Which Fish Benefit from the Combined Influence of Eutrophication and Warming in the Dnipro River (Ukraine)?
Abstract
1. Introduction
2. Materials and Methods
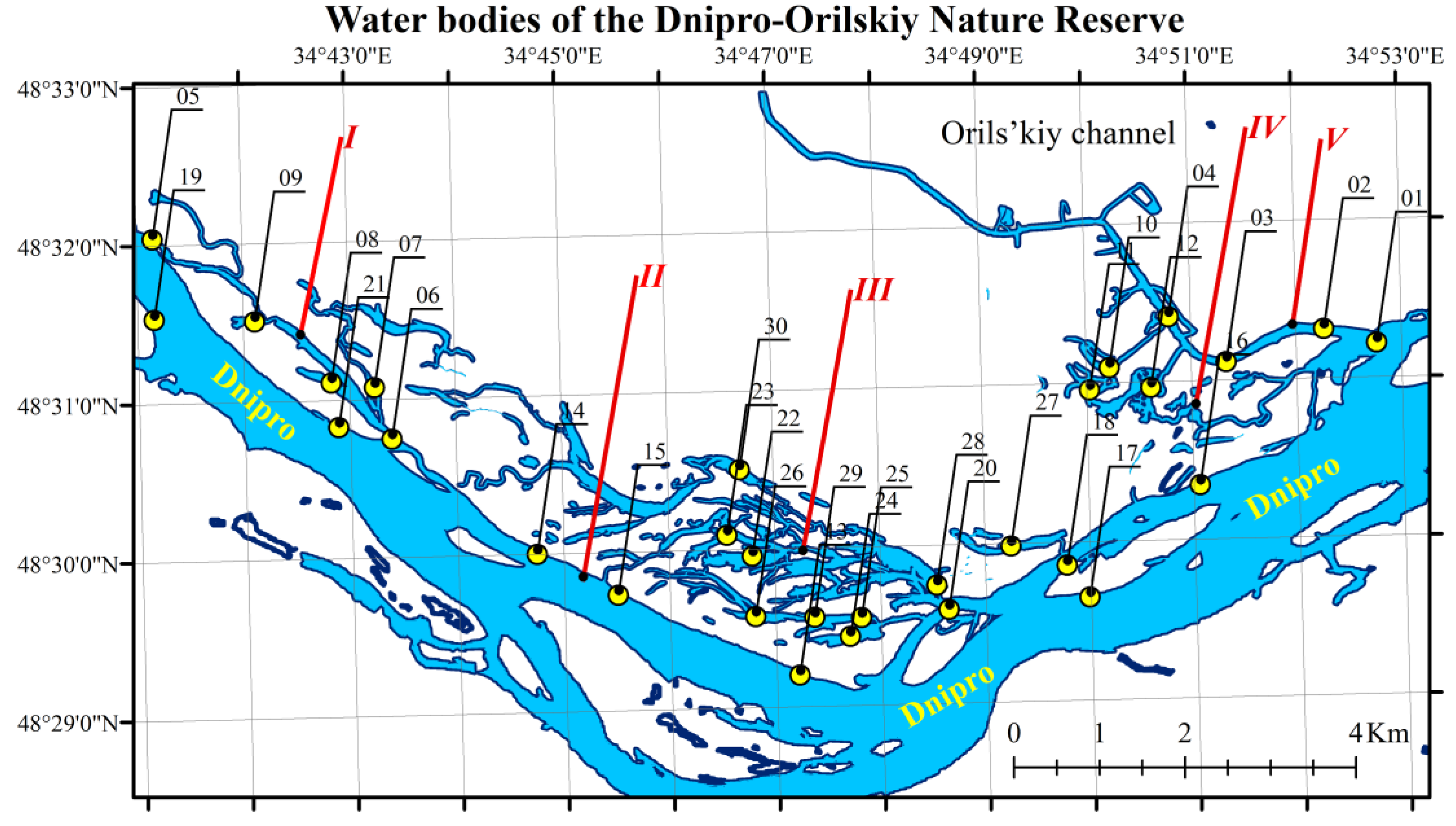
2.1. Study Sites
2.2. Fish Sampling
2.3. Fish Traits
2.4. Landsat 5/TM, 7/ETM, and 8 OLI Data
2.5. Remote Sensing Estimation of Chlorophyll-a Concentration
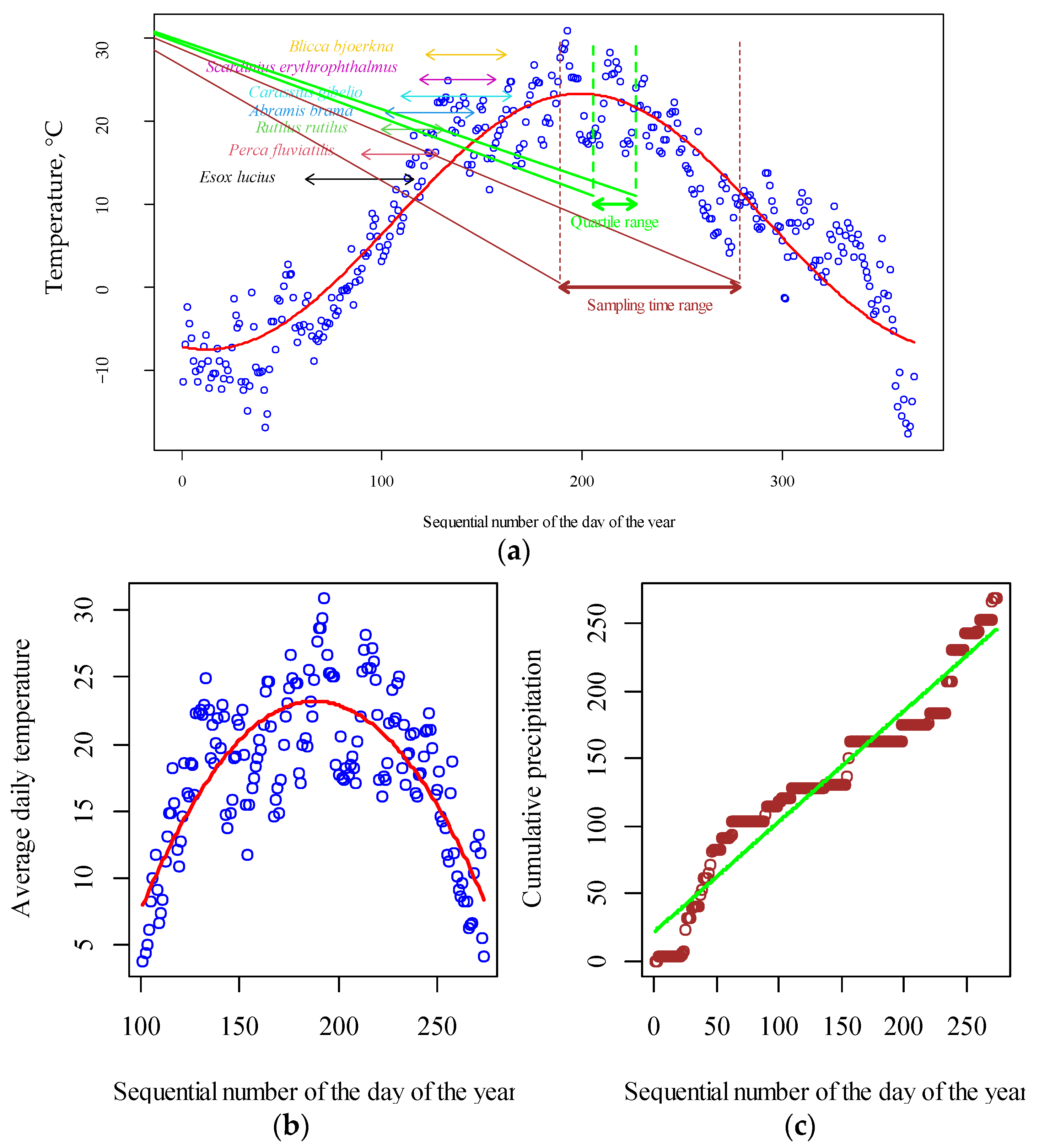
2.6. Climatic Data
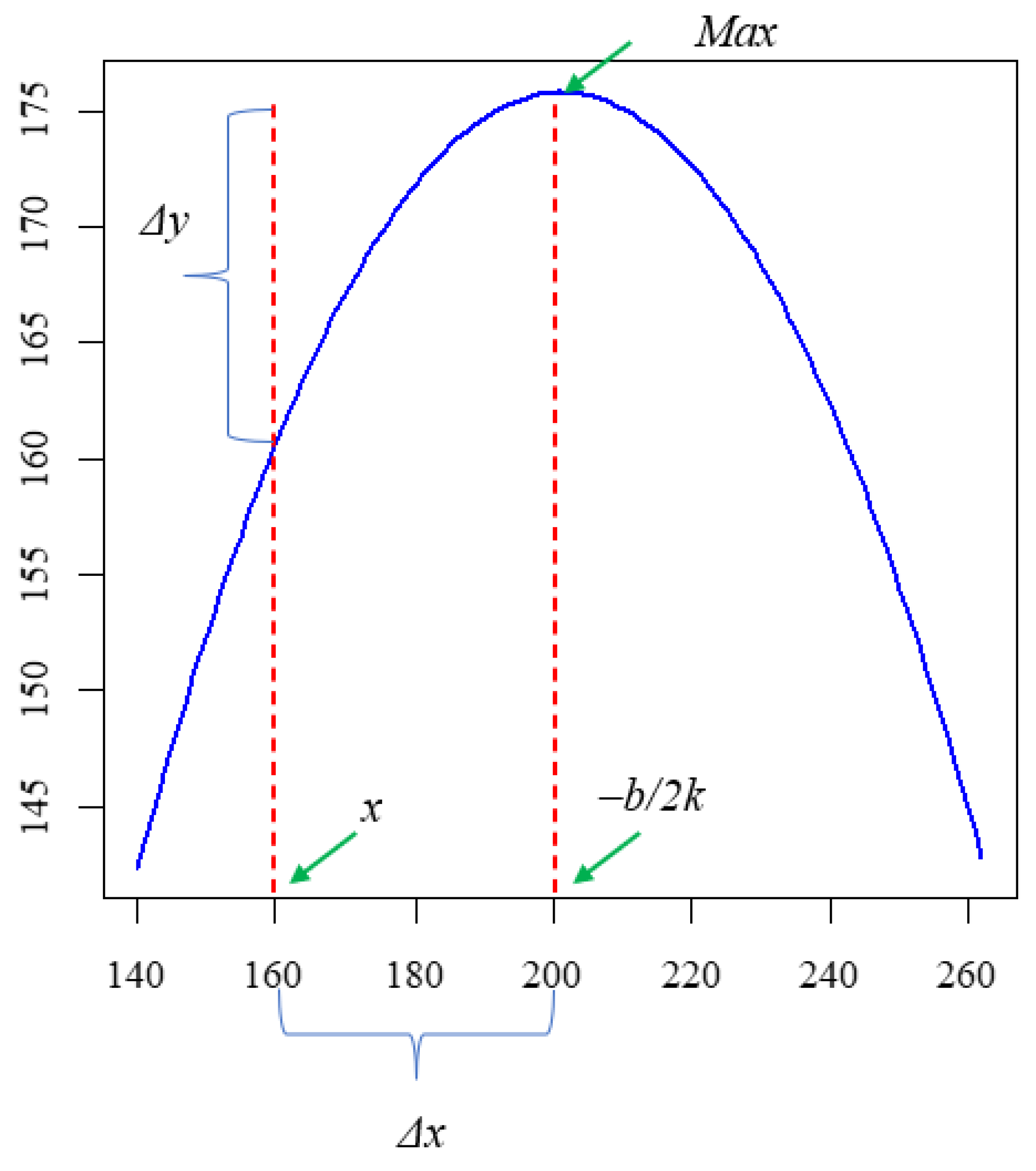
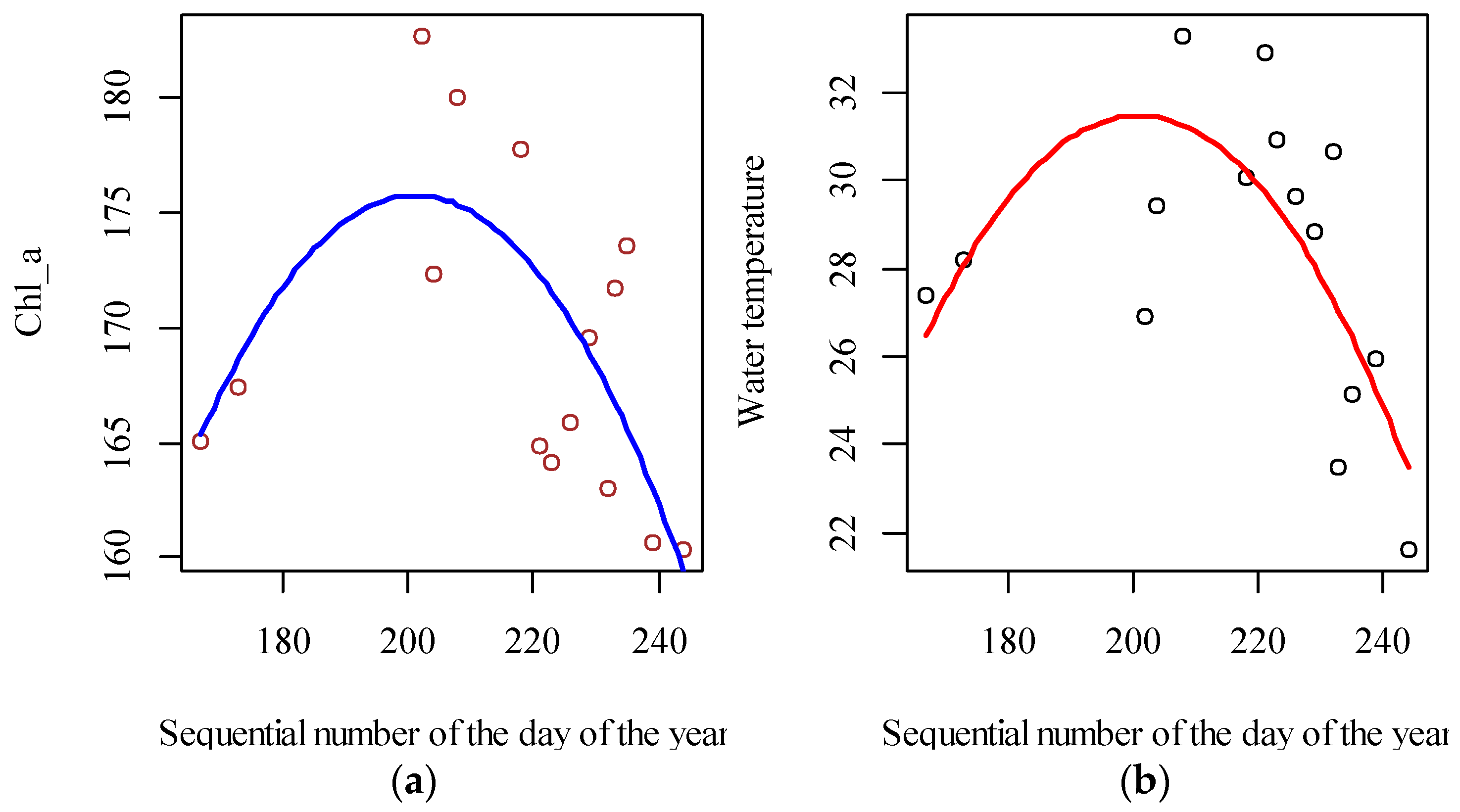
2.7. Chlorophyll-a Concentration and Water Temperature Correction
2.8. Data Analysis
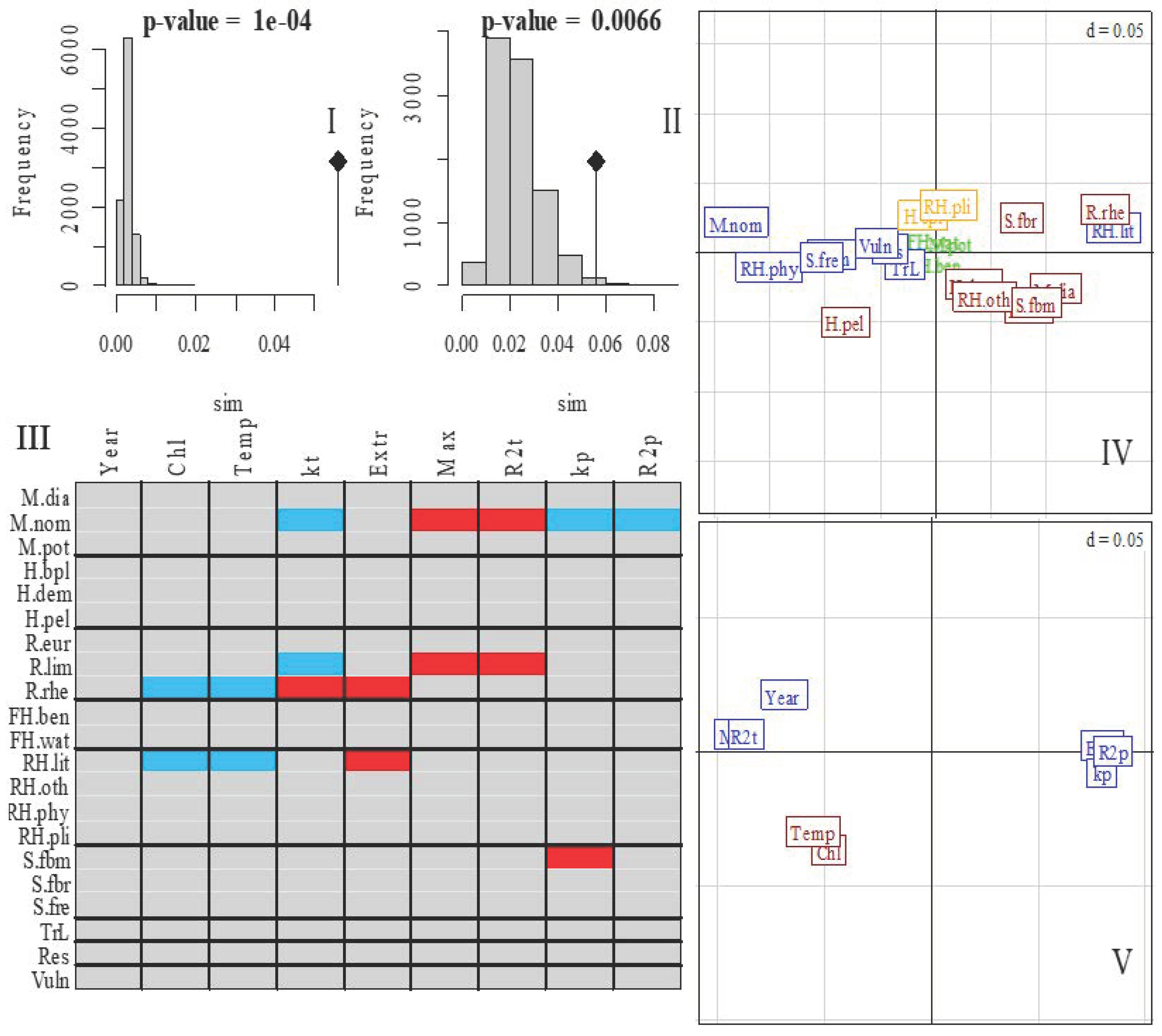
3. Results
3.1. Temporal Variability of Climatic Regime and Eutrophication Level of Water Bodies
3.2. Fish Community Diversity
3.3. Fish Community Ordination
3.4. Interpretation of Clusters in Terms of Species Traits
4. Discussion
4.1. The Role of Climatic Factors in the Dynamics of Eutrophication
4.2. Effects of Global Warming on Fish Communities
4.3. Effect of Eutrophication on the Fish Community
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Species | Migration (M) | Habitat Preference (H) | Water Velocity Preferences (R) | Feeding Habitat (FH) | Reproduction Habitat (RH) | Salinity (S) | Trophic Level (TrL) | Resilience (Res) | Vulnerability (Vuln) |
|---|---|---|---|---|---|---|---|---|---|
| Abramis brama (Linnaeus, 1758) | pot | bpl | rhe | ben | pli | fbr | 3.1 | 6.0 | 62 |
| Acipenser ruthenus Linnaeus, 1758 * | pot | dem | rhe | ben | lit | fbr | 3.6 | 9.5 | 64 |
| Alburnus alburnus (Linnaeus, 1758) | pot | bpl | rhe | wat | lit | fbr | 2.7 | 2.0 | 31 |
| Alosa immaculata Bennett, 1835 * | dia | pel | lim | wat | lit | fbm | 3.9 | 1.5 | 35 |
| Anguilla anguilla (Linnaeus, 1758) | dia | dem | eur | ben | oth | fbm | 3.6 | 7.9 | 64 |
| Atherina boyeri Risso, 1810 | dia | dem | lim | wat | pli | fbm | 3.2 | 1.3 | 20 |
| Babka gymnotrachelus (Kessler, 1857) | nom | dem | lim | ben | phy | fre | 3.5 | 2.9 | 29 |
| Ballerus ballerus (Linnaeus, 1758) | pot | bpl | lim | wat | phy | fbr | 3.2 | 3.9 | 49 |
| Benthophiloides brauneri Beling & Iljin, 1927 * | dia | dem | lim | ben | oth | fbr | 3.4 | 2.4 | 28 |
| Benthophilus stellatus (Sauvage, 1874) | pot | dem | lim | ben | lit | fbr | 3.7 | 3.4 | 34 |
| Blicca bjoerkna (Linnaeus, 1758) | pot | dem | lim | ben | phy | fre | 3.2 | 5.5 | 65 |
| Carassius carassius (Linnaeus, 1758) | pot | dem | lim | ben | phy | fre | 3.1 | 3.2 | 38 |
| Carassius gibelio (Bloch, 1782) | nom | bpl | lim | ben | pli | fre | 2.5 | 3.9 | 51 |
| Chondrostoma nasus (Linnaeus, 1758) * | pot | bpl | rhe | ben | lit | fre | 2.0 | 4.0 | 48 |
| Clupeonella cultriventris (Nordmann, 1840) | dia | pel | eur | wat | oth | fbm | 3.0 | 4.7 | 35 |
| Cobitis taenia Linnaeus, 1758 | pot | dem | lim | ben | pli | fre | 3.3 | 1.5 | 36 |
| Ctenopharyngodon idella (Valenciennes, 1844) * | pot | dem | rhe | wat | oth | fre | 2.0 | 6.0 | 65 |
| Cyprinus carpio Linnaeus, 1758 | pot | bpl | eur | ben | phy | fbr | 3.1 | 4.4 | 46 |
| Esox lucius Linnaeus, 1758 | pot | pel | eur | wat | phy | fre | 4.1 | 8.0 | 85 |
| Gasterosteus aculeatus Linnaeus, 1758 | dia | dem | lim | wat | oth | fbm | 3.3 | 2.1 | 10 |
| Gobio gobio (Linnaeus, 1758) * | pot | bpl | rhe | ben | psa | fbr | 3.1 | 2.9 | 31 |
| Gymnocephalus cernua (Linnaeus, 1758) | pot | dem | eur | ben | pli | fbr | 3.3 | 3.2 | 20 |
| Hypophthalmichthys molitrix (Valenciennes, 1844) * | pot | bpl | eur | wat | oth | fbr | 2.0 | 4.7 | 55 |
| Hypophthalmichthys nobilis (Richardson, 1845) * | pot | bpl | eur | wat | oth | fre | 2.8 | 5.3 | 66 |
| Lepomis gibbosus (Linnaeus, 1758) | pot | bpl | lim | wat | pli | fre | 3.3 | 3.0 | 32 |
| Leucaspius delineatus (Heckel, 1843) | pot | pel | lim | wat | phy | fre | 3.2 | 5.2 | 58 |
| Leuciscus aspius (Linnaeus, 1758) * | pot | bpl | rhe | wat | lit | fbr | 4.5 | 6.5 | 70 |
| Leuciscus idus (Linnaeus, 1758) * | pot | bpl | rhe | wat | pli | fbr | 3.8 | 7.0 | 63 |
| Leuciscus leuciscus (Linnaeus, 1758) | pot | bpl | rhe | wat | lit | fbr | 2.9 | 3.2 | 48 |
