Climate Change May Impact Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) Distribution in the Southeastern Arabian Peninsula through Range Contraction under Various Climate Scenarios
Abstract
:1. Introduction
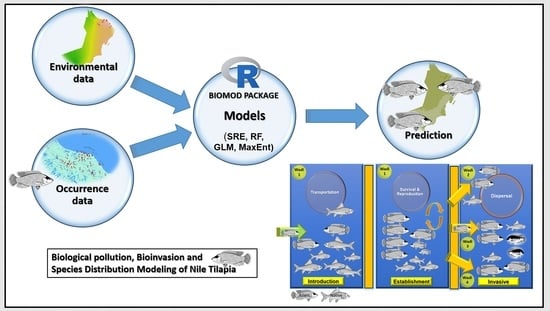
Why the Ensemble Model?
2. Material and Methods
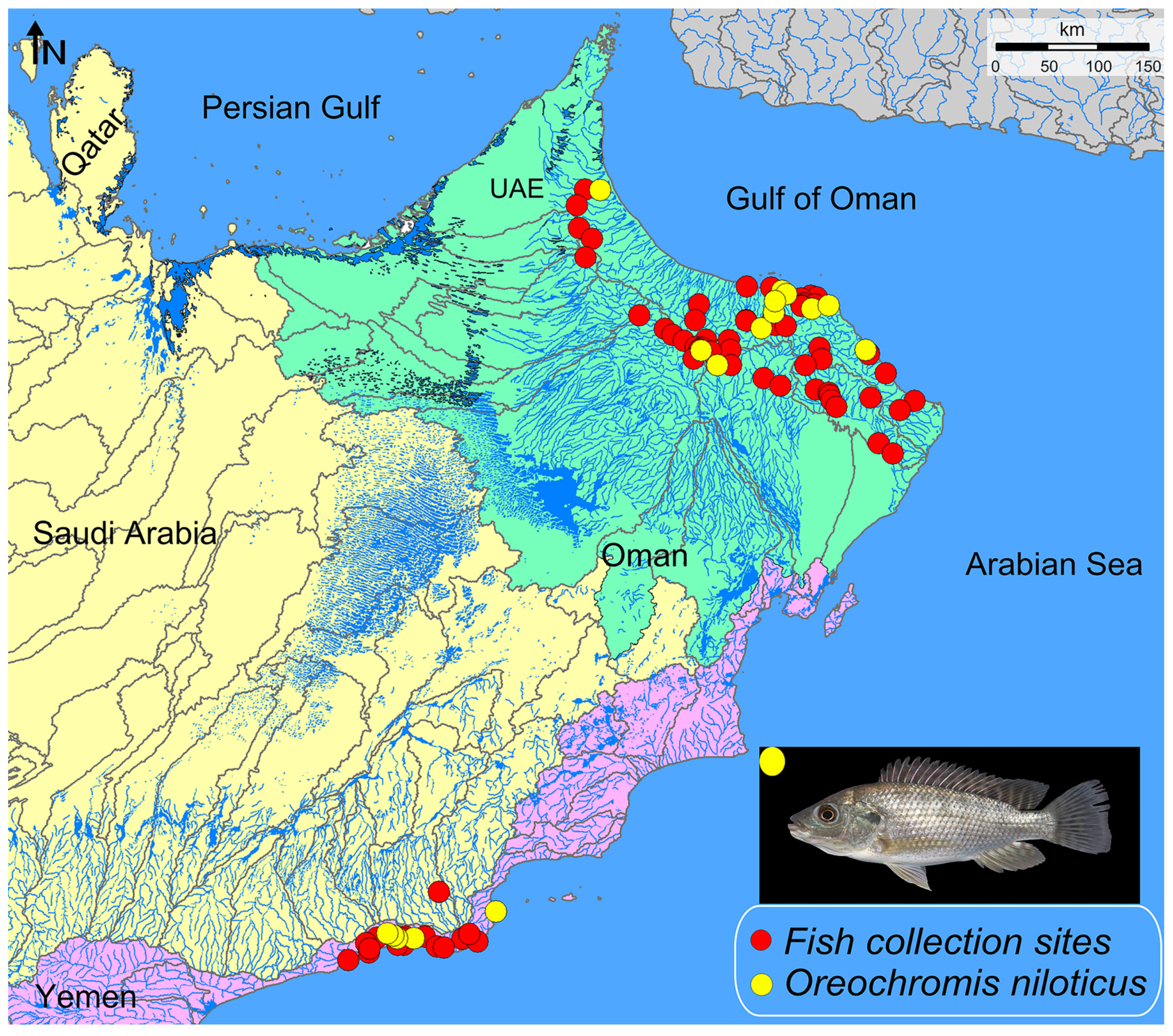
2.1. Specimen Data Sources and Occurrence Records
2.2. Environmental Variables
2.3. Establishment of Single-Species Distribution Model
2.4. Model Evaluation
2.5. Ensemble Model (EM) Construction
3. Results
3.1. Mapping the Occurrence Records
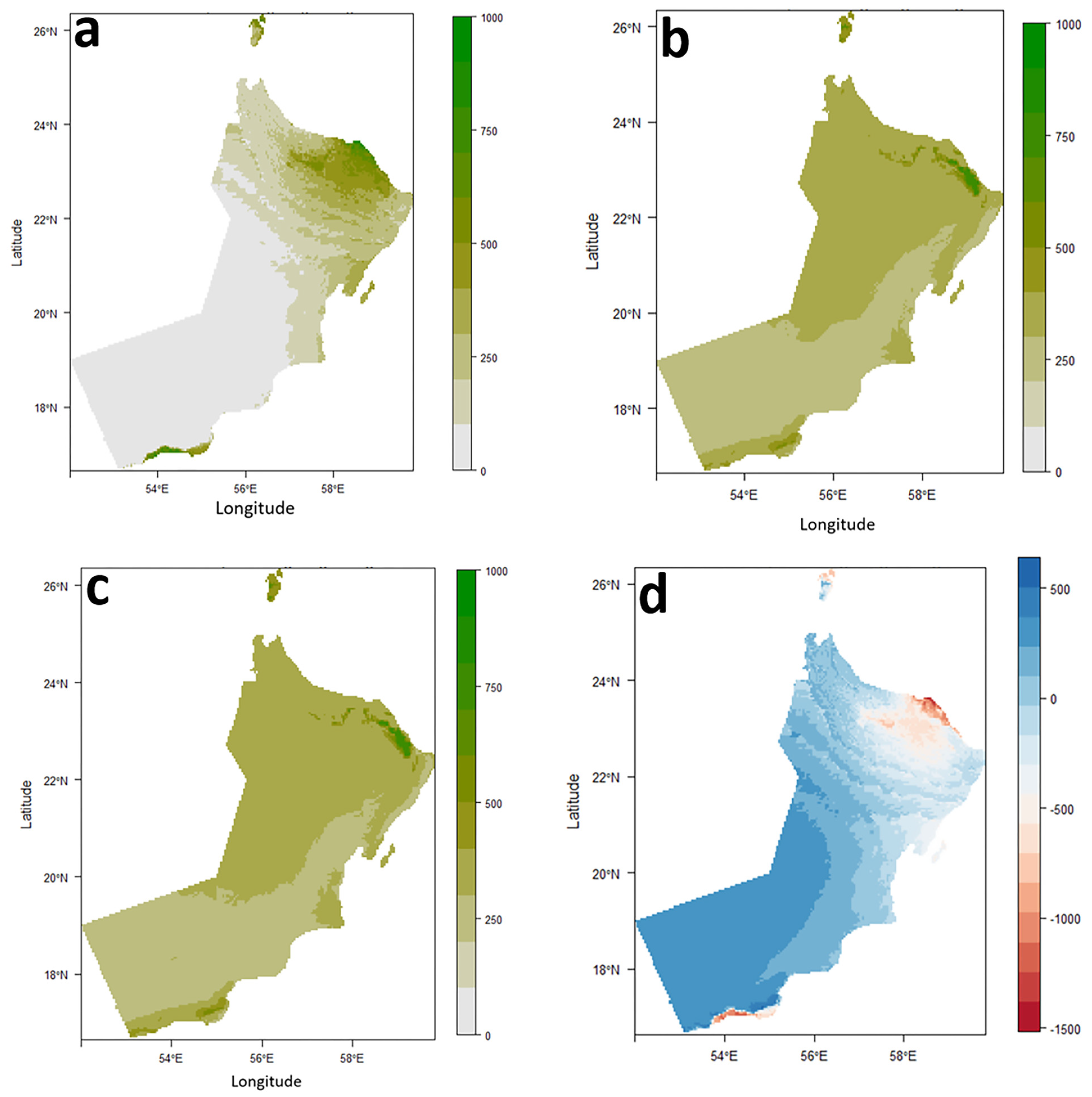
3.2. Ensemble Modeling and Predicted Current and Future Distribution
3.3. Nile Tilapia and Co-Occurrence with Indigenous Fishes
4. Discussion
4.1. Distribution Pattern
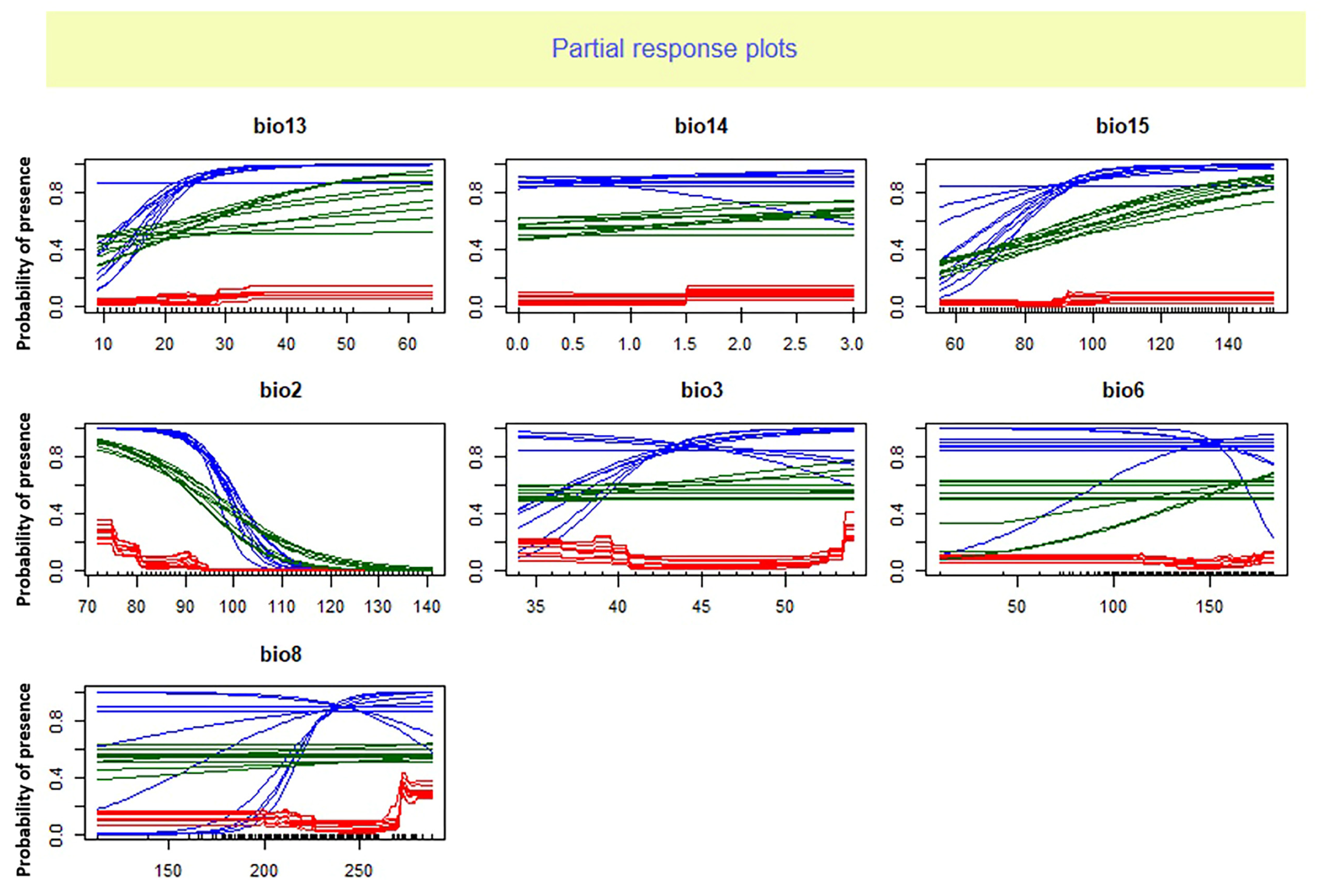
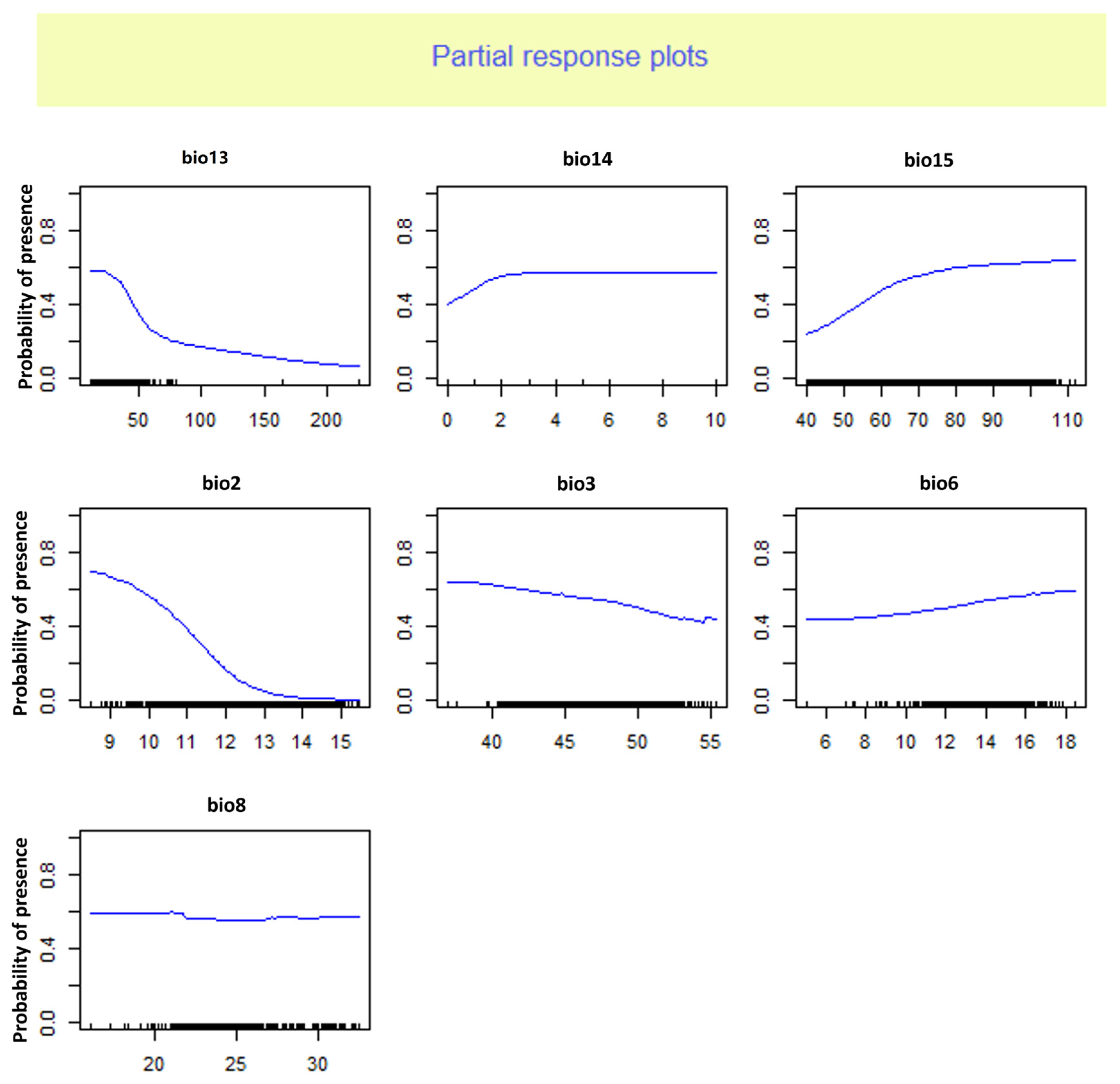
4.2. Ensemble Model Evaluation and Dominant Climatic Factor
4.3. Nile Tilapia as an Introduced Bioinvasive Species and Its Co-Occurrence with Other Fishes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunders, D.L.; Meeuwig, J.J.; Vincent, A.C.J. Freshwater protected areas: Strategies for conservation. Conserv. Biol. 2002, 16, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Leveque, C. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.A.; Ford, B.M.; Benson, J.A. Using species distribution modelling to identify ‘coldspots’ for conservation of freshwater fishes under a changing climate. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 576–590. [Google Scholar] [CrossRef]
