Eco-Dynamic Analysis of the Community Structure of Nekton in the Northern South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Fishery Data
2.2. Study Area
2.3. Data Analysis
2.3.1. Biomass of the Nekton (D)
2.3.2. EX and EXsp
2.3.3. PC and PL
2.3.4. The Trophic Level of Fish
3. Results
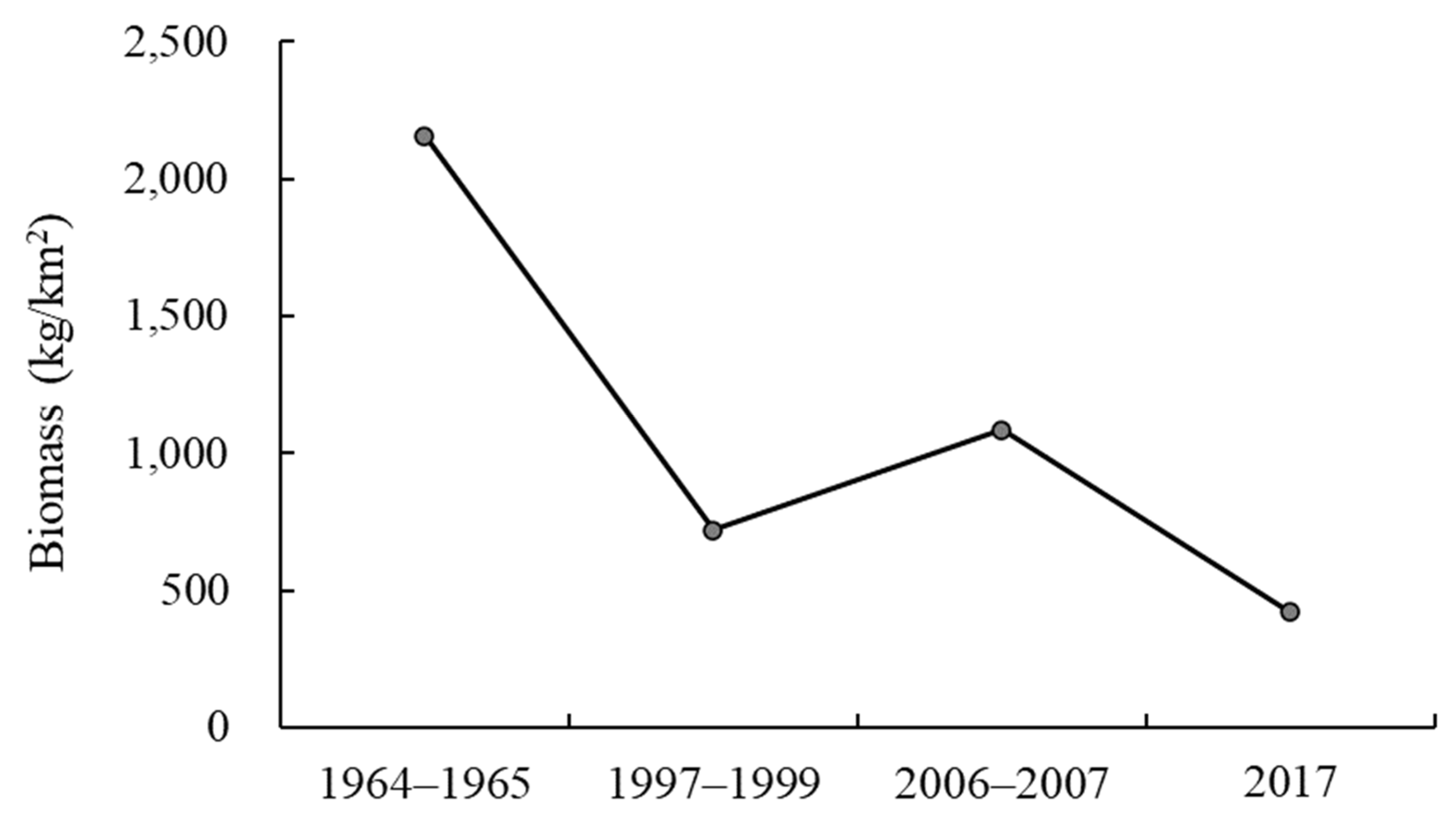
3.1. Fishery Resource Biomass
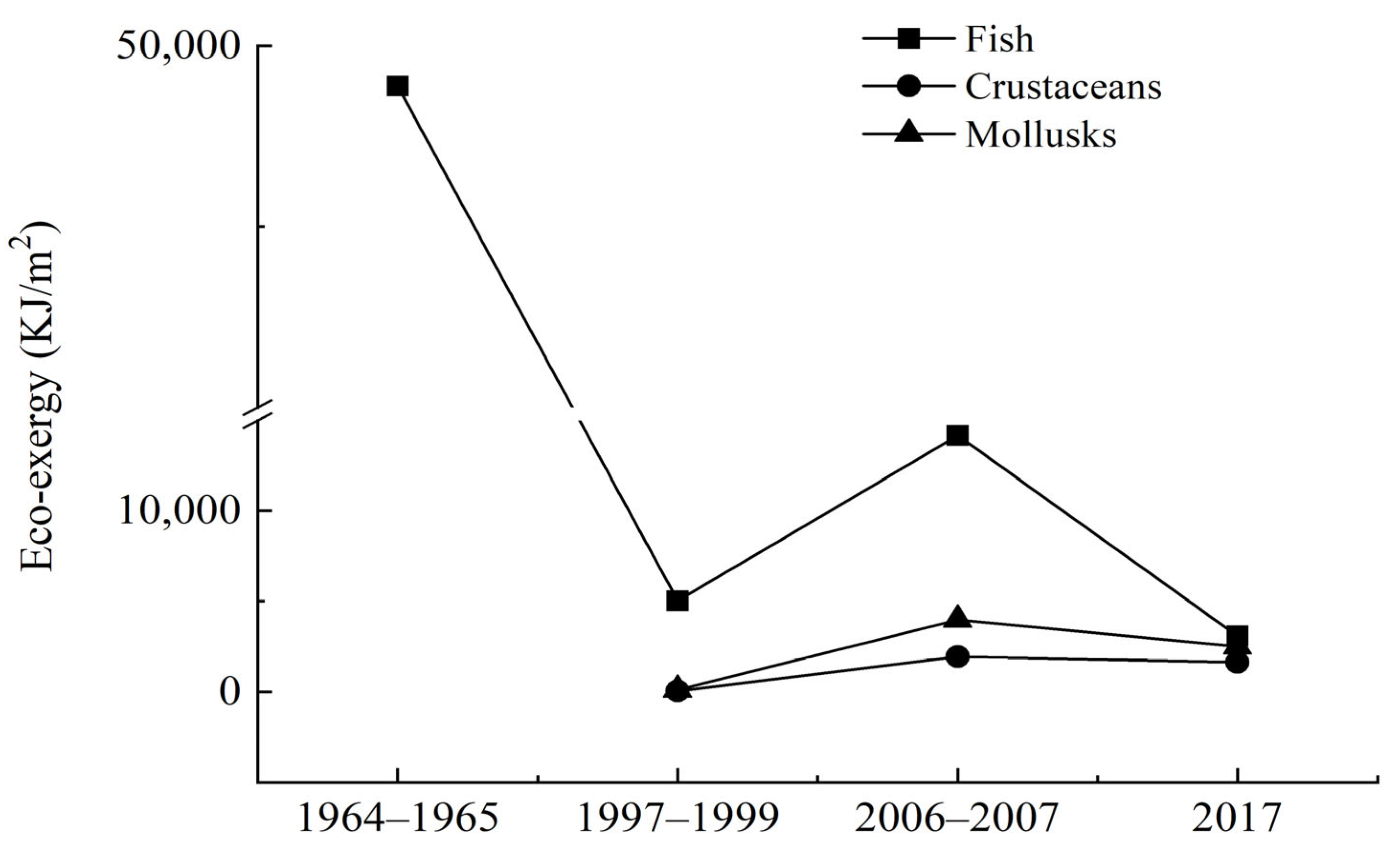
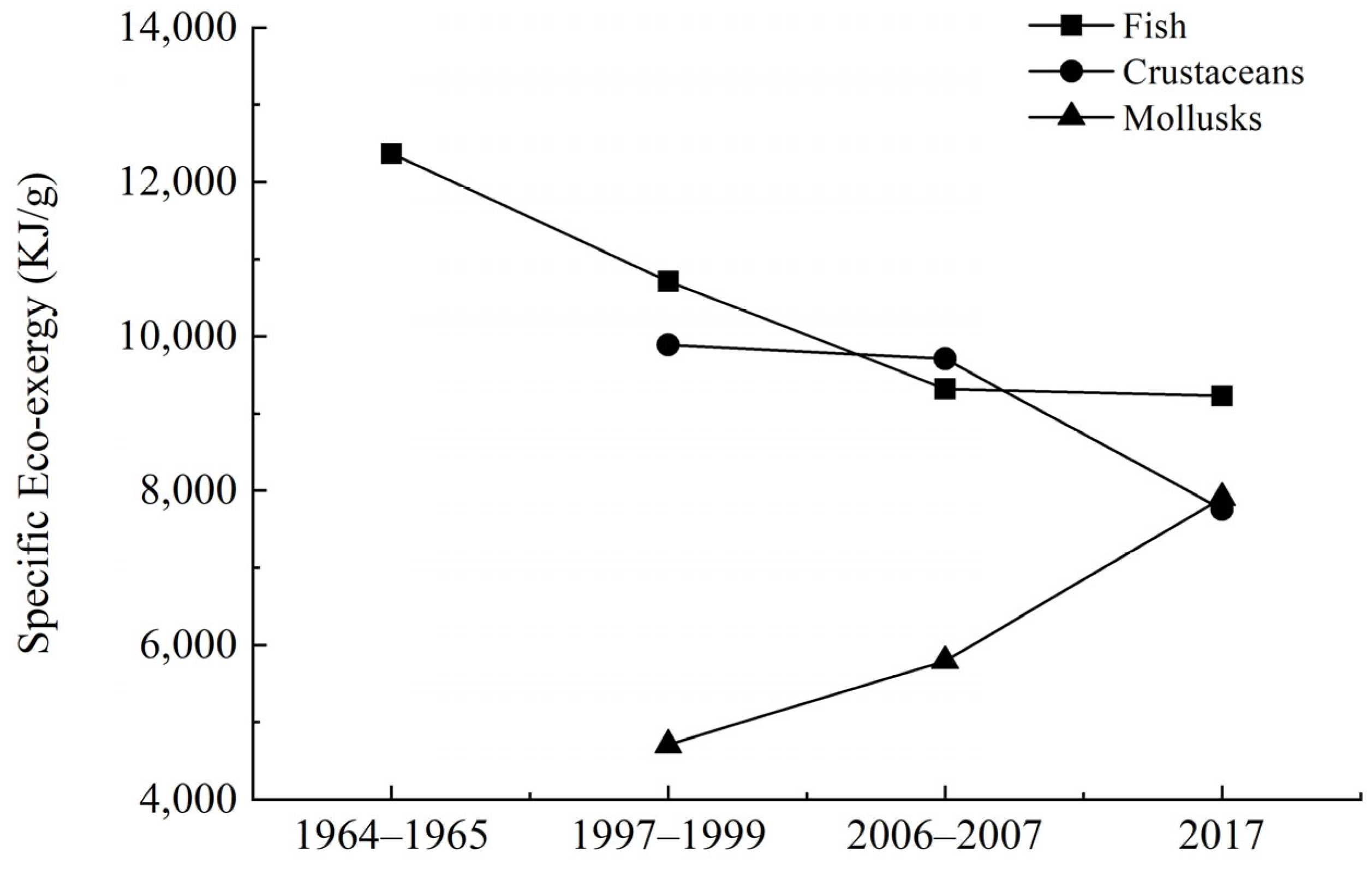
3.2. EX and EXsp
3.2.1. Dynamics of Fish Population Structure
3.2.2. Dynamics of Crustacean Population Structure
3.2.3. Dynamics of Cephalopod Population Structure
3.3. PC and PL
3.3.1. Changes in the Statuses of Species in the Nekton Community
3.3.2. Changes in the Statuses of Species in the Fish Population
3.3.3. Changes in the Statuses of Species in the Crustacean Population
3.3.4. Changes in the Statuses of Species in the Cephalopod Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, P.F.; Cui, X.Y.; Luo, H.H.; Peng, W.Q.; Gao, Y.X. Trophic structure and energy flow of the lianshi lake ecosystem. Trans. Oceanol. Limnol. 2021, 6, 124–132. [Google Scholar] [CrossRef]
- Pykh, Y.A.; Kennedy, E.T.; Grant, W.E. An overview of systems analysis methods in delineating environmental quality indices. Ecol. Model. 2000, 130, 25–38. [Google Scholar] [CrossRef]
- Molozzi, J.; Salas, F.; Callisto, M.; Marques, J.C. Thermodynamic oriented ecological indicators: Application of Eco-Exergy and Specific Eco-Exergy in capturing environmental changes between disturbed and non-disturbed tropical reservoirs. Ecol. Indic. 2013, 24, 543–551. [Google Scholar] [CrossRef]
- Fu, X.; Wu, G.; Liu, Y. Analytical theories of exergy and emergy for ecological research. Acta Ecol. Sin. 2004, 11, 2621–2626. [Google Scholar] [CrossRef]
- Xu, F.L.; Zhao, Z.Y.; Zhan, W.; Zao, S.S.; Dawson, R.W.; Tao, S. An ecosystem health index methodology (EHIM) for lake ecosystem health assessment. Ecol. Model. 2005, 188, 327–339. [Google Scholar] [CrossRef]
- Xu, F.; Yang, Z.F.; Chen, B.; Zhao, Y.W. Ecosystem Health Assessment of Baiyangdian Lake Based on Thermodynamic Indicators. Procedia Environ.Sci. 2012, 13, 2402–2413. [Google Scholar] [CrossRef][Green Version]
- Xu, F.L.; Yang, Z.F.; Chen, B.; Zhao, Y.W. Ecosystem health assessment of the plant-dominated Baiyangdian Lake based on eco-exergy. Ecol. Model. 2011, 222, 201–209. [Google Scholar] [CrossRef]
- Zhan, Q.P.; Dong, J.Y.; Sun, X.; Zhang, Y.Y.; Zhang, X.M. Impacts of artificial reef on community structure and functional traits of macrobenthos near Furong Island, Shandong, China. J. Chin. J. Appl. Ecol. 2023, 34, 796–804. [Google Scholar] [CrossRef]
- Cardoso, A.P.; Matos, M.R.; Rosa, R.S.; Alvarado, F.; Medeiros, A.P.; Santos, B.A. Increased fish diversity over day and night in structurally complex habitats of artificial reefs. J. Exp. Mar. Biol. Ecol. 2020, 522, 151244. [Google Scholar] [CrossRef]
- Chen, X.J.; Li, Y.S. Review of application of individual-based model to fishery ecosystem. J. Fish. China 2012, 4, 629–640. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2014. [Google Scholar]
- Baum, J.K.; Myers, R.A.; Kehler, D.C.; Worm, B.; Harley, S.J.; Doherty, P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science 2003, 299, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Mullon, C.; Fréon, P.; Cury, P. The dynamics of collapse in world fisheries. J. Fish Fish. 2005, 6, 111–120. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Jensen, O.P.; Ricard, D.; Palumbi, S.R. Unexpected patterns of fisheries collapse in the world’s oceans. Proc. Natl. Acad. Sci. USA 2011, 20, 8317–8322. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.A.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 423, 280–283. [Google Scholar] [CrossRef] [PubMed]