| Lota lota (Linnaeus, 1758)* | pot | dem | eur | ben | lit | fbr | 4.1 | 6.0 | 66 |
| Mesogobius batrachocephalus (Pallas, 1814) | dia | bpl | lim | ben | oth | fbr | 4.2 | 2.9 | 33 |
| Misgurnus fossilis (Linnaeus, 1758) | pot | dem | lim | ben | phy | fre | 3.4 | 3.4 | 31 |
| Neogobius fluviatilis (Pallas, 1814) | dia | bpl | rhe | ben | lit | fbr | 3.4 | 2.4 | 21 |
| Neogobius melanostomus (Pallas, 1814) | dia | dem | lim | ben | oth | fbm | 3.3 | 2.9 | 31 |
| Pelecus cultratus (Linnaeus, 1758) | dia | pel | lim | wat | oth | fbr | 3.6 | 4.8 | 50 |
| Perca fluviatilis Linnaeus, 1758 | dia | dem | eur | wat | pli | fre | 3.4 | 5.3 | 50 |
| Petroleuciscus borysthenicus (Kessler, 1859) | nom | bpl | lim | ben | phy | fre | 3.1 | 2.9 | 32 |
| Ponticola kessleri (Günther, 1861) | nom | bpl | rhe | wat | pli | fbr | 3.5 | 2.9 | 30 |
| Proterorhinus marmoratus (Pallas, 1814) | dia | dem | eur | ben | lit | fbm | 3.5 | 1.9 | 15 |
| Pseudorasbora parva (Temminck & Schlegel, 1846) | nom | bpl | lim | wat | phy | fre | 3.2 | 2.0 | 29 |
| Pungitius platygaster (Kessler, 1859) | dia | dem | lim | ben | oth | fbm | 3.5 | 2.3 | 14 |
| Rhodeus sericeus (Pallas, 1776) | pot | bpl | lim | ben | oth | fre | 2.1 | 2.0 | 19 |
| Rutilus rutilus (Linnaeus, 1758) | pot | bpl | rhe | wat | lit | fbr | 3.0 | 4.5 | 53 |
| Sander lucioperca (Linnaeus, 1758) | nom | pel | eur | wat | phy | fbr | 4.0 | 7.0 | 73 |
| Sander volgensis (Gmelin, 1789) * | nom | dem | lim | wat | pli | fbr | 4.1 | 7.3 | 52 |
| Scardinius erythrophthalmus (Linnaeus, 1758) | nom | bpl | lim | wat | phy | fbr | 3.6 | 5.5 | 67 |
| Silurus glanis Linnaeus, 1758 | nom | dem | eur | ben | phy | fbr | 4.4 | 7.3 | 84 |
| Squalius cephalus (Linnaeus, 1758) | pot | bpl | rhe | wat | lit | fbr | 3.0 | 6.5 | 80 |
| Syngnathus abaster Risso, 1827 | dia | dem | eur | wat | oth | fbm | 3.2 | 2.1 | 17 |
| Tinca tinca (Linnaeus, 1758) | pot | dem | lim | ben | phy | fre | 3.7 | 5.3 | 65 |
| Data | Sequential Number of the Day of the Year | Sampling Time Range | Image | Satellite | |
|---|---|---|---|---|---|
| 21.08.1997 | 233 | 219 | 253 | LT05_L2SP_178026_19970821_20200910_02_T1 | Landsat 5/TM |
| 23.07.1998 | 204 | 213 | 226 | LT05_L2SP_178026_19980723_20200908_02_T1 | Landsat 5/TM |
| 11.08.1999 | 223 | 221 | 274 | LT05_L2SP_178026_19990811_20211205_02_T1 | Landsat 5/TM |
| 13.08.2000 | 226 | 214 | 217 | LT05_L2SP_178026_20000813_20200906_02_T1 | Landsat 5/TM |
| 01.09.2001 | 244 | 212 | 221 | LT05_L2SP_178026_20010901_20200905_02_T1 | Landsat 5/TM |
| 16.06.2002 | 167 | 204 | 214 | LT05_L2SP_178026_20020616_20211209_02_T1 | Landsat 5/TM |
| 06.08.2003 | 218 | 212 | 220 | LT05_L2SP_178026_20030806_20200904_02_T1 | Landsat 5/TM |
| 21.06.2004 | 173 | 231 | 246 | LT05_L2SP_178026_20040621_20200903_02_T1 | Landsat 5/TM |
| 27.08.2005 | 239 | 217 | 217 | LT05_L2SP_178026_20050827_20200902_02_T1 | Landsat 5/TM |
| 14.08.2006 | 226 | 189 | 214 | LT05_L2SP_178026_20060814_20200831_02_T1 | Landsat 5/TM |
| 17.08.2007 | 229 | 208 | 241 | LT05_L2SP_178026_20070817_20200830_02_T1 | Landsat 5/TM |
| 19.08.2008 | 232 | 205 | 225 | LT05_L2SP_178026_20080819_20200829_02_T1 | Landsat 5/TM |
| 21.07.2009 | 202 | 199 | 219 | LT05_L2SP_178026_20090721_20200827_02_T1 | Landsat 5/TM |
| 09.08.2010 | 221 | 209 | 210 | LT05_L2SP_178026_20100809_20200823_02_T1 | Landsat 5/TM |
| 27.07.2011 | 208 | 214 | 222 | LT05_L2SP_178026_20110727_20200822_02_T1 | Landsat 5/TM |
| 22.08.2012 | 235 | 207 | 223 | LE07_L2SP_178026_20120822_20200908_02_T1 | Landsat 7/ETM |
| 17.08.2013 | 229 | 206 | 218 | LC08_L2SP_178026_20130817_20200913_02_T1 | Landsat 8 OLI |
| 20.08.2014 | 232 | 220 | 227 | LC08_L2SP_178026_20140820_20200911_02_T1 | Landsat 8 OLI |
| 23.08.2015 | 235 | 211 | 219 | LC08_L2SP_178026_20150823_20200908_02_T1 | Landsat 8 OLI |
Appendix B
Appendix C
| Species | Individuals | Biotope Groups * | Total (n = 570) | ||||
|---|---|---|---|---|---|---|---|
| I (n = 76) | II (n = 95) | III (n = 57) | IV (n = 171) | V (n = 171) | |||
