Stock Status of a Few Small Indigenous Fish Species Exploited in the River Ganga, India
Abstract
:1. Introduction
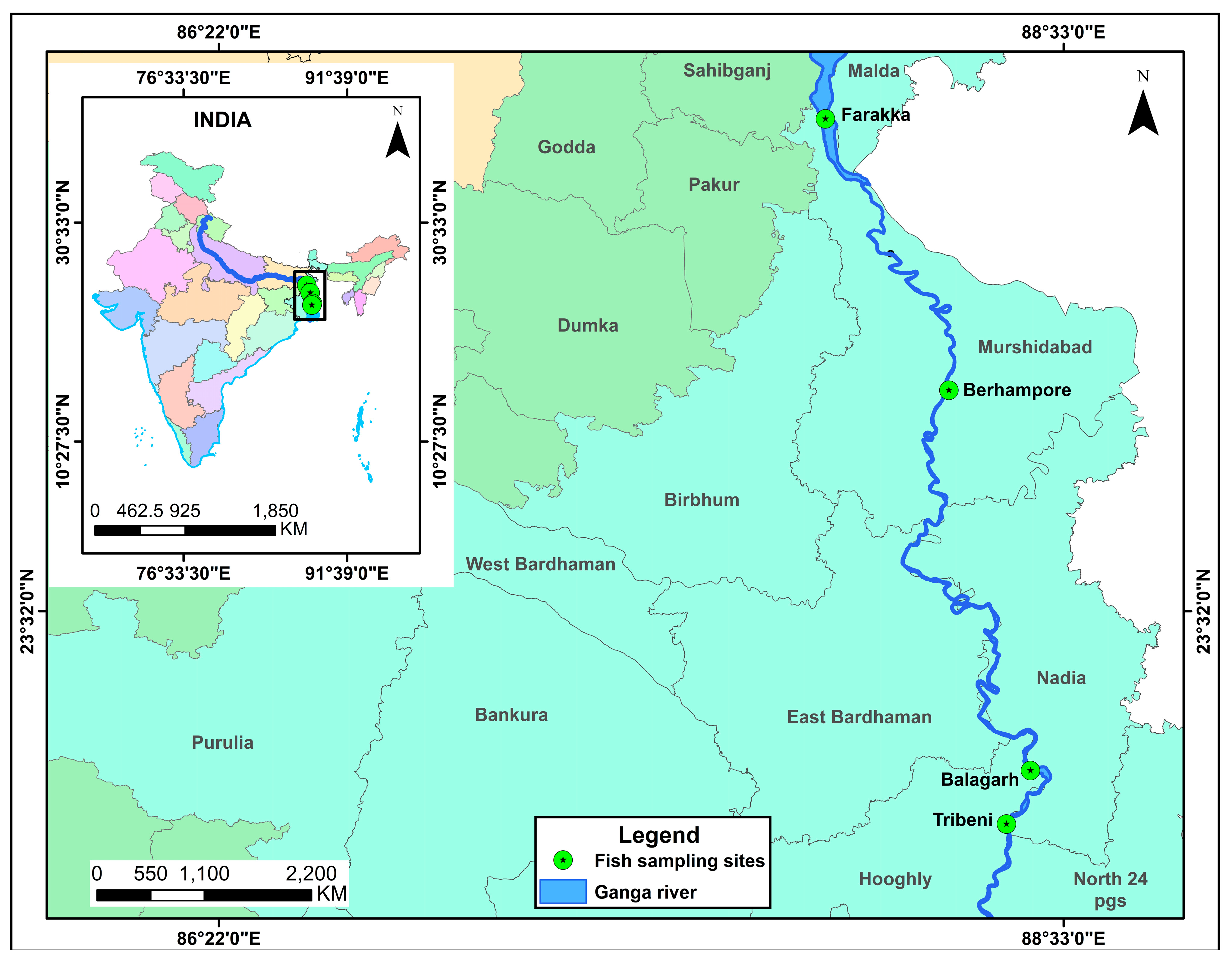
2. Materials and Methods
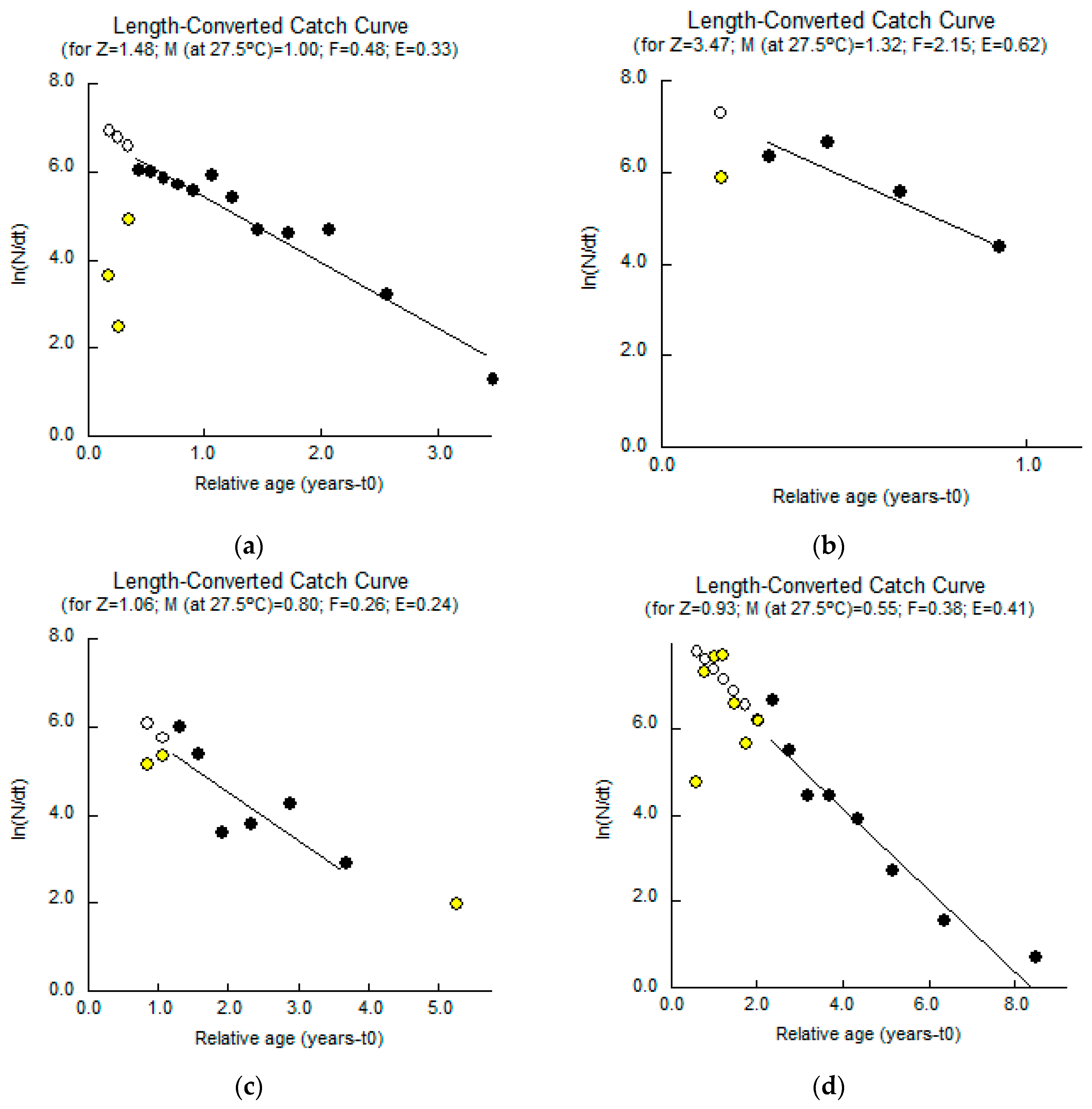
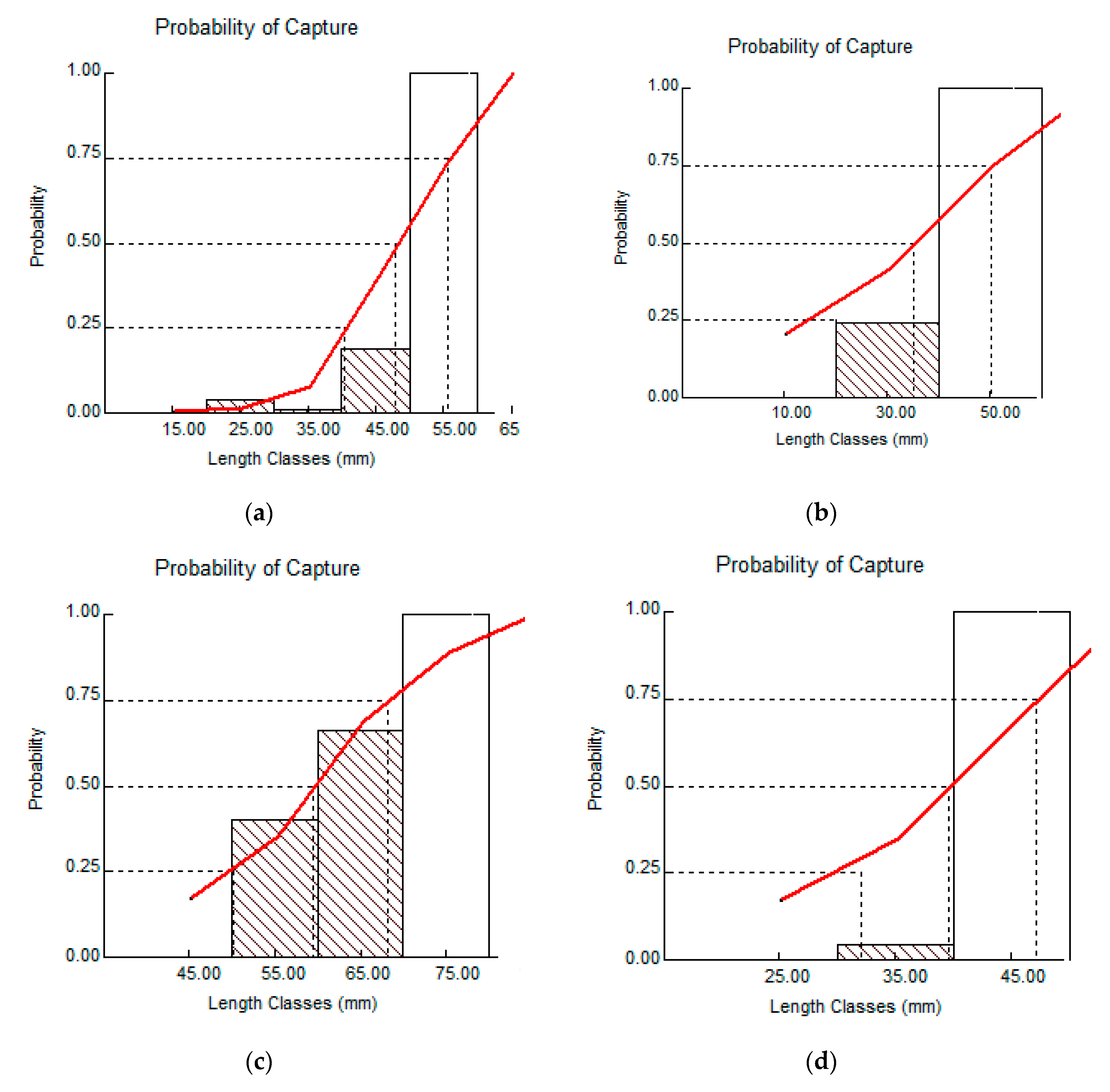
3. Results
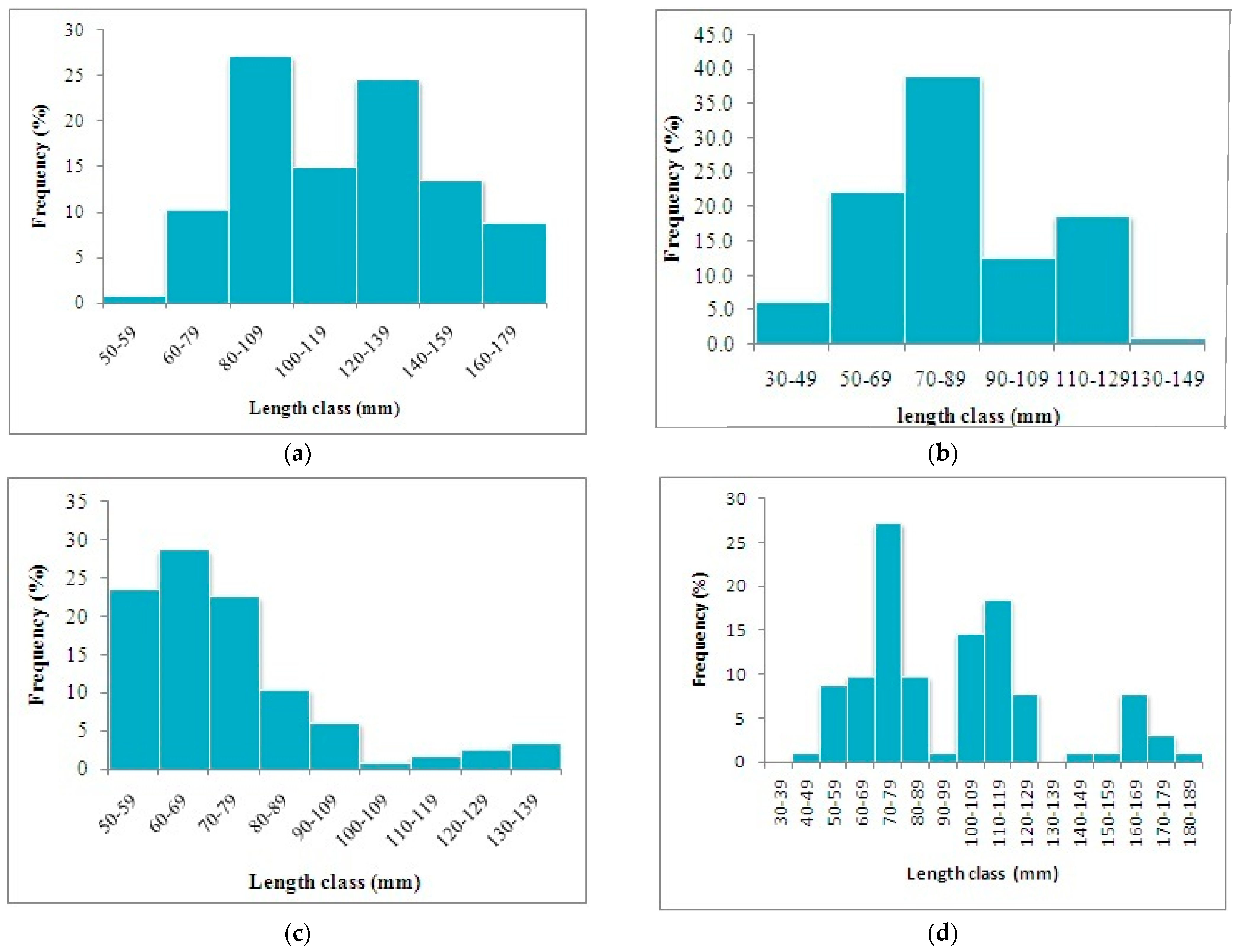
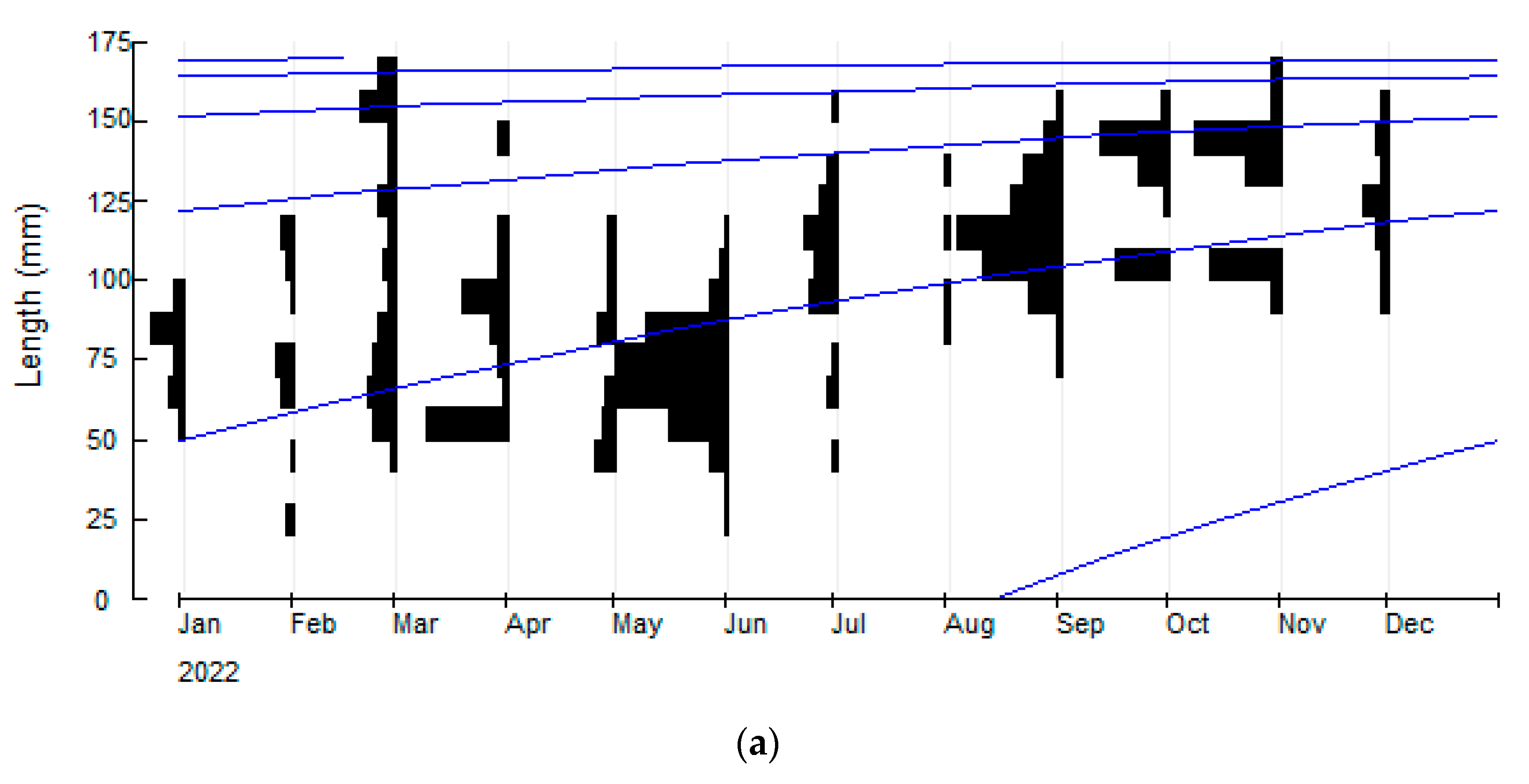
3.1. Johnius coitor (Hamilton, 1822)
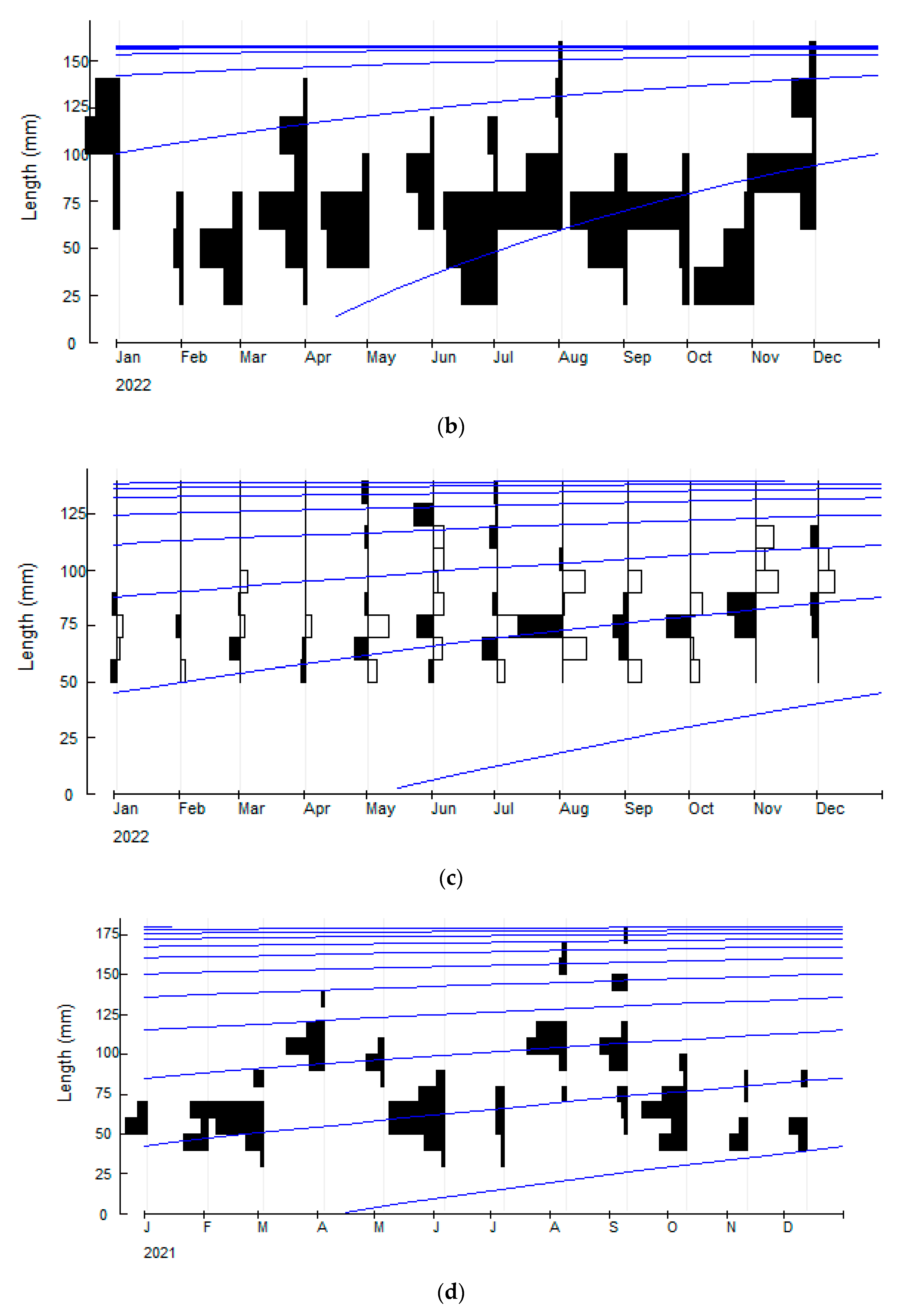
3.2. Cabdio morar (Hamilton, 1822)
3.3. Salmostoma bacaila (Hamilton, 1822)
3.4. Gudusia chapra (Hamilton, 1822)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welcomme, R.L. River Fisheries; FAO: Rome, Italy, 1985; ISBN 9251022992. [Google Scholar]
- Das, B.K.; Ray, A.; Johnson, C.; Verma, S.K.; Alam, A.; Baitha, R.; Manna, R.K.; Roy, S.; Sarkar, U.K. The Present Status of Ichthyofaunal Diversity of River Ganga India: Synthesis of Present v/s Past. Acta Ecol. Sin. 2023, 43, 307–332. [Google Scholar] [CrossRef]
- Sinha, M.; Khan, M.A. Impact of Environmental Aberrations on Fisheries of the Ganga (Ganges) River. Aquat. Ecosyst. Health Manag. 2001, 4, 493–504. [Google Scholar] [CrossRef]