- Davis, J.; O’Grady, A.P.; Dale, A.; Arthington, A.H.; Gell, P.A.; Driver, P.D.; Bond, N.; Casanova, M.; Finlayson, M.; Watts, R.J.; et al. When trends intersect: The challenge of protecting freshwater ecosystems under multiple land use and hydrological intensification scenarios. Sci. Total Environ. 2015, 534, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Saemi-Komsari, M.; Esmaeili, H.R.; Keshavarzi, B.; Abbasi, K.; Birami, F.A.; Nematollahi, M.J.; Tayefeh, F.H.; Busquets, R. Characterization of ingested MPs and their relation with growth parameters of endemic and invasive fish from a coastal wetland. Sci. Total Environ. 2023, 860, 160495. [Google Scholar] [CrossRef]
- Elliott, M. Biological pollutants and biological pollution—An increasing cause for concern. Mar. Pollut. Bull. 2003, 46, 275–280. [Google Scholar] [CrossRef]
- Santamarina, S.; Alfaro-Saiz, E.; Llamas, F.; Acedo, C. Different approaches to assess the local invasion risk on a threatened species: Opportunities of using high-resolution species distribution models by selecting the optimal model complexity. Glob. Ecol. Conserv. 2019, 20, e00767. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef]
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef]
- Renault, D.; Leclerc, C.; Colleu, M.A.; Boutet, A.; Hotte, H.; Colinet, H.; Chown, S.L.; Convey, P. The rising threat of climate change for arthropods from Earth’s cold regions: Taxonomic rather than native status drives species sensitivity. Glob. Chang. Biol. 2022, 28, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.L.; D’antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Ogden, N.H.; Wilson, J.R.; Richardson, D.M.; Hui, C.; Davies, S.J.; Kumschick, S.; Le Roux, J.J.; Measey, J.; Saul, W.C.; Pulliam, J.R. Emerging infectious diseases and biological invasions: A call for a One Health collaboration in science and management. R. Soc. Open Sci. 2019, 6, 181577. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.; Cuthbert, R.N.; Kouba, A.; Capinha, C.; Turbelin, A.; Hudgins, E.J.; Diagne, C.; Courchamp, F.; Haubrock, P.J. Global economic costs of herpetofauna invasions. Sci. Rep. 2022, 12, 10829. [Google Scholar] [CrossRef]
- Grammer, G.L.; Slack, W.T.; Peterson, M.S.; Dugo, M.A. Nile tilapia Oreochromis niloticus (Linnaeus, 1758) establishment in temperate Mississippi, USA: Multi-year survival confirmed by otolith ages. Aquat. Invasions 2012, 7, 367–376. [Google Scholar] [CrossRef]
- Freyhof, J.Ö.R.G.; Yoğurtçuoğlu, B. A proposal for a new generic structure of the killifish family Aphaniidae, with the description of Aphaniops teimorii (Teleostei: Cyprinodontiformes). Zootaxa 2020, 4810, 421–451. [Google Scholar] [CrossRef]
- Shuai, F.; Li, J. Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) Invasion Caused Trophic Structure Disruptions of Fish Communities in the South China River—Pearl River. Biology 2022, 11, 1665. [Google Scholar] [CrossRef]
- Zengeya, T.A.; Robertson, M.P.; Booth, A.J.; Chimimba, C.T. Ecological niche modeling of the invasive potential of Nile tilapia Oreochromis niloticus in African river systems: Concerns and implications for the conservation of indigenous congenerics. Biol. Invasions 2013, 15, 1507–1521. [Google Scholar] [CrossRef]
- Stauffer, J.R., Jr.; Chirwa, E.R.; Jere, W.; Konings, A.F.; Tweddle, D.; Weyl, O. Nile Tilapia, Oreochromis niloticus (Teleostei: Cichlidae): A threat to native fishes of Lake Malawi? Biol. Invasions 2022, 24, 1585–1597. [Google Scholar] [CrossRef]