- He, J. Enhancing Chinese law and practice to combat illegal, unreported and unregulated fishing and trade. Asia Pac. J. Environ. Law 2016, 1, 4–28. [Google Scholar] [CrossRef]
- Ju, H.L. Studies on the Decrement of Fishery Resource in the South China Sea. South. Asian Stud. 2012, 6, 51–55. [Google Scholar] [CrossRef]
- Wu, Z.C. Study on sustainable utilization of fishery resources in South China Sea. J. Agric. Technol. 2020, 23, 137–139. [Google Scholar] [CrossRef]
- Su, L.; Chen, Z.Z.; Zhang, K.; Xu, Y.W.; Qiu, Y.S. Establishment of Quality Status Evaluation System of Fishery Resources in Beibu Gulf Based on Bottom Trawl Survey Data. J. Guangdong Ocean Univ. 2021, 1, 10–16. [Google Scholar] [CrossRef]
- Zhang, K.; Liao, B.C.; Xu, Y.W.; Zhang, J.; Sun, M.S.; Qiu, Y.S.; Chen, Z.Z. Assessment for allowable catch of fishery resources in the South China Sea based on statistical data. J. Haiyang Xuebao 2017, 8, 25–33. [Google Scholar] [CrossRef]
- Zhu, X.G.; Fang, Y.Y.; Yan, L.J.; Zhang, G.; Huang, L. The Ecological Strategy Evolution of Marine Fishes under High Intensity Fishing Environment. Bull. Sci. Technol. 2009, 1, 51–55. [Google Scholar]
- Zhang, K.; Chen, Z.Z.; Yan, L.J.; Qiu, Y.S. Decadal changes in growth, mortality and maturity parameters of Evynnis cardinalis in Beibu Gulf. J. South China Fish. Sci. 2016, 6, 9–16. [Google Scholar] [CrossRef]
- Wang, S.S. Construction of the Regional Cooperation Mechanism for the Conservation of Fishery Resources in the South China Sea. J. Hainan Trop. Ocean Univ. 2022, 4, 13–22. [Google Scholar] [CrossRef]
- Zhu, M.H.; Qian, W.H. Reasons for the Decline of Fishery Resources in Zhoushan Fishery Ground and Countermeasures for Its Restoration. J. Rural Econ. Sci.-Technol. 2022, 9, 79–82. [Google Scholar] [CrossRef]
- Arena, P.T.; Jordan, L.K.B.; Spieler, R.E. Fish assemblages on sunken vessels and natural reefs in southeast Florida, USA. Hydrobiologia 2007, 8, 157–171. [Google Scholar] [CrossRef]
- Abelson, A.; Obolski, U.; Regoniel, P.; Hadany, L. Restocking Herbivorous Fish Populations As a Social-Ecological Restoration Tool in Coral Reefs. Front. Mar. Sci. 2016, 3, 138. [Google Scholar] [CrossRef]
- Becker, A.; Taylor, M.D.; Folpp, H.; Lowry, M.B. Managing the development of artificial reef systems: The need for quantitative goals. Fish Fish. 2018, 19, 740–752. [Google Scholar] [CrossRef]
- Lemoine, H.R.; Paxton, A.B.; Anisfeld, S.C.; Rosemond, R.C.; Peterson, C.H. Selecting the optimal artificial reefs to achieve fish habitat enhancement goals. Biol. Conserv. 2019, 238, 108200. [Google Scholar] [CrossRef]
- Wu, K. Analysis on the current situation, existing problems and prospects of marine ecological restoration. J. Technol. Wind 2020, 3, 131. [Google Scholar] [CrossRef]
- Wang, H.L.; Dong, Z.Z. Research on the Dynamics Mechanism of System of Marine Eco-economy. J. Ocean Technol. 2009, 1, 50–54. [Google Scholar] [CrossRef]
- Yan, L.; Tan, Y.G.; Yang, L.; Lian, X.P.; Yang, B.Z.; Zhang, P.; Chen, S.; Li, J. Catch composition and diversity of gillnet fishery in the Pearl River Estuary coastal waters of the South China Sea in autumn. J. South China Fish. Sci. 2016, 1, 111–119. [Google Scholar] [CrossRef]