| Abramis brama | 1357 | 1.96 ± 0.08 | 2.12 ± 0.08 | 2.75 ± 0.09 | 2.47 ± 0.05 | 2.50 ± 0.06 | 2.38 ± 0.03 |
| Alburnus alburnus | 1924 | 2.93 ± 0.14 | 3.78 ± 0.16 | 5.82 ± 0.16 | 2.92 ± 0.11 | 2.99 ± 0.09 | 3.38 ± 0.07 |
| Atherina boyeri | 682 | 1.34 ± 0.06 | 1.07 ± 0.05 | 1.18 ± 0.05 | 1.29 ± 0.04 | 1.11 ± 0.04 | 1.20 ± 0.02 |
| Babka gymnotrachelus | 1246 | 1.91 ± 0.08 | 2.25 ± 0.07 | 2.86 ± 0.09 | 1.58 ± 0.05 | 2.65 ± 0.05 | 2.19 ± 0.03 |
| Ballerus ballerus | 14 | – | – | – | 0.03 ± 0.03 | 0.05 ± 0.03 | 0.02 ± 0.01 |
| Benthophilus stellatus | 2 | – | – | – | 0.01 ± 0.01 | – | – |
| Blicca bjoerkna | 1791 | 2.61 ± 0.07 | 2.98 ± 0.10 | 5.12 ± 0.12 | 2.61 ± 0.05 | 3.35 ± 0.05 | 3.14 ± 0.04 |
| Carassius carassius | 47 | – | 0.11 ± 0.11 | 0.18 ± 0.18 | – | 0.16 ± 0.08 | 0.08 ± 0.03 |
| Carassius gibelio | 1639 | 2.03 ± 0.13 | 3.88 ± 0.15 | 4.46 ± 0.19 | 2.33 ± 0.09 | 2.71 ± 0.08 | 2.88 ± 0.06 |
| Clupeonella cultriventris | 104 | 0.05 ± 0.05 | 0.05 ± 0.05 | – | 0.53 ± 0.08 | 0.03 ± 0.02 | 0.18 ± 0.03 |
| Cobitis taenia | 922 | 1.59 ± 0.10 | 1.53 ± 0.09 | 3.30 ± 0.19 | 1.20 ± 0.05 | 1.53 ± 0.07 | 1.62 ± 0.04 |
| Cyprinus carpio | 13 | 0.05 ± 0.04 | – | – | 0.05 ± 0.03 | – | 0.02 ± 0.01 |
| Esox lucius | 1900 | 2.86 ± 0.10 | 2.55 ± 0.21 | 3.77 ± 0.15 | 2.43 ± 0.08 | 4.74 ± 0.13 | 3.33 ± 0.07 |
| Gasterosteus aculeatus | 140 | 0.12 ± 0.07 | 0.59 ± 0.20 | 0.30 ± 0.21 | 0.34 ± 0.08 | – | 0.25 ± 0.05 |
| Gymnocephalus cernua | 33 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.33 ± 0.21 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.06 ± 0.03 |
| Lepomis gibbosus | 519 | 0.99 ± 0.11 | 1.08 ± 0.09 | 1.14 ± 0.14 | 0.43 ± 0.07 | 1.19 ± 0.07 | 0.91 ± 0.04 |
| Leucaspius delineatus | 1215 | 1.66 ± 0.11 | 1.93 ± 0.12 | 3.19 ± 0.14 | 1.34 ± 0.07 | 2.89 ± 0.09 | 2.13 ± 0.05 |
| Leuciscus aspius | 512 | 0.86 ± 0.09 | 0.85 ± 0.08 | 0.75 ± 0.08 | 1.28 ± 0.06 | 0.61 ± 0.05 | 0.90 ± 0.03 |
| Leuciscus leuciscus | 6 | – | – | – | 0.04 ± 0.02 | – | 0.01 ± 0.01 |
| Mesogobius batrachocephalus | 71 | 0.17 ± 0.10 | 0.11 ± 0.11 | 0.25 ± 0.17 | 0.20 ± 0.06 | – | 0.12 ± 0.03 |
| Misgurnus fossilis | 14 | – | – | 0.09 ± 0.09 | – | 0.05 ± 0.03 | 0.02 ± 0.01 |
| Neogobius fluviatilis | 1012 | 1.70 ± 0.10 | 1.13 ± 0.06 | 1.56 ± 0.14 | 2.75 ± 0.08 | 1.27 ± 0.05 | 1.78 ± 0.05 |
| Neogobius melanostomus | 603 | 2.38 ± 0.16 | 1.05 ± 0.10 | 0.63 ± 0.08 | 1.07 ± 0.06 | 0.60 ± 0.04 | 1.06 ± 0.04 |
| Pelecus cultratus | 3 | – | – | – | 0.02 ± 0.01 | – | 0.01 ± 0.00 |
| Perca fluviatilis | 1688 | 1.96 ± 0.12 | 2.43 ± 0.10 | 2.96 ± 0.18 | 2.23 ± 0.08 | 4.43 ± 0.11 | 2.96 ± 0.06 |
| Petroleuciscus borysthenicus | 1908 | 1.70 ± 0.09 | 3.91 ± 0.08 | 3.67 ± 0.10 | 2.08 ± 0.06 | 4.93 ± 0.06 | 3.35 ± 0.06 |
| Ponticola kessleri | 448 | 1.09 ± 0.07 | 0.61 ± 0.06 | 0.42 ± 0.07 | 1.22 ± 0.05 | 0.44 ± 0.04 | 0.79 ± 0.03 |
| Proterorhinus marmoratus | 2067 | 2.66 ± 0.09 | 4.26 ± 0.19 | 4.37 ± 0.23 | 2.96 ± 0.09 | 4.12 ± 0.11 | 3.63 ± 0.06 |
| Pseudorasbora parva | 919 | 1.29 ± 0.09 | 2.16 ± 0.12 | 1.63 ± 0.14 | 1.79 ± 0.08 | 1.27 ± 0.06 | 1.61 ± 0.04 |
| Pungitius platygaster | 871 | 2.51 ± 0.11 | 0.96 ± 0.06 | 5.44 ± 0.27 | 0.69 ± 0.05 | 0.94 ± 0.07 | 1.53 ± 0.07 |
| Rhodeus sericeus | 2187 | 2.50 ± 0.12 | 3.72 ± 0.15 | 5.84 ± 0.25 | 2.91 ± 0.09 | 4.75 ± 0.12 | 3.84 ± 0.07 |
| Rutilus rutilus | 2378 | 2.75 ± 0.11 | 4.78 ± 0.28 | 5.79 ± 0.33 | 3.04 ± 0.10 | 5.06 ± 0.17 | 4.17 ± 0.10 |
| Sander lucioperca | 26 | – | 0.03 ± 0.03 | – | 0.12 ± 0.04 | 0.02 ± 0.02 | 0.05 ± 0.01 |
| Scardinius erythrophthalmus | 2068 | 2.26 ± 0.15 | 4.22 ± 0.34 | 5.18 ± 0.40 | 2.31 ± 0.15 | 4.71 ± 0.23 | 3.63 ± 0.12 |
| Silurus glanis | 41 | 0.03 ± 0.03 | – | 0.09 ± 0.09 | 0.01 ± 0.01 | 0.19 ± 0.09 | 0.07 ± 0.03 |
| Squalius cephalus | 684 | 1.03 ± 0.17 | 0.79 ± 0.18 | 1.32 ± 0.37 | 2.37 ± 0.17 | 0.29 ± 0.09 | 1.20 ± 0.09 |
| Syngnathus abaster | 1242 | 2.66 ± 0.12 | 1.89 ± 0.35 | 2.98 ± 0.44 | 1.35 ± 0.14 | 2.68 ± 0.26 | 2.18 ± 0.12 |
| Tinca tinca | 1326 | 1.92 ± 0.14 | 1.76 ± 0.11 | 3.70 ± 0.16 | 0.87 ± 0.07 | 3.82 ± 0.15 | 2.33 ± 0.08 |

| Eigenvalue Order | Eigenvalue and Projected Inertia | Covar | sdR | sdQ | Corr |