- Lodge, D.M.; Williams, S.; MacIsaac, H.J.; Hayes, K.R.; Leung, B.; Reichard, S. Biological invasions: Recommendations for US policy and management. Ecol. Appl. 2006, 16, 2035–2054. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Hamza, W.; Munawar, M. Protecting and managing the Arabian Gulf: Past, present and future. Aquat. Ecosyst. Health Manag. 2009, 12, 429–439. [Google Scholar] [CrossRef]
- Esmaeili, H.R.; Al Jufaili, S.; Masoumi, A.H.; Zarei, F. Ichthyodiversity in southeastern Arabian Peninsula: Annotated checklist, taxonomy, short description and distribution of iInland fishes of Oman. Zootaxa 2022, 5134, 451–503. [Google Scholar] [CrossRef]
- Esmaeili, H.R.; Hamidan, N. Inland fishes of the Arabian Peninsula: Review and a revised checklist. Zootaxa 2023, 5330, 201–226. [Google Scholar] [CrossRef]
- De Oliveira da Conceição, E.; Mantovano, T.; de Campos, R.; Rangel, T.F.; Martens, K.; Bailly, D.; Higuti, J. Mapping the observed and modelled intracontinental distribution of non-marine ostracods from South America. Hydrobiologia 2020, 847, 1663–1687. [Google Scholar] [CrossRef]
- Franklin, J. Predictive vegetation mapping: Geographic modelling of biospatial patterns in relation to environmental gradients. Prog. Phys. Geogr. 1995, 19, 474–499. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Modell. 2020, 135, 147–186. [Google Scholar] [CrossRef]
- Guisan, A.; Graham, C.H.; Elith, J.; Huettmann, F. The NCEAS Species Distribution Modelling Group. Sensitivity of predictive species distribution models to change in grain size. Divers. Distrib. 2017, 13, 332–340. [Google Scholar] [CrossRef]
- Ruiz-Navarro, A.; Gillingham, P.K.; Britton, J.R. Predicting shifts in the climate space of freshwater fishes in Great Britain due to climate change. Biol. Conserv. 2016, 203, 33–42. [Google Scholar] [CrossRef]