- Ji, Y.L.; Zhao, N.; Wang, Z.Z.; Ji, X.X.; Yang, C.P.; Yu, Z.S. Macrobenthic community structure of intertidal zone of Rushan Bay in spring. J. Chin. J. Appl. Ecol. 2015, 2, 609–615. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.F.; Chen, J.H. A study on seasonal changes of fish communities in the East China Sea and Huanghai Sea. Acta Oceanol. Sin. 2006, 4, 108–114. [Google Scholar] [CrossRef]
- Tang, G.L.; Liu, Y.; Wu, P.; Sun, D.R.; Xiao, Y.Y.; Wang, T.; Xie, Y.F.; Li, C.R.; Shi, J.; Zhong, Z.H.; et al. Community structure of fishery resources and its relationship to environmental factors in the Wanshan Islands Sea of the Pearl River Estuary in spring. J. Fish. Sci. China 2022, 8, 1198–1209. [Google Scholar]
- Li, Y.; Li, B.; Li, T. Distribution pattern and influence factors of benthic foraminifera in the surface sediments of northern South China Sea. J. Mar. Geol. Front. 2022, 38, 30–36. [Google Scholar] [CrossRef]
- Liu, G.H.; Li, J.; Chen, D.H.; Liu, J. Geochemistry of Surface Sediment in the Taixinan (Southwestern Taiwan) Sea Area in the Northeastern South China Sea. Mar. Geol. Quat. Geol. 2006, 5, 61–67. [Google Scholar] [CrossRef]
- Yang, H.Q.; Lin, Z.H.; Zhang, F.Y.; Lin, X.T.; Ji, F.W. The Distribution Characteristics of Heavy Minerals in the East of South China Sea and Their Controlling Factors. J. Ocean Univ. Qindao. 2002, 6, 956–964. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, B.; Yan, W.; Chen, M.H.; Yang, H.N.; Gu, S.C.; Li, Y. Distribution, chemical characteristics and source area of volcanic glass in the South China Sea. Acta Oceanol. Sin. 2005, 5, 73–81. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Liu, J.G.; Chen, H.; Yan, W.; Zhong, Y. Distribution of surface sediment types and sedimentary environment divisions in the northern South China Sea. J. Tropi. Oceano. 2014, 33, 54–61. [Google Scholar] [CrossRef]
- Li, J.D.; Zhao, Q.; Liu, Y.H.; Zhang, P.D. Study on the characteristics of fishery biological community structure and factors influencing biomass increments in different artificial reefs in the Yellow Sea and Bohai Sea. J. Fish. Sci. China 2023, 3, 371–383. [Google Scholar] [CrossRef]
- Wang, Y.B. Advances in Ecosystem Health Assessment in Baiyangdian Wetland. Environ. Sci. Manag. 2015, 8, 165–168. [Google Scholar] [CrossRef]
- Xu, F.L.; Dawson, R.W.; Tao, S.; Cao, J.; Li, B. A method for lake ecosystem health assessment: An ecological modelling method (EMM) and its application. Hydrobiologia 2001, 443, 159–175. [Google Scholar] [CrossRef]
- Jørgensen, S.E. Description of aquatic ecosystem’s development by eco-exergy and exergy destruction. Ecol. Model. 2007, 204, 22–28. [Google Scholar] [CrossRef]
- Cabral, D.C.; Zrzavá, M.; Kubíc, S.; Rendón, P.; Marec, F. The Role of Satellite DNAs in Genome Architecture and Sex Chromosome Evolution in Crambidae Moths. Front. Genet. 2021, 12, 661417. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.C.; Marques, J.C.; Paiva, A.A.; Freitas, A.M.; Madeira, V.M.C.; Rgensen, S.E. Nuclean DNA in the determination of weighing factors to estim ate exergy from organisms biomass. Ecol. Model. 2000, 126, 179–189. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.S.; Lu, H.F. Eco-exergy analysis of structural dynamics of Schima wallichii plantation in Heshan. Ecol. Environ. Sci. 2012, 11, 1822–1829. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Dong, J.Y.; Sun, X.; Zhan, Q.P.; Wang, L.L.; Zhang, X.M. Trophic Structure of Fishery Assemblage in Surrounding Waters of Lingshan Island Based on Stable Isotope Analysis. J. Period. Ocean Univ. China 2022, 8, 40–51. [Google Scholar] [CrossRef]