|---|---|---|---|---|---|
| 1 | 0.0524 (93.5%) | 0.23 | 2.34 | 1.64 | 0.06 |
| 2 | 0.0029 (5.15%) | 0.05 | 1.27 | 1.72 | 0.02 |
| 3 | 0.00039 (0.70%) | 0.02 | 0.54 | 1.50 | 0.02 |
| 4 | 0.00023 (0.42%) | 0.02 | 0.64 | 1.00 | 0.02 |
| Environmental Characteristics (Matrix R) | Inertia | Max Inertia | Ratio |
|---|---|---|---|
| First RLQ axis | 5.49 | 5.50 | 0.99 |
| First and second RLQ axes | 7.10 | 7.37 | 0.96 |
| 1, 2, and 3 RLQ axes | 7.40 | 7.79 | 0.95 |
| 1, 2, 3 and 4 RLQ axes | 7.81 | 8.15 | 0.96 |
| Species traits (Matrix Q) | Inertia | Max inertia | Ratio |
| First RLQ axis | 2.71 | 4.04 | 0.67 |
| First and second RLQ axes | 5.65 | 6.96 | 0.81 |
| 1, 2, and 3 RLQ axes | 7.91 | 9.29 | 0.85 |
| 1, 2, 3 and 4 RLQ axes | 8.91 | 10.54 | 0.85 |
| Species matrix (Matrix L) | Corr | Max correlation | Ratio |
| RLQ axis 1 | 0.059 | 0.19 | 0.32 |
| RLQ axis 2 | 0.025 | 0.18 | 0.14 |
| RLQ axis 3 | 0.024 | 0.16 | 0.15 |
| RLQ axis 4 | 0.024 | 0.15 | 0.16 |
References
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of Excess Nutrient Inputs on Freshwater, Marine, and Terrestrial Ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, E.M. Adding Climate Change to the Mix: Responses of Aquatic Ectotherms to the Combined Effects of Eutrophication and Warming. Biol. Lett. 2021, 17, 20210442. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied Attack: Climate Change and Eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; DelSontro, T.; Downing, J.A. Eutrophication Will Increase Methane Emissions from Lakes and Impoundments during the 21st Century. Nat. Commun. 2019, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H. Eutrophication of Freshwater and Coastal Marine Ecosystems a Global Problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.J.; Vonlanthen, P.; Seehausen, O. Does Eutrophication-Driven Evolution Change Aquatic Ecosystems? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160041. [Google Scholar] [CrossRef]
- Pinkina, T.; Zymaroieva, A.; Matkovska, S.; Svitelskyi, M.; Ishchuk, O.; Fediuchka, M. Trophic Characteristics of Lymnaea stagnalis (Mollusca: Gastropoda: Lymnaeidae) in Toxic Environment. Ekológia 2019, 38, 292–300. [Google Scholar] [CrossRef]
- Egertson, C.J.; Downing, J.A. Relationship of Fish Catch and Composition to Water Quality in a Suite of Agriculturally Eutrophic Lakes. Can. J. Fish. Aquat. Sci. 2004, 61, 1784–1796. [Google Scholar] [CrossRef]
- Shimoda, Y.; Azim, M.E.; Perhar, G.; Ramin, M.; Kenney, M.A.; Sadraddini, S.; Gudimov, A.; Arhonditsis, G.B. Our Current Understanding of Lake Ecosystem Response to Climate Change: What Have We Really Learned from the North Temperate Deep Lakes? J. Great Lakes Res. 2011, 37, 173–193. [Google Scholar] [CrossRef]
- Jacobson, P.C.; Hansen, G.J.A.; Bethke, B.J.; Cross, T.K. Disentangling the Effects of a Century of Eutrophication and Climate Warming on Freshwater Lake Fish Assemblages. PLoS ONE 2017, 12, e0182667. [Google Scholar] [CrossRef]
- Schindler, D.W. The Cumulative Effects of Climate Warming and Other Human Stresses on Canadian Freshwaters in the New Millennium. Can. J. Fish. Aquat. Sci. 2001, 58, 18–29. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.J.; De Meester, L.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of Climate Warming on Lake Fish Community Structure and Potential Effects on Ecosystem Function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Meerhoff, M.; Clemente, J.M.; de Mello, F.T.; Iglesias, C.; Pedersen, A.R.; Jeppesen, E. Can Warm Climate-Related Structure of Littoral Predator Assemblies Weaken the Clear Water State in Shallow Lakes? Glob. Change Biol. 2007, 13, 1888–1897. [Google Scholar] [CrossRef]
- Godinho, F.N.; Segurado, P.; Franco, A.; Pinheiro, P.; Pádua, J.; Rivaes, R.; Ramos, P. Factors Related to Fish Kill Events in Mediterranean Reservoirs. Water Res. 2019, 158, 280–290. [Google Scholar] [CrossRef]