- Rathore, P.; Roy, A.; Karnatak, H. Modelling the vulnerability of Taxus wallichiana to climate change scenarios in South East Asia. Ecol. Indic. 2019, 102, 199–207. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Xu, B.; Xue, Y.; Ren, Y. How to predict biodiversity in space? An evaluation of modeling approaches in marine ecosystems. Divers. Distrib. 2019, 25, 1697–1708. [Google Scholar] [CrossRef]
- Pearson, R.G.; Thuiller, W.; Araújo, M.B.; Martínez-Meyer, E.; Brotons, L.; McClean, C.; Miles, L.; Segurado, P.; Dawson, T.; Lees, D.C. Model-based uncertainty in species range prediction. J. Biogeogr. 2006, 33, 1704–1711. [Google Scholar] [CrossRef]
- Ardestani, E.G.; Rigi, H.; Honarbakhsh, A. Predicting optimal habitats of Haloxylon persicum for ecosystem restoration using ensemble ecological niche modeling under climate change in southeast Iran. Restor. Ecol. 2021, 29, e13492. [Google Scholar] [CrossRef]
- Thuiller, W. BIOMOD-Optimizing predictions of species distributions and projecting potential future shifts under global change. Glob. Chang. Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, X.; Zhang, J.; Zhao, J.; Ge, Z.; Zhang, Z. Predicting the Potential Suitable Distribution of Larix principis-rupprechtii Mayr under Climate Change Scenarios. Forests 2022, 13, 1428. [Google Scholar] [CrossRef]
- Lehner, B.; Grill, G. Global river hydrography and network routing: Baseline data and new approaches to study the world’s large river systems. Hydrol. Process. 2013, 27, 2171–2186. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Petrie, R.; Denvil, S.; Ames, S.; Levavasseur, G.; Fiore, S.; Allen, C.; Antonio, F.; Berger, K.; Bretonnière, P.A.; Cinquini, L. Coordinating an operational data distribution network for CMIP6 data. Geosci. Model Dev. 2021, 14, 629–644. [Google Scholar] [CrossRef]