- Zhang, X.C.; Ma, C.; Gu, S.; Yang, J.; Song, B.Y.; He, X.D.; Gao, Y.B. Complexity of Ecosystem Dynamics. J. Acta Sci. Nat. Univ. Nankaiensis 2009, 2, 99–104. [Google Scholar] [CrossRef]
- Shu, J.L.; Tang, Q.S. A new Direction for China’s Research on Marine Ecosystem-International Trend and National Needs. Adv. Earth Sci. 2005, 2, 139–143. [Google Scholar] [CrossRef]
- Zhang, J.J.; Gurkan, Z.; Jorgensen, S.E. Application of eco-exergy for assessment of ecosystem health and development of structurally dynamic models. Ecol. Model. 2010, 221, 693–702. [Google Scholar] [CrossRef]
- Linares, M.S.; Callisto, M.; Marques, J.C. Thermodynamic based indicators illustrate how a run-of-river impoundment in neotropical savanna attracts invasive species and alters the benthic macroinvertebrate assemblages’ complexity. Ecol. Indic. 2018, 88, 693–702. [Google Scholar] [CrossRef]
- Sun, J.F.; Yuan, X.Z.; Liu, H.; Liu, G.D. Emergy and eco-exergy evaluation of wetland reconstruction based on ecological engineering approaches in the three Gorges Reservoir, China. Ecol. Indic. 2021, 122, 107278. [Google Scholar] [CrossRef]
- Xu, F.L. Exergy and structural exergy as ecological indicators for the development state of the Lake Chaohu ecosystem. Ecol. Model. 1997, 99, 41–49. [Google Scholar] [CrossRef]
- Zhang, F.J.; Tong, F.C.; Xie, Z.F.; Lu, J.J. Application of Exergy as an indicator in the restoration of benthic fauna communities. Acta Ecol. Sin. 2007, 27, 1910–1916. [Google Scholar] [CrossRef]
- Jørgensen, S.E.; Nielsen, S.N. Application of exergy as thermodynamic indicators in ecology. Energy 2007, 32, 673–685. [Google Scholar] [CrossRef]
- Lu, H.F.; Wang, Z.H.; Campbell, D.E.; Ren, H.; Wang, J. Emergy and eco-exergy evaluation of four forest restoration modes in southeast China. Ecol. Eng. 2011, 37, 277–285. [Google Scholar] [CrossRef]
- Jørgensen, S.E.; Odum, H.T.; Brown, M.T. Emergy and exergy stored in genetic information. Ecol. Model. 2004, 178, 11–16. [Google Scholar] [CrossRef]
- Ludovisi, A. Use of thermodynamic indices as ecological indicators of the development state of lake ecosystems: Specific dissipation. Ecol. Indic. 2006, 6, 30–42. [Google Scholar] [CrossRef]
- Jørgensen, S.E. The application of ecological indicators to assess the ecological condition of a lake. Lakes Reserv. Res. Manag. 1995, 1, 177–182. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.B.; Ma, S.W.; Huang, Y.B.; Gao, L.P.; Du, G.Y.; Wu, Q.E. Analysis of the index weights of the order evaluation system during the summer fishing moratorium in the South China Sea. J. Shanghai Ocean Univ. 2022, 31, 491–501. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, H.L.; Xu, F. Overview of artificial reef construction. J. Agric. Technol. Serv. 2017, 34, 149–151. [Google Scholar] [CrossRef]
- Ding, D.W.; Suo, A.N. Theoretical thinking of artificial ecosystem for modern marine ranching. Bull. Chin. Acad. Sci. 2022, 9, 1335–1346. [Google Scholar] [CrossRef]
- Wang, W.J. Evaluation of Effect of Five Artificial Reefs Area in Coastal Area of Guangdong. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2018. [Google Scholar]
- Wang, H.; Chen, P.M.; Zhang, S.Y.; Jia, X.P. Effect on fishery resources multiplication of artificial reefs. J. Guangdong Agric. Sci. 2009, 8, 18–21. [Google Scholar] [CrossRef]
- Lu, L.Y.; Zeng, J.W.; Lin, K.; Zhang, J.; Wang, X.F. Environmental Influences on Seasonal Variations of Fish Community Structure in Off-sea of Eastern Hainan, Northern South China Sea. J. Guangdong Ocean Univ. 2021, 3, 28–35. [Google Scholar]