- Stillman, J.H. Heat Waves, the New Normal: Summertime Temperature Extremes Will Impact Animals, Ecosystems, and Human Communities. Physiology 2019, 34, 86–100. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Bennion, H.; Moss, B.; Jeppesen, E.; Carvalho, L.; Demeester, L.; Feuchtmayr, H.; Friberg, N.; Gessner, M.O.; Hefting, M.; Lauridsen, T.L.; et al. Interaction of Climate Change and Eutrophication. Clim. Change Impacts Freshw. Ecosyst. 2010, 17, 119–151. [Google Scholar] [CrossRef]
- Glibert, P.M. Harmful Algae at the Complex Nexus of Eutrophication and Climate Change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate: Blooms like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Orlov, I.-I.O.; Fedoniuk, O.O.; Iakushenko, T.P.; Danylyk, D.M.; Kish, I.M.; Ya, R.; Zimaroieva, A.; Khant, A.A. Distribu-Tion and Ecological Growth Conditions of Utricularia Australis R. Br. in Ukraine. J. Water Land Dev. 2021, 48, 32–47. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, S.; Zhang, J.; Wu, Y.; Zhao, C.; Lian, Z.; Huang, X. Eutrophication Indirectly Reduced Carbon Sequestration in a Tropical Seagrass Bed. Plant Soil 2018, 426, 135–152. [Google Scholar] [CrossRef]
- Jameson, B.D.; Berg, P.; Grundle, D.S.; Stevens, C.J.; Juniper, S.K. Continental Margin Sediments Underlying the NE Pacific Oxygen Minimum Zone Are a Source of Nitrous Oxide to the Water Column. Limnol. Oceanogr. Lett. 2021, 6, 68–76. [Google Scholar] [CrossRef]
- Vasenko, O.G. Environmental Situation in the Lower Dnipro River Basin. Water Qual. Res. J. 1998, 33, 457–488. [Google Scholar] [CrossRef]
- Borysova, O.; Kondakov, A.; Paleari, S.; Rautalahti-Miettinen, E.; Stolberg, F.; Daler, D. Eutrophication in the Black Sea Region; Impact Assessment and Causal Chain Analysis. In Global International Waters Assessment; University of Kalmar: Kalmar, Sweden, 2005. [Google Scholar]
- Strokal, M.; Kroeze, C. Nitrogen and Phosphorus Inputs to the Black Sea in 1970–2050. Reg. Environ. Change 2013, 13, 179–192. [Google Scholar] [CrossRef]
- Ghilarov, A.M. Report on Eutrophication Studies in the U.S.S.R. Water Res. 1983, 17, 607–611. [Google Scholar] [CrossRef]
- Violle, C.; Reich, P.B.; Pacala, S.W.; Enquist, B.J.; Kattge, J. The Emergence and Promise of Functional Biogeography. Proc. Natl. Acad. Sci. USA 2014, 111, 13690–13696. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding Community Ecology from Functional Traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using Plant Functional Traits to Understand Ecological Processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Beukhof, E.; Frelat, R.; Pecuchet, L.; Maureaud, A.; Dencker, T.S.; Sólmundsson, J.; Punzón, A.; Primicerio, R.; Hidalgo, M.; Möllmann, C.; et al. Marine Fish Traits Follow Fast-Slow Continuum across Oceans. Sci. Rep. 2019, 9, 17878. [Google Scholar] [CrossRef]
- Côte, J.; Morisseau, O.; Pilisi, C.; Veyssière, C.; Perrault, A.; Jezequel, C.; Loot, G.; Blanchet, S.; Jean, S.; Jacquin, L. Urbanisation and Eutrophication as Drivers of Morphological and Physiological Divergence among Riverine Fish Populations. Freshw. Biol. 2021, 66, 669–682. [Google Scholar] [CrossRef]
- Hohausová, E. Exchange Rate and Small-Scale Movements of Fish between a River and Its Backwater. Arch. Hydrobiol. 2000, 147, 485–504. [Google Scholar] [CrossRef]
- Schmutz, S.; Kaufmann, M.; Vogel, B.; Jungwirth, M.; Muhar, S. A Multi-Level Concept for Fish-Based, River-Type-Specific Assessment of Ecological Integrity. Hydrobiologia 2000, 422, 279–289. [Google Scholar] [CrossRef]
- Grift, R.E.; Buijse, A.D.; Van Densen, W.L.T.; Machiels, M.A.M.; Kranenbarg, J.; Klein Breteler, J.G.P.; Backx, J.J.G.M. Suitable Habitats for 0-Group Fish in Rehabilitated Floodplains along the Lower River Rhine. River Res. Appl. 2003, 19, 353–374. [Google Scholar] [CrossRef]
- Tucker, T.R.; Roseman, E.F.; DeBruyne, R.L.; Pritt, J.J.; Bennion, D.H.; Hondorp, D.W.; Boase, J.C. Long-Term Assessment of Ichthyoplankton in a Large North American River System Reveals Changes in Fish Community Dynamics. Can. J. Fish. Aquat. Sci. 2018, 75, 2255–2270. [Google Scholar] [CrossRef]