- Vass, K.K.; Das, M.K.; Srivastava, P.K.; Dey, S. Assessing the Impact of Climate Change on Inland Fisheries in River Ganga and Its Plains in India. Aquat. Ecosyst. Health Manag. 2009, 12, 138–151. [Google Scholar] [CrossRef]
- Tripathi, S.; Gopesh, A.; Dwivedi, A.C. Fish and Fisheries in the Ganga River: Current Assessment of the Fish Community, Threats and Restoration. J. Exp. Zool. India 2017, 2, 907–912. [Google Scholar]
- Le Cren, E.D. The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- King, M. Fisheries Biology, Assessment and Management; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 111868804X. [Google Scholar]
- Sparre, P. Introduction to Tropical Fish Stock Assessment. Part 1. Manual. FAO Fish. Tech. Paper 1998, 306, 1–407. [Google Scholar]
- Chung, K.; Woo, N.Y.S. Age and Growth by Scale Analysis of Pomacanthus imperator (Teleostei: Pomacanthidae) from Dongsha Islands, Southern China. Environ. Biol. Fishes 1999, 55, 399–412. [Google Scholar] [CrossRef]
- Bhakta, D.; Das, S.K.; Das, B.K.; Nagesh, T.S.; Samanta, R. Growth, Mortality and Exploitation Status of Otolithoides pama (Hamilton, 1822) from Hooghly-Matlah Estuary of West Bengal, India. Reg. Stud. Mar. Sci. 2020, 39, 101451. [Google Scholar] [CrossRef]
- Johnson, C.; Sarkar, U.K.; Koushlesh, S.K.; Das, A.K.; Das, B.K.; Naskar, B.K. Population Structure of Nile Tilapia and Its Impact on Fisheries of a Tropical Impacted Reservoir, Central India. Environ. Sci. Pollut. Res. 2020, 27, 29091–29099. [Google Scholar] [CrossRef]
- Chirwatkara, B.B.; Das, S.K.; Bhakta, D.; Nagesha, T.S.; Beheraa, S. Growth, Mortality and Stock Assessment of Arius arius (Hamilton, 1822) from Hooghly-Matlah Estuary, West Bengal. Indian J. Geo-Mar Sci. 2022, 50, 302–309. [Google Scholar]
- Bhakta, D.; Das, S.K.; Das, B.K.; Nagesh, T.S.; Behera, B.K. Fishery and Population Dynamics of Otolithoides pama (Hamilton, 1822) from Hooghly-Matlah Estuary of West Bengal, India. Aquat. Ecosyst. Health Manag. 2022, 25, 36–43. [Google Scholar] [CrossRef]