- Su, L.; Xu, Y.W.; Zhang, K.; Chen, Z.Z. Development trend of trawl fishery and its impact on fishery resources in South China Sea. J. South China Fish. Sci. 2023, 4, 41–48. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Qiu, Y.S. Assessment of the food-web structure, energy flows, and system attribute of northern South China Sea ecosystem. Acta Ecol. Sin. 2010, 30, 4855–4865. [Google Scholar]
- Liu, W.Y.; Huang, J.Q.; Dai, G.X.; Liu, G.Q.; Lei, X.T.; Zhou, Y.D.; Ye, Y.C. RDA analysis between fish community structure and environmental factors in the Lingdingyang Estuary. J. Fish. Inf. Strategy 2023, 1, 32–41. [Google Scholar] [CrossRef]
- Ji, W.W.; Li, S.F.; Chen, X.Z. Application of fish trophic level in marine ecosystem. J. Fish. Sci. China 2010, 4, 878–887. [Google Scholar]
- Wang, Y.Z.; Yuan, W.W. Changes of demersal trawl fishery resources in northern South China Sea as revealed by demersal trawling. J. South China Fish. Sci. 2008, 4, 26–33. [Google Scholar] [CrossRef]
- Wei, P.; Wang, X.H.; Ma, S.W.; Zhou, Y.B.; Huang, Y.B.; Su, Y.J.; Wu, Q.E. Analysis of current status of marine fishing in South China Sea. J. Shanghai Ocean Univ. 2019, 28, 976–982. [Google Scholar] [CrossRef]
- Yan, L.; Tan, Y.G.; Yang, B.Z.; Zhang, P.; Li, J.; Yang, L. Comparison on resources community of stow-net fishery before and after fishing off season in Huangmaohai Estuary. J. South China Fish. Sci. 2016, 12, 1–8. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, H.Q.; Xu, H.G.; Francisco, A.; Manuel, J.; Pablo, D.M.; William, J. Trophic controls of jellyfish blooms and links with fisheries in the East China Sea. Ecol. Model. 2008, 212, 492–503. [Google Scholar] [CrossRef]
- Jin, X.S.; Shan, X.J.; Li, X.S.; Wang, J.; Cui, Y.; Zuo, T. Long-term changes in the fishery ecosystem structure of Laizhou Bay, China. Sci. China (Earth Sci.) 2013, 3, 366–374. [Google Scholar] [CrossRef]
- Tang, Q.S.; Su, J.L. Study on marine ecosystem dynamics and living resources sustainable utilization. Adv. Earth Sci. 2001, 16, 5–11. [Google Scholar] [CrossRef]
- Shi, M.J.; Cheng, Y.Y.; Zhang, W.T.; Xia, X.Q. The evolutionary mechanism of genome size. Chin. Sci. Bull. 2016, 61, 3188–3195. [Google Scholar] [CrossRef]
- Williams-Grove, L.J.; Szedlmayer, S.T. Depth preferences and three-dimensional movements of red snapper, Lutjanus campechanus, on an artificial reef in the northern Gulf of Mexico. Fish. Res. 2017, 190, 61–70. [Google Scholar] [CrossRef]

| Survey Time | Net Width (m) | Net Length (m) | Net Port Mesh Size (cm) | Cod End Mesh Size (cm) |
|---|---|---|---|---|
| 1964–1965 | 10.7 | 29 | 6.4 | 2.5 |
| 1997–1999 | 18 | 78.2 | 20 | 2.4 |
| 2006–2007 | 24.4 | 60.6 | 10 | 4 |
| 2017 | 16 | 32 | 10 | 5 |
| Species | PC (%) | |||
|---|---|---|---|---|
| 1964–1965 | 1997–1999 | 2006–2007 | 2017 | |
| Carcharhinus amblyrhynchos Bleeker, 1856 | 13.100 | — | — | — |
| Navodon septentrionalis (Günther, 1874) | 5.592 | 0.057 | 0.001 | — |
| Nemipteras bathybius Snyder,1911 | 4.568 | 1.132 | 3.465 | 2.280 |
| Lutjanus erythopterus (Cuvier, 1828) | 4.066 | 0.137 | — | 0.013 |
| Dasyatis zugei (Müller & Henle, 1841) | 3.723 | 0.101 | — | 0.014 |
| Pristipomoides typus Bleeker, 1852 | 3.446 | 0.101 | 0.062 | — |
| Saurida tumbil (Bloch, 1795) | 3.112 | 9.986 | 1.283 | 2.086 |
| Upeneus moluccensis (Bleeker, 1855) | 2.621 | 0.072 | — | — |
| Argyrosomus microcephalus (Tang, 1937) | 2.063 | 0.217 | 0.026 | 0.149 |
| Saurida filamentosa Ogilby, 1910 | 1.929 | 0.683 | 0.571 | 0.704 |