- Sandström, A.; Karås, P. Effects of Eutrophication on Young-of-the-Year Freshwater Fish Communities in Coastal Areas of the Baltic. Environ. Biol. Fishes 2002, 63, 89–101. [Google Scholar] [CrossRef]
- Casselman, J.M.; Penczak, T.; Carl, L.; Mann, R.H.K.; Holcik, J.; Woitowich, W.A. An Evaluation of Fish Sampling Methodologies for Large River Systems. Pol. Arch. Hydrobiol. 1990, 37, 521–551. [Google Scholar]
- Schlosser, I.J. The Role of Predation in Age- and Size-Related Habitat Use by Stream Fishes. Ecology 1987, 68, 651–659. [Google Scholar] [CrossRef]
- Schiemer, F.; Spindler, T.; Wintersberger, H.; Schneider, A.; Chovanec, A. Fish Fry: Important Indicators for the Ecological Status of Large Rivers. Int. Organ. Theor. Appl. Limnol. 1991, 24, 2497–2500. [Google Scholar] [CrossRef]
- Jurajda, P.; Reichard, M.; Hohausová, E.; Černý, J. Comparsion of 0+ Fish Communities between Regulated–Channelized and Floodplain Stretches of the River Morava. Arch. Hydrobiol. 2001, 135, 187–202. [Google Scholar]
- Cattanéo, F. Does Hydrology Constrain the Structure of Fish Assemblages in French Streams? Local Scale Analysis. Arch. Hydrobiol. 2005, 164, 345–365. [Google Scholar] [CrossRef]
- Jurajda, P.; Slavík, O.; White, S.; Adámek, Z. Young-of-the-Year Fish Assemblages as an Alternative to Adult Fish Monitoring for Ecological Quality Evaluation of Running Waters. Hydrobiologia 2010, 644, 89–101. [Google Scholar] [CrossRef]
- Zhukov, O.; Kunakh, O.; Bondarev, D.; Chubchenko, Y. Extraction of Macrophyte Community Spatial Variation Allows to Adapt the Macrophyte Biological Index for Rivers to the Conditions of the Middle Dnipro River. Limnologica 2022, 97, 126036. [Google Scholar] [CrossRef]
- Arsan, O.M.; Davydov, O.Y.; Dyachenko, T.M. Methods of Hydroecological Research of Surface Waters; Romanenko, V.D., Ed.; Logos: Kyiv, Ukraine, 2006. [Google Scholar]
- Žiliukas, V.; Žiliukienė, V.; Repečka, R. Temporal Variation in Juvenile Fish Communities of Kaunas Reservoir Littoral Zone, Lithuania. Cent. Eur. J. Biol. 2012, 7, 858–866. [Google Scholar] [CrossRef]
- Pierce, C.L.; Rasmussen, J.B.; Leggett, W.C. Sampling Littoral Fish with a Seine: Corrections for Variable Capture Efficiency. Can. J. Fish. Aquat. Sci. 1990, 47, 1004–1010. [Google Scholar] [CrossRef]
- Paradis, Y.; Mingelbier, M.; Brodeur, P.; Magnan, P. Comparisons of Catch and Precision of Pop Nets, Push Nets, and Seines for Sampling Larval and Juvenile Yellow Perch. N. Am. J. Fish. Manag. 2008, 28, 1554–1562. [Google Scholar] [CrossRef]
- Kratochvíl, M.; Čech, M.; Vašek, M.; Kubečka, J.; Hejzlar, J.; Matěna, J.; Peterka, J.; Macháček, J.; Seďa, J. Diel Vertical Migrations of Age 0+ Percids in a Shallow, Well-Mixed Reservoir. J. Limnol. 2010, 69, 305. [Google Scholar] [CrossRef]
- Treasurer, J.W. Sampling Larval and Juvenile Fish Populations in Freshwater. Aquac. Res. 1978, 9, 6–17. [Google Scholar] [CrossRef]
- Chugunova, N.I. Guidelines for the Study of the Age and Growth of Fish (Methodological Guide to Ichthyology); Pavlovsky, E.N., Moiseyev, P.A., Eds.; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1959. [Google Scholar]
- Pfauserová, N.; Brabec, M.; Slavík, O.; Horký, P.; Žlábek, V.; Hladík, M. Effects of Physical Parameters on Fish Migration between a Reservoir and Its Tributaries. Sci. Rep. 2022, 12, 8612. [Google Scholar] [CrossRef]
- Binder, T.R.; Cooke, S.J.; Hinch, S.G. The Biology of Fish Migration. In Encyclopedia of Fish Physiology; Farrell, A.P., Ed.; Elsevier: London, UK, 2011; pp. 1921–1927. [Google Scholar]
- Thurow, R. Life Histories of Potamodromous Fishes. In An Introduction to Fish Migration; Morais, P., Daverat, F., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 29–54. [Google Scholar]
- Lin, H.-Y.; Brown, C.J.; Dwyer, R.G.; Harding, D.J.; Roberts, D.T.; Fuller, R.A.; Linke, S.; Possingham, H.P. Impacts of Fishing, River Flow and Connectivity Loss on the Conservation of a Migratory Fish Population. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 45–54. [Google Scholar] [CrossRef]