- Sarkar, U.K.; Naskar, M.; Roy, K.; Sudheesan, D.; Gupta, S.; Bose, A.K.; Srivastava, P.K.; Nandy, S.K.; Verma, V.K.; Sarkar, S. Das Baseline Information of Reproduction Parameters of an Amphidromous Croaker Johnius coitor (Hamilton, 1822) from Ganga River Basin, India with Special Reference to Potential Influence of Climatic Variability. Aquat. Living Resour. 2018, 31, 4. [Google Scholar] [CrossRef]
- Vinci, G.K.; Suresh, V.R.; Bandyopadhyaya, M.K. Biology of Gudusia chapra (Hamilton-Buchanan) from a Floodplain Wetland in West Bengal. Indian J. Fish. 2005, 52, 73–79. [Google Scholar]
- Kumari, S.; Sandhya, K.M.; Karnatak, G.; Sarkar, U.K.; Panda, D.; Mishal, P. Length-Weight Relationship and Condition Factor of Gudusia chapra (Hamilton, 1822) from Panchet Reservoir, Jharkhand, India. Indian J. Fish. 2019, 66, 138–141. [Google Scholar] [CrossRef]
- Dwivedi, A.C. Ecological Assessment of Fishes and Population Dynamics of Labeo rohita (Hamilton), Tor tor (Hamilton) and Labeo calbasu (Hamilton) in the Paisuni River. Aquacult 2009, 10, 249–259. [Google Scholar]