| Pampus cinereus (Bloch, 1795) | 1.742 | 0.268 | 0.338 | 0.002 |
| Muraenesox cinereus (Forsskål, 1775) | 1.742 | 0.862 | 1.117 | 2.541 |
| Pomadasys hasta (Bloch, 1790) | 1.610 | 0.104 | — | — |
| Gymnocranius griseus (Temminck & Schlegel, 1843) | 1.483 | 0.102 | — | — |
| Saurida undosquamis (Richardson, 1848) | 1.432 | 8.418 | 1.002 | 2.068 |
| Priacanthus macracanthus Cuvier, 1829 | 1.235 | 1.677 | 0.352 | 0.070 |
| Nemipteras virgatus (Houttuyn, 1782) | 1.012 | 2.873 | 0.613 | 1.821 |
| Parargyrops edita Tanaka, 1916 | 0.958 | 1.877 | 1.026 | 0.395 |
| Trichiurus haumela (Forsskål, 1775) | 0.910 | 3.852 | 1.053 | 1.120 |
| Polynemus sextarius Bloch & Schneider, 1801 | 0.595 | 0.027 | 2.363 | 2.701 |
| Upeneus bensasi (Temminck & Schlegel, 1843) | 0.451 | 0.237 | 1.790 | 0.600 |
| Siganus oramin (Bloch & Schneider, 1801) | — | 2.955 | 0.219 | 4.043 |
| Thamnaconus hypargyreus (Cope, 1871) | — | 2.499 | 2.387 | 0.449 |
| Trachinocephalus myops (Forster, 1801) | — | 0.108 | 1.486 | 2.217 |
| Trichiurus brevis Wang & You, 1992 | — | 3.908 | 0.219 | — |
| Raja hollandi Jordan & Richardson, 1909 | — | 2.093 | 0.222 | 0.750 |
| Champsodon capensis Regan, 1908 | — | 2.010 | 0.986 | 0.347 |
| Apogon semilineatus Temminck & Schlegel, 1842 | — | 1.957 | 0.269 | 0.226 |
| Synodus kaianus (Günther, 1880) | — | 1.580 | 0.540 | — |
| Trachurus japonicus (Temminck & Schlegel, 1844) | — | 0.122 | 2.461 | 0.859 |
| Malakichthys griseus Döderlein, 1883 | — | 0.004 | 2.039 | — |
| Lophiomus setigerus (Vahl, 1797) | — | 0.343 | 1.399 | 0.822 |
| Arnoglossus tenuis Günther, 1880 | — | 0.063 | 0.037 | 5.491 |
| Penaeus monodon Fabricius, 1798 | — | 0.004 | 0.130 | 0.186 |
| Penaeus japonicus Spence Bate, 1888 | — | 0.009 | 0.003 | 0.114 |
| Penaeus penicillatus Alcock, 1905 | — | 0.001 | 0.006 | 0.084 |
| Ibacus novemdentatus Gibbes, 1850 | — | 0.029 | 0.057 | 0.001 |
| Solenocera crassicornis (H. Milne Edwards, 1837) | — | 0.025 | 3.751 | 1.900 |
| Portunus sanguinolentus (Herbst, 1783) | — | 0.043 | 3.602 | 0.906 |
| Portunus trituberculatus (Miers, 1876) | — | 0.011 | 0.131 | 0.143 |
| Portunus gracilimanus (Stimpson, 1858) | — | 0.001 | 0.006 | 0.866 |
| Portunus argentatus (Alcock, 1899) | — | 0.419 | 0.121 | 0.459 |
| Portunus haanii (Stimpson, 1858) | — | 0.072 | 0.294 | 0.273 |
| Portunus pelagicus (Linnaeus, 1758) | — | 0.001 | 0.008 | 0.015 |
| Calappa philargius (Linnaeus, 1758) | — | 0.018 | 0.334 | 0.472 |
| Charybdis feriatus (Linnaeus, 1758) | — | 0.176 | 2.188 | 0.433 |
| Charybdis miles (De Haan, 1835) | — | 0.849 | 0.197 | 1.843 |
| Oratosquilla nepa (Serville & Guérin, 1828) | — | 0.001 | 0.146 | 0.039 |
| Harpiosquilla raphidea (Fabricius, 1798) | — | 0.010 | 0.017 | 0.006 |
| Oratosquilla oratoria (De Haan, 1844) | — | 0.001 | 0.414 | 0.223 |
| Oratosquilla inornata (Tate, 1883) | — | 0.004 | 0.002 | 0.019 |
| Loligo edulis Hoyle, 1885 | — | 1.551 | 1.261 | 2.930 |
| Loligo duvaucelii Loligo Lamarck, 1798 | — | 0.152 | 0.860 | 3.443 |
| Loligo chinensis Gray, 1849 | — | 0.047 | 1.474 | 1.777 |
| Loligo tagoi Sasaki, 1929 | — | 0.024 | 0.047 | 2.659 |
| Sepioteuthis lessoniana Sepioteuthis Blainville, 1824 | — | 0.022 | 0.129 | 0.039 |
| Sepia esculenta Hoyle, 1885 | — | 0.158 | 0.388 | 0.807 |
| Euprymna berryi Sasaki, 1929 | — | 0.011 | 0.015 | 0.121 |
| Sepia pharaonic Ehrenberg, 1831 | — | 0.170 | 0.392 | 0.004 |
| Sepia lycidas Gray, 1849 | — | 0.041 | 0.234 | 0.017 |
| Octopus variabilis (Sasaki, 1929) | — | 0.025 | 0.179 | 0.187 |