- Verhelst, P.; Reubens, J.; Buysse, D.; Goethals, P.; Van Wichelen, J.; Moens, T. Toward a Roadmap for Diadromous Fish Conservation: The Big Five Considerations. Front. Ecol. Environ. 2021, 19, 396–403. [Google Scholar] [CrossRef]
- Schmidt-Kloiber, A.; Hering, D. Www.Freshwaterecology.Info—An Online Tool That Unifies, Standardises and Codifies More than 20,000 European Freshwater Organisms and Their Ecological Preferences. Ecol. Indic. 2015, 53, 271–282. [Google Scholar] [CrossRef]
- McDowall, R. Different Kinds of Diadromy: Different Kinds of Conservation Problems. ICES J. Mar. Sci. 1999, 56, 410–413. [Google Scholar] [CrossRef]
- Britton, J.R.; Pegg, J. Ecology of European Barbel Barbus Barbus: Implications for River, Fishery, and Conservation Management. Rev. Fish. Sci. 2011, 19, 321–330. [Google Scholar] [CrossRef]
- van Treeck, R.; Van Wichelen, J.; Wolter, C. Fish Species Sensitivity Classification for Environmental Impact Assessment, Conservation and Restoration Planning. Sci. Total Environ. 2020, 708, 135173. [Google Scholar] [CrossRef]
- Hondorp, D.W.; Breitburg, D.L.; Davias, L.A. Eutrophication and Fisheries: Separating the Effects of Nitrogen Loads and Hypoxia on the Pelagic-to-Demersal Ratio and Other Measures of Landings Composition. Mar. Coast. Fish. 2010, 2, 339–361. [Google Scholar] [CrossRef]
- Perivolioti, T.-M.; Tušer, M.; Frouzova, J.; Znachor, P.; Rychtecký, P.; Mouratidis, A.; Terzopoulos, D.; Bobori, D. Estimating Environmental Preferences of Freshwater Pelagic Fish Using Hydroacoustics and Satellite Remote Sensing. Water 2019, 11, 2226. [Google Scholar] [CrossRef]
- Cheng, L.; Lek, S.; Lek-Ang, S.; Li, Z. Predicting Fish Assemblages and Diversity in Shallow Lakes in the Yangtze River Basin. Limnologica 2012, 42, 127–136. [Google Scholar] [CrossRef]
- Hekstra, G.P. The Biodiversity Convention and Coastal Regions. Limnologica 1999, 29, 339–345. [Google Scholar] [CrossRef][Green Version]
- Okun, N.; Mendonca, R.; Mehner, T. Diel Shifts in Community Composition and Feeding of Juvenile Fishes in the Pelagic Area of a Large Shallow Lake. Limnologica 2005, 35, 70–77. [Google Scholar] [CrossRef]
- Wegscheider, B.; Linnansaari, T.; Monk, W.A.; Ndong, M.; Haralampides, K.; St-Hilaire, A.; Schneider, M.; Curry, R.A. Quantitative Modelling of Fish Habitat in a Large Regulated River in a Changing Climate. Ecohydrology 2022, 15, e2318. [Google Scholar] [CrossRef]
- Stoffers, T.; Buijse, A.D.; Verreth, J.A.J.; Nagelkerke, L.A.J. Environmental Requirements and Heterogeneity of Rheophilic Fish Nursery Habitats in European Lowland Rivers: Current Insights and Future Challenges. Fish Fish. 2022, 23, 162–182. [Google Scholar] [CrossRef]
- Logez, M.; Bady, P.; Melcher, A.; Pont, D. A Continental-Scale Analysis of Fish Assemblage Functional Structure in European Rivers. Ecography 2013, 36, 80–91. [Google Scholar] [CrossRef]
- Meyer, A.; Alric, B.; Dézerald, O.; Billoir, E.; Coulaud, R.; Larras, F.; Mondy, C.P.; Usseglio-Polatera, P. Linking Micropollutants to Trait Syndromes across Freshwater Diatom, Macroinvertebrate, and Fish Assemblages. Water 2022, 14, 1184. [Google Scholar] [CrossRef]
- Schinegger, R.; Trautwein, C.; Schmutz, S. Pressure-Specific and Multiple Pressure Response of Fish Assemblages in European Running Waters. Limnologica 2013, 43, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Magoulick, D.D. Effects of Predation Risk on Habitat Selection by Water Column Fish, Benthic Fish and Crayfish in Stream Pools. Hydrobiologia 2004, 527, 209–221. [Google Scholar] [CrossRef]
- Smith, B.; Collie, J.; Lengyel, N. Effects of Chronic Bottom Fishing on the Benthic Epifauna and Diets of Demersal Fishes on Northern Georges Bank. Mar. Ecol. Prog. Ser. 2013, 472, 199–217. [Google Scholar] [CrossRef]
- Choat, J.H. Fish Feeding and the Structure of Benthic Communities in Temperate Waters. Annu. Rev. Ecol. Syst. 1982, 13, 423–449. [Google Scholar] [CrossRef]
- Negro, G.; Fenoglio, S.; Quaranta, E.; Comoglio, C.; Garzia, I.; Vezza, P. Habitat Preferences of Italian Freshwater Fish: A Systematic Review of Data Availability for Applications of the MesoHABSIM Model. Front. Environ. Sci. 2021, 9, 305. [Google Scholar] [CrossRef]