- Krishna, M.; Suresh, V.R.; Biswas, D.K.; Biswas, B.K. Biology, Population Dynamics and Fishery of Puntius conchonius (Hamilton) in a Floodplain Wetland in Ganga River Basin, India. J. Inland Fish. Soc. India 2011, 43, 16–24. [Google Scholar]
- Dwivedi, A.C. Population Dynamics, Age, Growth and Sex Ratio of Labeo bata (Hamilton) from the Middle Stretch of the Ganga River, India. Flora Founa 2013, 19, 133–137. [Google Scholar]
- Rizvi, A.F.; Singh, K.P.; Kumar, N. Stock Assessment of a Non-Commercial Fish Sciaena coitor (Hamilton, 1822) from Middle Stretch of River Ganga. Natl. Acad. Sci. Lett. 2015, 38, 9–12. [Google Scholar] [CrossRef]
- Sarkar, U.K.; Johnson, C.; Kumari, S.; Bakshi, S.; Karnatak, G.; Das Ghosh, B.; Lianthuamluaia, M.P.; Das, B.K. Population Dynamics of Indian River Shad Gudusia chapra (Hamilton, 1822) Using Length Frequency Analysis for Fisheries Management in a Floodplain Wetland of Ganga River Basin, India. Lakes Reserv. Res. Manag. 2021, 26, e12365. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List of Threatened SpeciesTM. Version 2020-1. 2020. Available online: https://www.iucnredist.org (accessed on 2 March 2023).