| Octopus ocellatus Gray, 1849 | — | 0.001 | 0.171 | 0.603 |
| Octopus variabilis (Sasaki, 1929) | — | 0.004 | 0.049 | 2.792 |
| Other species | 42.611 | 45.694 | 56.064 | 44.869 |
| Total | 100 | 100 | 100 | 100 |
| Species | PL (%) | |||
|---|---|---|---|---|
| 1964–1965 | 1997–1999 | 2006–2007 | 2017 | |
| Carcharhinus menisorrah Bleeker, 1856 | 13.100 | — | — | — |
| Saurida tumbil (Bloch, 1795) | 3.112 | 10.658 | 1.625 | 3.046 |
| Saurida undosquamis (Richardson, 1848) | 1.432 | 8.985 | 1.269 | 3.019 |
| Arnoglossus tenuis Günther, 1880 | — | 0.067 | 0.046 | 8.017 |
| Siganus oramin (Bloch & Schneider, 1801) | — | 3.154 | 0.278 | 5.903 |
| Navodon septentrionalis (Günther, 1874) | 5.592 | 0.0002 | 0.001 | — |
| Nemipteras bathybius Snyder,1911 | 4.568 | 1.208 | 4.389 | 3.329 |
| Trichiurus brevis Wang & You, 1992 | — | 4.171 | 0.278 | — |
| Trichiuru haumela (Forsskål, 1775) | 0.910 | 4.111 | 1.334 | 1.635 |
| Lutjanus erythopterus (Cuvier, 1828) | 4.066 | 0.307 | — | 0.020 |
| Polynemus sextarius Bloch & Schneider, 1801 | 0.595 | 0.028 | 2.993 | 3.944 |
| Acropoma japonicum Günther, 1859 | — | 3.780 | 0.540 | 1.609 |
| Dasyatis zugei (Müller & Henle, 1841) | 3.723 | 0.115 | — | 0.021 |
| Muraenesox cinereus (Forsskål, 1775) | 1.742 | 0.920 | 1.415 | 3.710 |
| Dactyloptena peterseni (Nystrom, 1908) | — | 0.065 | 3.599 | — |
| Leiognathus bindus Valenciennes, 1835 | 0.904 | 3.535 | 0.250 | 0.011 |
| Pristipomoides typus Bleeker, 1852 | 3.446 | 0.042 | 0.079 | — |
| Trachinocephalus myops (Forster, 1801) | — | 0.751 | 1.882 | 3.238 |
| Trachurus japonicus (Temminck & Schlegel, 1844) | — | 1.062 | 3.117 | 1.254 |
| Nemipteras virgatus (Houttuyn, 1782) | 1.012 | 3.066 | 0.776 | 2.658 |
| Navodon xanthopterus (Cope, 1871) | — | 2.667 | 3.023 | 0.656 |
| Urolophus marmoratus Chu, Hu & Li, 1981 | — | — | 2.990 | — |
| Upeneus moluccensis (Bleeker, 1855) | 2.621 | 0.047 | — | — |
| Malakichthys griseus Döderlein, 1883 | — | 0.005 | 2.583 | — |
| Crossorhombus azureus (Alcock, 1889) | — | 0.001 | 0.004 | 2.478 |
| Upeneus japonicus Houttuyn, 1782 | — | — | — | 2.447 |
| Upeneus bensasi (Temminck & Schlegel, 1843) | 0.451 | 0.253 | 2.267 | 0.877 |
| Raja hollandi Jordan & Richardson, 1909 | — | 2.234 | 0.281 | 1.095 |
| Sparus latus (Houttuyn, 1782) | — | 0.107 | 0.475 | 2.216 |
| Champsodon capensis Regan, 1908 | — | 2.146 | 1.249 | 0.506 |
| Apogon semilineatus Temminck & Schlegel, 1842 | — | 2.088 | 0.340 | 0.329 |
| Argyrosomus microcephalus (Tang, 1937) | 2.063 | 0.232 | 0.033 | 0.217 |
| Parargyrops edita Tanaka, 1916 | 0.958 | 2.003 | 1.300 | 0.576 |
| Acropoma hanedai (Matsubara, 1953) | — | 0.0001 | 1.799 | — |
| Priacanthus macracanthus Cuvier, 1829 | 1.235 | 1.790 | 0.446 | 0.103 |
| Lophiomus setigerus (Vahl, 1797) | 0.609 | 0.366 | 1.772 | 1.200 |
| Pampus cinereus (Bloch, 1795) | 1.742 | 0.019 | 0.429 | 0.003 |
| Synodus kaianus (Günther, 1880) | — | 1.687 | 0.684 | — |
| Raja kenojei (Müller et Henle,1841) | — | 0.070 | 1.626 | — |
| Pomadasys hasta (Bloch, 1790) | 1.610 | 0.007 | — | — |
| Harpodon nehereus Hamilton, 1822 | — | 0.005 | 1.518 | 0.355 |
| Gymnocranius griseus (Temminck & Schlegel, 1843) | 1.483 | 0.020 | — | — |
| Saurida elongate (Temminck & Schlegel, 1846) | — | 0.729 | 0.724 | 1.028 |
| Other species | 43.028 | 37.497 | 52.586 | 44.502 |
| Total | 100 | 100 | 100 | 100 |
| Species | PL (%) | ||
|---|---|---|---|
| 1997–1999 | 2006–2007 | 2017 | |
| Charybdis miles (De Haan, 1835) | 30.794 | 1.411 | 12.417 |
| Portunus sanguinolentus (Herbst, 1783) | 1.545 | 25.784 | 6.106 |
