.; Collet, B.; Secombes, C.J. Molecular characterization of IRF3 and IRF7 in rainbow trout, Oncorhynchus mykiss: Functional analysis and transcriptional modulation. Mol. Immunol. 2008, 46, 269–285. [Google Scholar] [CrossRef]
- Lin, R.; Mamane, Y.; Hiscott, J. Multiple regulatory domains control IRF-7 activity in response to virus infection. J. Biol. Chem. 2000, 275, 34320–34327. [Google Scholar] [CrossRef] [PubMed]
- Wickramaarachchi, W.D.; Wan, Q.; Lim, B.S.; Jung, H.B.; De Zoysa, M.; Park, M.A.; Lee, J.; Whang, I. Genomic characterization of interferon regulatory factor 5 from rock bream (Oplegnathus fasciatus) and its role in antiviral defense. Fish Shellfish Immunol. 2014, 37, 256–267. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, C.; Shan, S.; Zhang, F.; Li, H.; An, L.; Yang, G. Characterization of common carp (Cyprinus carpio L.) interferon regulatory factor 5 (IRF5) and its expression in response to viral and bacterial challenges. BMC Vet. Res. 2016, 12, 127. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, X.; Luo, T.; Ao, J.; Ai, C.; Chen, X. Molecular characterization of the interferon regulatory factor (IRF) family and functional analysis of IRF11 in the large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2020, 107, 218–229. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, Mucosal Immunity and Vaccination in Teleost Fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.B.; Lou, H.M.; Dong, X.Z.; Liu, Q.M.; Zhang, S.C. Characteristics of the interferon regulatory factor 5 (IRF5) and its expression in response to LCDV and poly I:C challenges in Japanese flounder, Paralichthys olivaceus. Dev. Comp. Immunol. 2012, 38, 377–382. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Sequence of Primer (5′−3′) | Usage |
|---|---|---|
| PmIRF5-F1 | ATGAGCGTGCAGCCTCGGA | Amplification for reaffirmation of full-length CDS |
| PmIRF5-R1 | TGTCCCTCCGGTGGACAGAC | |
| PmIRF5-F2 | AGGGACATTTGGACCTCCTC | |
| PmIRF5-R2 | TGTAGAACCGCTGCTTCTGG | |
| PmIRF5-F3 | GCGGTTCTACACTGAGGCCC | |
| PmIRF5-R3 | TCAGGGGACATTGGGGGTCC | |
| PmIRF6-F1 | ATGTCAGTCACCCCTCGGC | |
| PmIRF6-R1 | CTCTTTGGGCCAGACCTCGT | |
| PmIRF6-F2 | TCAGACTCCTCCATGCAGCC | |
| PmIRF6-R2 | TGGGCTATCACCCCACTGAG | |
| PmIRF6-F3 | TTTTCTCAGTGGGGTGATAG | |
| PmIRF6-R3 | TCACTGTCCCTGCATAAC | |
| qPCR-PmIRF5-F | ACCTGTTTGGACCTGTCACC | RT-qPCR amplification |
| qPCR-PmIRF5-R | AGCAGGGCCTCAGTGTAGAA | |
| qPCR-PmIRF6-F | CTCTGCCAGTGCAAGGTGTA | |
| qPCR-PmIRF6-R | GGCTATCACCCCACTGAGAA | |
| qPCR-PmEF-1α-F | ACGTGTCCGTCAAGGAAATC | |
| qPCR-PmEF-1α-R | TGATGACCTGAGCGTTGAAG | |
| qPCR-RSIV-Meg 1041-F | CCACCAGATGGGAGTAGAC | RSIV copy number determination [32] |
| qPCR-RSIV-Meg 1139-R | GGTTGATATTGCCCATGTCCA | |
| qPCR-RSIV-Meg 1079-P | [FAM]CCTACTA[i-EBQ]CTTTGCGCCCAGCATG[phosphate] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Joo, M.-S.; Kang, G.; Woo, W.-S.; Sohn, M.-Y.; Son, H.-J.; Park, C.-I. Characterization of Red Sea Bream (Pagrus major) Interferon Regulatory Factor 5 and 6 Genes and Their Expression in Response to RSIV Infection. Fishes 2023, 8, 114. https://doi.org/10.3390/fishes8020114
Kim K-H, Joo M-S, Kang G, Woo W-S, Sohn M-Y, Son H-J, Park C-I. Characterization of Red Sea Bream (Pagrus major) Interferon Regulatory Factor 5 and 6 Genes and Their Expression in Response to RSIV Infection. Fishes. 2023; 8(2):114. https://doi.org/10.3390/fishes8020114
Chicago/Turabian StyleKim, Kyung-Ho, Min-Soo Joo, Gyoungsik Kang, Won-Sik Woo, Min-Young Sohn, Ha-Jeong Son, and Chan-Il Park. 2023. "Characterization of Red Sea Bream (Pagrus major) Interferon Regulatory Factor 5 and 6 Genes and Their Expression in Response to RSIV Infection" Fishes 8, no. 2: 114. https://doi.org/10.3390/fishes8020114
APA StyleKim, K.-H., Joo, M.-S., Kang, G., Woo, W.-S., Sohn, M.-Y., Son, H.-J., & Park, C.-I. (2023). Characterization of Red Sea Bream (Pagrus major) Interferon Regulatory Factor 5 and 6 Genes and Their Expression in Response to RSIV Infection. Fishes, 8(2), 114. https://doi.org/10.3390/fishes8020114