- Talwar, P.K.; Jhingran, A.G. Inland Fishes of India and Adjacent Countries; IBH Pub. Co. Pvt. Ltd.: New Delhi, India, 1991. [Google Scholar]
- Jayaram, K.C. The Freshwater Fishes of the Indian Region, 2nd ed.; Nrendra Publishing: New Delhi, India, 2010. [Google Scholar]
- Gayanilo, F.C.; Sparre, P. FAO-ICLARM Stock Assessment Tools II: User’s Guide; Food & Agriculture Organization: Rome, Italy, 2005; ISBN 9251053006. [Google Scholar]
- Pauly, D. On the Interrelationships between Natural Mortality, Growth Parameters, and Mean Environmental Temperature in 175 Fish Stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Silvestre, G.T.; Garces, L.R. Population Parameters and Exploitation Rate of Demersal Fishes in Brunei Darussalam (1989–1990). Fish. Res. 2004, 69, 73–90. [Google Scholar] [CrossRef]
- Berverton, R.J.H.; Holt, S.J. Manual of Methods for Fish Stock Assessment. Part II. Tables of yield function. In FAO Fish Biology Technical Paper; FAO: Rome, Italy, 1966; Volume 10. [Google Scholar]
- Rizvi, A.F.; Singh, K.P.; Kumar, N. Population Dynamics of Non-Commercial Fishes in Rivers around Allahabad and Possibility of Their Commercialisation to Enhance Socio-Economic Status of Fishermen. Natl. Acad. Sci. Lett. 2011, 34, 437–448. [Google Scholar]
- Sarmina, M.S.; Hossain, M.Y.; Islama, M.A.; Rahmana, M.A.; Khatuna, D.; Mawa, Z.; Chowdhurya, A.A.; Ohtomib, J. Estimation of Population Parameters for a Data Deficient Salmostoma bacaila (Hamilton 1822) Stock from the Mahananda River (Tributary of the Ganges) in NW Bangladesh. Indian J. Geo-Mar Sci. 2022, 50, 403–409. [Google Scholar]
- Rahman, M.A.; Haque, M.M.; Khan, S. Food and feeding habits of Chapila, Gudusia chapra (Hamilton-Buchanan) from Rajdhala reservoir. J. Inland Fish. Soc. India 2008, 40, 13–20. [Google Scholar]
- Ahmed, Z.F.; Smith, C.; Ahamed, F.; Hossain, M.Y. Growth and Reproduction of the Indian River Shad, Gudusia chapra (Clupeidae). Folia Zool. 2007, 56, 429. [Google Scholar]
- Kumari, S.; Sarkar, U.K.; Mandhir, S.K.; Lianthuamluaia, L.; Panda, D.; Chakraborty, S.K.; Karnatak, G.; Kumar, V.; Puthiyottil, M. Studies on the Growth and Mortality of Indian River Shad, Gudusia chapra (Hamilton, 1822) from Panchet Reservoir, India. Environ. Sci. Pollut. Res. 2018, 25, 33768–33772. [Google Scholar] [CrossRef]
- Samad, M.A.; Rahman, M.A.; Yeasmin, S.M.; Rahman, M.H.; Hossain, M.Y. Assessment of Stock Status, Metal Contents with Human Health Risk of Gudusia chapra from Oxbow Lake, Bangladesh. Heliyon 2023, 9, e1944. [Google Scholar] [CrossRef]
- Sinha, M.; Mukhopadhyay, M.K.; Mitra, P.M.; Bagchi, M.M.; Karamkar, H.C. Impact of Farakka Barrage on the Hydrology and Fishery of Hoogly Estuary. Estuaries 1996, 19, 710–722. [Google Scholar] [CrossRef]
- Sinha, M. Farakka Barrage and Its Impact on the Hydrology and Fishery of Hooghly Estuary. In The Ganges Water Diversion: Environmental Effects and Implications; Springer: Berlin/Heidelberg, Germany, 2004; pp. 103–124. [Google Scholar]
- Islam, A.R.M.T.; Talukdar, S.; Akhter, S.; Eibek, K.U.; Rahman, M.M.; Pal, S.; Naikoo, M.W.; Rahman, A.; Mosavi, A. Assessing the Impact of the Farakka Barrage on Hydrological Alteration in the Padma River with Future Insight. Sustainability 2022, 14, 5233. [Google Scholar] [CrossRef]
- Pal, R.; Pani, P. Seasonality, Barrage (Farakka) Regulated Hydrology and Flood Scenarios of the Ganga River: A Study Based on MNDWI and Simple Gumbel Model. Model. Earth Syst. Environ. 2016, 2, 57. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Saha, M.; Takada, H.; Bhattacharya, A.; Mishra, P.; Bhattacharya, B. Water Quality Management in the Lower Stretch of the River Ganges, East Coast of India: An Approach through Environmental Education. J. Clean. Prod 2007, 15, 1559–1567. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Carranza, A.; Vilela, R.; Casadevall, M. A Rapid Assessment of Trends in the Multispecies Small-Scale Fishery of Palamós (Catalonia, Spain). ICES J. Mar. Sci. 2015, 72, 2638–2649. [Google Scholar] [CrossRef]
- Jobling, M. Environmental Factors and Rates of Development and Growth. Handb. Fish Biol. Fish. 2002, 1, 97–122. [Google Scholar]
- Carlander, K.D. Handbook of Freshwater Fishery Biology; Iowa State University Press: Ames, IA, USA, 1997; Volume 3, ISBN 0813829992. [Google Scholar]
- Froese, R. Length-weight Relationships for 18 Less-studied Fish Species. J. Appl. Ichthyol. 1998, 14, 117–118. [Google Scholar] [CrossRef]
- Froese, R.; Froese, D.P.R.; Pauly, D. (Eds.) FishBase. 2016. Available online: https://www.fishbase.org (accessed on 2 June 2023).