| Charybdis feriatus (Linnaeus, 1758) | 6.403 | 15.663 | 2.921 |
| Solenocera crassicornis (H. Milne Edwards, 1837) | 0.923 | 26.850 | 12.802 |
| Portunus argentatus (Alcock, 1899) | 15.197 | 0.868 | 3.093 |
| Parapenaeus fissuroides Crosnier, 1986 | 8.851 | 0.169 | — |
| Portunus gracilimanus (Stimpson, 1858) | 0.026 | 0.046 | 5.836 |
| Charybdis variegate (Fabricius, 1798) | 0.010 | 0.030 | 5.141 |
| Metapenaeopsis barbata (De Haan, 1844) | 2.479 | 0.034 | 5.019 |
| Heterocarpoides laevicarina (Bate, 1888) | 4.226 | — | — |
| Metapenaeopsis palmensis (Haswell, 1879) | 0.156 | 0.498 | 3.509 |
| Dromia dehaani Rathbun, 1923 | 0.008 | 0.079 | 3.301 |
| Calappa philargius (Linnaeus, 1758) | 0.670 | 2.391 | 3.177 |
| Parapenaeopsis hardwickii (Miers, 1878) | — | 0.026 | 3.130 |
| Ibacus ciliatus (von Siebold, 1824) | 1.627 | 3.112 | — |
| Oratosquilla oratoria (De Haan, 1844) | 0.034 | 2.966 | 1.500 |
| Charybdis japonica (A. Milne-Edwards, 1861) | 0.015 | 2.826 | 1.678 |
| Portunus haanii (Stimpson, 1858) | 2.620 | 2.107 | 1.842 |
| Solenocera koelbeli De Man, 1911 | 2.511 | 0.012 | — |
| Trachypenaeus longipes (Paulson, 1875) | 2.404 | 0.129 | 0.278 |
| Portunus hastatoides Fabricius, 1798 | 2.340 | 0.003 | 0.701 |
| Metapenaeus affinis (H. Milne Edwards, 1837) | — | 2.269 | 0.443 |
| Philyra pisum De Haan, 1841 | — | — | 2.185 |
| Charybdis truncate (Fabricius, 1798) | 2.066 | 0.306 | 2.016 |
| Harpiosquilla harpax (de Haan, 1844) | — | 1.511 | 1.988 |
| Oratosquilla nepa (Serville & Guérin, 1828) | 0.020 | 1.042 | 0.265 |
| Portunus trituberculatus (Miers, 1876) | 0.417 | 0.939 | 0.966 |
| Other species | 14.657 | 8.930 | 19.687 |
| Total | 100 | 100 | 100 |
| Species | PL (%) | ||
|---|---|---|---|
| 1997–1999 | 2006–2007 | 2017 | |
| Loligo edulis Hoyle, 1885 | 43.654 | 17.819 | 17.578 |
| Loligo chinensis Gray, 1849 | 22.123 | 20.827 | 10.663 |
| Loligo duvaucelii Loligo Lamarck, 1798 | 4.277 | 12.146 | 20.658 |
| Sepia esculenta Hoyle, 1885 | 4.447 | 5.486 | 4.840 |
| Sepia lycidas Gray, 1849 | 1.158 | 3.303 | 0.103 |
| Octopus variabilis (Sasaki, 1929) | 0.708 | 2.533 | 1.124 |
| Sepioteuthis lessoniana d’Orbigny, 1826 | 0.612 | 1.822 | 0.235 |
| Todarodes pacificus (Steenstrup, 1880) | 14.080 | 3.236 | — |
| Sepia pharaonis Ehrenberg, 1831 | 4.794 | 5.536 | 0.025 |
| Loligo tagoi Sasaki, 1929 | 0.676 | 0.664 | 15.955 |
| Octopus vulgaris Cuvier, 1797 | 0.104 | 6.148 | 0.394 |
| Octopus ocellatus Gray, 1849 | 0.039 | 2.410 | 3.620 |
| Euprymna berryi Sasaki, 1929 | 0.299 | 0.213 | 0.725 |
| Octopus variabilis (Sasaki, 1929) | 0.113 | 0.693 | 16.753 |
| Other species | 2.919 | 17.163 | 7.327 |
| Total | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Lin, Z.; Chen, Y.; Chen, P. Eco-Dynamic Analysis of the Community Structure of Nekton in the Northern South China Sea. Fishes 2023, 8, 578. https://doi.org/10.3390/fishes8120578
Yuan H, Lin Z, Chen Y, Chen P. Eco-Dynamic Analysis of the Community Structure of Nekton in the Northern South China Sea. Fishes. 2023; 8(12):578. https://doi.org/10.3390/fishes8120578
Chicago/Turabian StyleYuan, Huarong, Zhaojin Lin, Yuxiang Chen, and Pimao Chen. 2023. "Eco-Dynamic Analysis of the Community Structure of Nekton in the Northern South China Sea" Fishes 8, no. 12: 578. https://doi.org/10.3390/fishes8120578
APA StyleYuan, H., Lin, Z., Chen, Y., & Chen, P. (2023). Eco-Dynamic Analysis of the Community Structure of Nekton in the Northern South China Sea. Fishes, 8(12), 578. https://doi.org/10.3390/fishes8120578