- Hamed, M.M.; Nashwan, M.S.; Shahid, S.; bin Ismail, T.; Wang, X.J.; Dewan, A.; Asaduzzaman, M. Inconsistency in historical simulations and future projections of temperature and rainfall: A comparison of CMIP5 and CMIP6 models over Southeast Asia. Atmos. Res. 2022, 265, 105927. [Google Scholar] [CrossRef]
- Wu, T.; Yu, R.; Lu, Y.; Jie, W.; Fang, Y.; Zhang, J.; Zhang, L.; Xin, X.; Li, L.; Wang, Z. BCC-CSM2-HR: A high-resolution version of the Beijing Climate Center Climate System Model. Geosci. Model Dev. 2021, 14, 2977–3006. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F.; Georges, M.D.; Thuiller, C.W. Package ‘biomod2’. Species Distribution Modeling within an Ensemble Forecasting Framework. Ecography 2016, 32, 369–373. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Xu, X. GC-LSTM: Graph convolution embedded LSTM for dynamic network link prediction. Appl. Intell. 2022, 52, 7513–7528. [Google Scholar] [CrossRef]
- Sayyadzadeh, G.; Al Jufaili, S.M.; Esmaeili, H.R. Species diversity deflation: Insight into taxonomic validity of Garra species (Teleostei: Cyprinidae) from Dhofar Region in the Arabian Peninsula using an integrated morpho-molecular approach. Zootaxa 2023, 5230, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Zarei, F.; Jufaili, S.M.A.; Esmaeili, H.R. Oxyurichthys omanensis sp. nov., a new Eyebrow Goby (Teleostei: Gobiidae) from Oman. Zootaxa 2022, 5182, 361–376. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. 2023. Available online: www.fishbase.org (accessed on 21 August 2023).
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. 2023. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 20 March 2023).