- Jisr, N.; Younes, G.; Sukhn, C.; El-Dakdouki, M.H. Length-Weight Relationships and Relative Condition Factor of Fish Inhabiting the Marine Area of the Eastern Mediterranean City, Tripoli-Lebanon. Egypt. J. Aquat. Res. 2018, 44, 299–305. [Google Scholar] [CrossRef]
- Gulland, J.A. The Fish Resources of the Ocean; Fishing News Ltd.: Surrey, UK, 1971; Volume 255. [Google Scholar]
- Borah, S.; Landge, A.T.; Bhattacharjya, B.K.; Chakraborty, S.K.; Ramteke, K.K.; Barman, J.; Bhagawati, K.; Saud, B.J. Variation in Morphometric and Meristic Traits of Aspidoparia morar from Brahmaputra and Barak Rivers of Assam, India. J. Appl. Nat. Sci. 2014, 6, 262–266. [Google Scholar] [CrossRef]
- Radkhah, A.R.; Eagderi, S.; Mouludi-Saleh, A. Morphological Variation, Length-Weight Relationship and Condition Factor of Cabdio morar (Hamilton, 1822) Populations in Southeast of Iran. J. Wildl. Biodivers. 2023, 7, 58–68. [Google Scholar]
- Baitha, R.; Karna, S.K.; Ray, A.; Chanu, T.N.; Swain, H.S.; Ramteke, M.H.; Bayen, S.; Manna, R.K.; Das, B.K. Length–Weight and Length–Length Relationships of Eight Fish Species from River Ganga, India. J. Appl. Ichthyol. 2018, 34, 1052–1054. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Rahman, M.M.; Bahkali, A.H.; Yahya, K.; Arefin, M.; Hossain, M.I.; Elgorban, A.M.; Hossen, M.A.; Islam, M.M.; Masood, Z. Temporal Variations of Sex Ratio, Length-Weight Relationships and Condition Factor of Cabdio morar (Cyprinidae) in the Jamuna (Brahmaputra River Distributary) River, Northern Bangladesh. Pak. J. Zool. 2016, 48, 1099–1107. [Google Scholar]
- Sharpe, D.M.T.; Hendry, A.P. Synthesis: Life History Change in Commercially Exploited Fish Stocks: An Analysis of Trends across Studies. Evol. Appl. 2009, 2, 260–275. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Arefin, M.S.; Mohmud, M.S.; Hossain, M.I.; Jewel, M.A.S.; Rahman, M.M.; Ahamed, F.; Ahmed, Z.F.; Ohtomi, J. Length-weight Relationships, Condition Factor, Gonadosomatic Index-based Size at First Sexual Maturity, Spawning Season and Fecundity of Aspidoparia morar (Cyprinidae) in the Jamuna River (Brahmaputra River Distributary), Northern Bangladesh. J. Appl. Ichthyol. 2013, 29, 1166–1169. [Google Scholar] [CrossRef]
- Masud, S.; Singh, K.P. Studies on Length–Weight Relationship and Condition Factor of Salmophasia bacaila (Hamilton) from the Lower Stretch of River Yamuna at Allahabad. Int. J. Fish. Aquat. Stud. 2015, 2, 147–150. [Google Scholar]
- Ahamed, F.; Ahmed, Z.F.; Ohtomi, J. Seasonal Variations in the Population Biology of Salmostoma bacaila (Cyprinidae) from a Tributary of the Payra River, Bangladesh. Zool. Ecol. 2019, 29, 113–119. [Google Scholar] [CrossRef]
- Karna, S.K.; Katselis, G.N.; Jawad, L.A. Length–Weight Relations of 24 Fish Species (Actinopterygii) from Hirakud Reservoir, Odisha State of India. Acta Ichthyol. Piscat. 2018, 48, 83–86. [Google Scholar] [CrossRef]
- Rahman, A.K.A. Freshwater Fishes of Bangladesh; Zoological Society of Bangladesh: Dhaka, Bangladesh, 1989. [Google Scholar]
- Sheikh, J.; Singha, N.; Nag, R.; Deka, P. Length-Weight Relationship and Relative Condition Factor of Gudusia chapra (Hamilton, 1822) of Dalani Beel (Wetland) of Assam, India. Int. J. Fish. Aquat. Stud. 2017, 5, 485–489. [Google Scholar]
- Rahman, M.A.; Haque, M.M. Population Dynamics and Stock Assessment of Gudusia chapra (Hamilton-Buchanan) in the Rajdhala Reservoir, Netrakona, Bangladesh. Asian Fish. Sci. 2007, 19, 281–292. [Google Scholar] [CrossRef]
- Kabir, A.; Hossain, M.A.; Rahmatullah, S.M.; Dewan, S.; Islam, M.S. Studies on the Gonadosomatic Index and Fecundity of Chapila (Gudusia chapra Ham.). Bangladesh J. Fish. Res. 1998, 2, 195–200. [Google Scholar]
- Chen, Y.; Chen, L.; Stergiou, K.I. Impacts of Data Quantity on Fisheries Stock Assessment. Aquat. Sci. 2003, 65, 92–98. [Google Scholar] [CrossRef]
- Maunder, M.N.; Starr, P.J. Bayesian Assessment of the SNA1 Snapper (Pagrus auratus) Stock on the North-east Coast of New Zealand. N. Z. J. Mar. Freshw. Res. 2001, 35, 87–110. [Google Scholar] [CrossRef]
- Maunder, M.N. Review and Evaluation of Likelihood Functions for Composition Data in Stock-Assessment Models: Estimating the Effective Sample Size. Fish. Res. 2011, 109, 311–319. [Google Scholar] [CrossRef]
- Hamilton, F. An Account of the Fishes Found in the River Ganges and Its Branches; Archibald Constable: London, UK, 1822; Volume 1. [Google Scholar]


| Population Parameters | J. coitor | C. morar | S. bacaila | G. chapra |
|---|---|---|---|---|
| TL range (mm) | 24–168 | 34–139 | 50–134 | 30–179 |
| Intercept (a) | 0.007 | 0.005 | 0.122 | 0.017 |
| Slope (b) | 2.93 | 3.11 | 2.84 | 2.50 |
| Coefficient of determination (r2) | 0.926 | 0.975 | 0.904 | 0.923 |
| 95% CL of a | 0.005–0.008 | 0.004–0.005 | 0.105–0.142 | 0.013–0.023 |
| 95% CL of b | 2.84–3.03 | 3.04–3.17 | 2.66–3.02 | 2.34–2.64 |
| Growth type | Negative allometric | Positive allometric | Negative allometric | Negative allometric |
| Asymptotic length (L∞ mm) | 173.25 | 157.5 | 141.75 | 183.75 |
| Growth coefficient (K yr−1) | 0.88 | 1.30 | 0.58 | 0.31 |
| Natural mortality (M yr−1) | 1.00 | 1.32 | 0.80 | 0.55 |
| Fishing mortality (F yr−1) | 0.48 | 2.15 | 0.26 | 0.38 |
| Total mortality (Z yr−1) | 1.48 | 3.47 | 1.06 | 0.93 |
| Exploitation ratio (E) | 0.33 | 0.62 | 0.24 | 0.41 |
| L25 | 40.29 | 20.11 | 50.26 | 32.03 |
| Length at first capture (L50) | 48.02 | 35.11 | 59.37 | 39.53 |
| L75 | 55.76 | 50.15 | 68.14 | 47.13 |
| Isopleth ratio (Lc/L∞) | 0.28 | 0.22 | 0.42 | 0.22 |
| M/K | 1.14 | 1.02 | 1.52 | 1.61 |
| Fishing rate at 10% (E10) | 0.415 | 0.407 | 0.506 | 0.358 |
| Fishing rate at 50% (E50) | 0.310 | 0.303 | 0.339 | 0.284 |
| Maximum yield exploitation rate (Emax) | 0.505 | 0.479 | 0.625 | 0.463 |
| Species | Author | Region | L∞ (mm) | K (yr−1) | ϕ | Z (yr−1) | M (yr−1) | F (yr−1) | E |
|---|---|---|---|---|---|---|---|---|---|
| J. coitor | Rizvi et al. (2011) [29] | River Ganga and Yamuna, Allahabad, India | 215 | 1.51 | - | 4.97 | 2.56 | 2.41 | 0.48 |
| Rizvi et al. (2015) [20] | River Ganga, Allahabad, India | 198 | 0.868 | 4.53 | 3.31 | 1.76 | 1.55 | 0.46 | |
| Present study | River Ganga | 173.25 | 0.88 | - | 1.48 | 1.0 | 0.48 | 0.33 | |
| S. bacaila | Sarmina et al. (2021) [30] | Mahananda River, Bangladesh | 126.6 | 0.60 | 1.98 | 1.57 | 0.92 | 0.65 | 0.41 |
| Present study | River Ganga | 141.75 | 0.58 | - | 1.06 | 0.26 | 0.88 | 0.24 | |
| G. chapra | Rahman and Haque (2006) [31] | Rajdhala reservoir, Bangladesh | 160.8 | 0.51 | - | 2.71 | 1.34 | 1.37 | 0.51 |
| Ahmed et al. (2007) [32] | Perennial pond, Bangladesh | Male—140.2 Female—145.39 | Male—1.352 Female—1.30 | - | - | - | - | - | |
| Rizvi et al. (2011) [29] | River Ganga and Yamuna, India | 180 | 2.83 | - | 9.60 | 4.07 | 5.54 | 0.57 | |
| Kumari et al. (2018) [33] | Panchet Reservoir, India | 194 | 1.23 | 2.69 | 6.63 | 2.45 | 4.18 | 0.63 | |
| Sarkar et al. (2021) [21] | Mathura Oxbow Lake, West Bengal, India | 165.5 | 1.10 | 2.48 | 2.72 | 1.25 | 1.47 | 0.54 | |
| Samad et al. (2023) [34] | Bukvora oxbow lake, Bangladesh | 153.8 | 0.70 | 2.20 | 2.25 | 1.71 | 0.55 | 0.24 | |
| Present study | River Ganga | 183.75 | 0.31 | - | 0.93 | 0.55 | 0.38 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, A.; Das, B.K.; Bhakta, D.; Johnson, C.; Roy, S.; Gupta, S.D.; Panda, S.P.; Baitha, R. Stock Status of a Few Small Indigenous Fish Species Exploited in the River Ganga, India. Fishes 2023, 8, 572. https://doi.org/10.3390/fishes8120572
Ray A, Das BK, Bhakta D, Johnson C, Roy S, Gupta SD, Panda SP, Baitha R. Stock Status of a Few Small Indigenous Fish Species Exploited in the River Ganga, India. Fishes. 2023; 8(12):572. https://doi.org/10.3390/fishes8120572
Chicago/Turabian StyleRay, Archisman, Basanta Kumar Das, Dibakar Bhakta, Canciyal Johnson, Shreya Roy, Subhadeep Das Gupta, Soumya Prasad Panda, and Raju Baitha. 2023. "Stock Status of a Few Small Indigenous Fish Species Exploited in the River Ganga, India" Fishes 8, no. 12: 572. https://doi.org/10.3390/fishes8120572
APA StyleRay, A., Das, B. K., Bhakta, D., Johnson, C., Roy, S., Gupta, S. D., Panda, S. P., & Baitha, R. (2023). Stock Status of a Few Small Indigenous Fish Species Exploited in the River Ganga, India. Fishes, 8(12), 572. https://doi.org/10.3390/fishes8120572