- Shuai, F.; Li, J.; Lek, S. Nile tilapia (Oreochromis niloticus) invasion impacts trophic position and resource use of commercially harvested piscivorous fishes in a large subtropical river. Ecol. Process. 2023, 12, 22. [Google Scholar] [CrossRef]
- Trewevas, E. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis and Danakilia; British Museum Natural History: London, UK, 1983; p. 583. [Google Scholar]
- Lind, C.E.; Agyakwah, S.K.; Attipoe, F.Y.; Nugent, C.; Crooijmans, R.P.; Toguyeni, A. Genetic diversity of Nile tilapia (Oreochromis niloticus) throughout West Africa. Sci. Rep. 2019, 9, 16767. [Google Scholar] [CrossRef]
- Lake, T.A.; Briscoe Runquist, R.D.; Moeller, D.A. Predicting range expansion of invasive species: Pitfalls and best practices for obtaining biologically realistic projections. Divers. Distrib. 2020, 26, 1767–1779. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Wei, H.; Ran, Q.; Liu, J.; Zhang, Q.; Gu, W. Potential distribution of Notopterygium incisum Ting ex H.T. Chang and its predicted responses to climate change based on a comprehensive habitat suitability model. Ecol. Evol. 2020, 10, 3004–3016. [Google Scholar] [CrossRef]
- Chege, S.; Walingo, T. Multiplexing capacity of hybrid PD-SCMA heterogeneous networks. IEEE Trans. Veh. Technol. 2022, 71, 6424–6438. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, S.C.; Verma, P. MaxEnt distribution modeling for predicting Oreochromis niloticus invasion into the Ganga river system, India and conservation concern of native fish biodiversity. Aquat. Ecosyst. Health Manag. 2021, 24, 43–51. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Wang, X.; Wang, M.; He, R.; Wang, M. Distribution Pattern of Fish Richness in the Yarlung Zangbo River Basin. Diversity 2022, 14, 1142. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lymbery, A.J.; Morine, M.; Kanani, H.G.; Beatty, S.J.; Morgan, D.L. Co-invaders: The effects of alien parasites on native hosts. Int. J. Parasitol. Parasites Wildl. 2014, 3, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gozlan, R.E.; Andreou, D.; Asaeda, T.; Beyer, K.; Bouhadad, R.; Burnard, D.; Caiola, N.; Cakic, P.; Djikanovic, V.; Esmaeili, H.R.; et al. Pan-continental invasion of Pseudorasbora parva: Towards a better understanding of freshwater fish invasions. Fish Fish. 2010, 11, 315–340. [Google Scholar] [CrossRef]
- Esmaeili, H.R.; Teimori, A.; Owfi, F.; Abbasi, K.; Brian, W.C. Alien and invasive freshwater fish species in Iran: Diversity, environmental impacts and management. Iran. J. Ichthyol. 2014, 1, 61–72. [Google Scholar]
- Esmaeili, H.R. Exotic, and Invasive Freshwater Fishes in the Tigris-Euphrates River System. In Tigris and Euphrates Rivers: Their Environment from Headwaters to Mouth; Springer: Cham, Switzerland, 2021; pp. 1103–1140. [Google Scholar]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2016, 44, 32–38. [Google Scholar] [CrossRef]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M. The effects of introduced tilapias on native biodiversity. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Tilapia Culture; CABI Publishing: Wallingford, UK, 2006. [Google Scholar]
- Suresh, V.; Bhujel, C. Tilapias. Aquaculture: Farming Aquatic Animals and Plants, 2nd ed.; Wiley-Blackwell Publishing: Chichester, UK, 2012; pp. 338–364. [Google Scholar]
- Rairat, T.; Liu, Y.K.; Hsu, J.C.N.; Hsieh, C.Y.; Chuchird, N.; Chou, C.C. Combined effects of temperature and salinity on the pharmacokinetics of florfenicol in Nile tilapia (Oreochromis niloticus) reared in brackish water. Front. Vet. Sci. 2022, 9, 826586. [Google Scholar] [CrossRef]
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Rejmanek, M.; Westbrooks, R. Introduced species: A significant component of human-caused global change. N. Z. J. Ecol. 1997, 21, 1–16. [Google Scholar]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. The effects of invasive species on the decline in species richness: A global meta-analysis. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2017; Volume 56, pp. 61–83. [Google Scholar]
- Mooney, H.A.; Hobbs, R.J. Invasive Species in a Changing World; Island Press: Washington, DC, USA, 2000; 384p. [Google Scholar]
- Catford, J.A.; Bode, M.; Tilman, D. Introduced species that overcome life history tradeoffs can cause native extinctions. Nat. Commun. 2018, 9, 2131. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Seedling establishment of a boreal tree species (Pinus sylvestris) at its southernmost distribution limit: Consequences of being in a marginal Mediterranean habitat. J. Ecol. 2004, 92, 266–277. [Google Scholar] [CrossRef]
- Gu, D.E.; Ma, G.M.; Zhu, Y.J.; Xu, M.; Luo, D.; Li, Y.Y.; Wei, H.; Mu, X.D.; Luo, J.R.; Hu, Y.C. The impacts of invasive Nile tilapia (Oreochromis niloticus) on the fisheries in the main rivers of Guangdong Province, China. Biochem. Syst. Ecol. 2015, 59, 1–7. [Google Scholar] [CrossRef]
| GLM | MAXENT | RF | SRE | Relative Importance | |
|---|---|---|---|---|---|
| Bio13 | 0.1766 | 0.0182 | 0.0435 | 0.3693 | 0.6076 |
| Bio14 | 0.3833 | 0 | 0.0087 | 0.2147 | 0.151675 |
| Bio15 | 0.4243 | 0.0198 | 0.0503 | 0.3726 | 0.21675 |
| Bio2 | 0.892 | 0.9965 | 0.2334 | 0.7814 | 0.725825 |
| Bio3 | 0.0954 | 0.0014 | 0.1253 | 0.0215 | 0.0609 |
| Bio6 | 0.0295 | 0.0003 | 0.088 | 0.2022 | 0.08 |
| Bio8 | 0.0041 | 0 | 0.0594 | 0.1347 | 0.04955 |
| Order | Family | Species | Authorship/s | Status | |
|---|---|---|---|---|---|
| 1 | Cypriniformes | Cyprinidae | Cyprinion muscatense * | (Boulenger, 1888) | Native |
| 2 | Cyprinidae | Garra barreimiae | (Fowler & Steinitz, 1956) | Native | |
| 3 | Cyprinidae | Garra dunsirei * | (Banister, 1987) | Endemic | |
| 4 | Cyprinidae | Garra gallagheri * | (Krupp, 1988) | Endemic | |
| 5 | Cyprinidae | Garra longipinnis * | (Banister & Clarke, 1977) | Endemic | |
| 6 | Cyprinidae | Garra shamal * | (Kirchner, Kruckenhauser, Pichler, Borkenhagen & Freyhof 2020) | Endemic | |
| 7 | Cyprinidae | Garra sharq * | (Kirchner, Kruckenhauser, Pichler, Borkenhagen & Freyhof 2020) | Endemic | |
| 8 | Cyprinodontiformes | Aphaniidae | Aphaniops kruppi * | (Freyhof, Weissenbacher & Geiger, 2017) | Endemic |
| 9 | Aphaniidae | Aphaniops stoliczkanus * | (Day, 1872) | Native | |
| 10 | Poeciliidae | Poecilia latipinna * | (Lesueur, 1821) | Exotic/Invasive | |
| 11 | Gobiiformes | Gobiidae | Awaous jayakari * | (Boulenger 1888) | Native |
| 12 | Gobiidae | Cryptocentroides arabicus * | (Gmelin, 1789) | Native | |
| 13 | Gobiidae | Favonigobius melanobranchus | (Fowler, 1934) | Native | |
| 14 | Gobiidae | Glossogobius tenuiformis * | (Fowler, 1934) | Native | |
| 15 | Gobiidae | Oxyurichthys omanensis | (Zarei, Al Jufaili & Esmaeili, 2022) | ||
| 16 | Eleotridae | Ophiocara porocephala | (Valenciennes, 1837) | Native | |
| 17 | Eleotridae | Eleotris acanthompus | (Bleeker, 1853) | Native | |
| 18 | Centrarchiformes | Terapontidae | Terapon jarbua * | (Forsskål, 1775) | Native |
| 19 | Cichliformes | Ambassidae | Ambassis gymnocephalus * | (Lacepède, 1802) | Native |
| 20 | Cichlidae | Oreochromis niloticus | (Linnaeus, 1758) | Exotic/Invasive | |
| 21 | Gonorynchiformes | Chanidae | Chanos chanos | (Forsskål, 1775) | Native |
| 22 | Mugiliformes | Mugilidae | Planiliza macrolepis | (Smith, 1846) | Native |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmaeili, H.R.; Eslami Barzoki, Z. Climate Change May Impact Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) Distribution in the Southeastern Arabian Peninsula through Range Contraction under Various Climate Scenarios. Fishes 2023, 8, 481. https://doi.org/10.3390/fishes8100481
Esmaeili HR, Eslami Barzoki Z. Climate Change May Impact Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) Distribution in the Southeastern Arabian Peninsula through Range Contraction under Various Climate Scenarios. Fishes. 2023; 8(10):481. https://doi.org/10.3390/fishes8100481
Chicago/Turabian StyleEsmaeili, Hamid Reza, and Zohreh Eslami Barzoki. 2023. "Climate Change May Impact Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) Distribution in the Southeastern Arabian Peninsula through Range Contraction under Various Climate Scenarios" Fishes 8, no. 10: 481. https://doi.org/10.3390/fishes8100481
APA StyleEsmaeili, H. R., & Eslami Barzoki, Z. (2023). Climate Change May Impact Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) Distribution in the Southeastern Arabian Peninsula through Range Contraction under Various Climate Scenarios. Fishes, 8(10), 481. https://doi.org/10.3390/fishes8100481