- Li, Q.; Dai, X.; Liu, Y.; Devlin, A.T.; Lai, G.; Wang, W. Potential Spawning Grounds of Phytophilic Fish under a Shifting Hydrological Regime in Poyang Lake, China. Fish. Manag. Ecol. 2022, 29, 597–607. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, F.; Song, C.; Gao, Y.; Chai, Y.; Zhong, L.; Zhuang, P. Hatchery Technology Restores the Spawning Ground of Phytophilic Fish in the Urban River of Yangtze Estuary, China. Urban Ecosyst. 2020, 23, 1087–1098. [Google Scholar] [CrossRef]
- Looby, A.; Reynolds, L.K.; Adams, C.R.; Martin, C.W. Submerged Aquatic Vegetation Patch Size Affects Fish Communities in a Turbid-Algal Lake. Front. Conserv. Sci. 2021, 2, 657691. [Google Scholar] [CrossRef]
- Fieseler, C.; Wolter, C. A Fish-Based Typology of Small Temperate Rivers in the Northeastern Lowlands of Germany. Limnologica 2006, 36, 2–16. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase; Version (12/2019); World Wide Web Electronic Publication: Washington, DC, USA, 2019. [Google Scholar]
- Oto, Y. Preference for Saline Water of an Amphidromous Goby Maintained during Migration to Upstream Freshwater Areas. J. Fish Biol. 2020, 97, 202–211. [Google Scholar] [CrossRef]
- Arai, T.; Ueno, D.; Kitamura, T.; Goto, A. Habitat Preference and Diverse Migration in Threespine sticklebacks, Gasterosteus aculeatus and G. nipponicus. Sci. Rep. 2020, 10, 14311. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Christensen, V. Primary Production Required to Sustain Global Fisheries. Nature 1995, 374, 255–257. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; Liu, Y.; Sui, X.; Feng, X.; Zhu, R.; Chen, Y. Understanding Trophic Structure Variation in Fish Assemblages of Subtropical Shallow Lakes: Combined Effects of Ecosystem Size, Productivity, and Disturbance. Ecol. Indic. 2021, 129, 107924. [Google Scholar] [CrossRef]
- Mason, J.G.; Eurich, J.G.; Lau, J.D.; Battista, W.; Free, C.M.; Mills, K.E.; Tokunaga, K.; Zhao, L.Z.; Dickey-Collas, M.; Valle, M.; et al. Attributes of Climate Resilience in Fisheries: From Theory to Practice. Fish Fish. 2022, 23, 522–544. [Google Scholar] [CrossRef]
- Mohamed, K.S.; Sathianandan, T.V.; Vivekanandan, E.; Kuriakose, S.; Ganga, U.; Pillai, S.L.; Nair, R.J. Application of Biological and Fisheries Attributes to Assess the Vulnerability and Resilience of Tropical Marine Fish Species. PLoS ONE 2021, 16, e0255879. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Engelhard, G.H.; Norris, N.J.; Theophille, D.; Sebastien, R.D. Assessing Vulnerability and Adaptive Capacity of the Fisheries Sector in Dominica: Long-Term Climate Change and Catastrophic Hurricanes. ICES J. Mar. Sci. 2019, 76, 1353–1367. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Pitcher, T.J.; Pauly, D. A Fuzzy Logic Expert System to Estimate Intrinsic Extinction Vulnerabilities of Marine Fishes to Fishing. Biol. Conserv. 2005, 124, 97–111. [Google Scholar] [CrossRef]
- Strona, G. Assessing Fish Vulnerability: IUCN vs. FishBase. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 153–154. [Google Scholar] [CrossRef]
- Boettiger, C.; Lang, D.T.; Wainwright, P.C. Rfishbase: Exploring, Manipulating and Visualizing FishBase Data from R. J. Fish Biol. 2012, 81, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Free, G.; Bresciani, M.; Pinardi, M.; Ghirardi, N.; Luciani, G.; Caroni, R.; Giardino, C. Detecting Climate Driven Changes in Chlorophyll-a in Deep Subalpine Lakes Using Long Term Satellite Data. Water 2021, 13, 866. [Google Scholar] [CrossRef]
- Geological Survey (U.S.). EROS Data Center Earth Explorer. Available online: https://earthexplorer.usgs.gov/ (accessed on 10 June 2022).
- Duan, H.; Zhang, Y.; Zhang, B.; Song, K.; Wang, Z. Assessment of Chlorophyll-a Concentration and Trophic State for Lake Chagan Using Landsat TM and Field Spectral Data. Environ. Monit. Assess. 2007, 129, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Kloiber, S.M.; Brezonik, P.L.; Olmanson, L.G.; Bauer, M.E. A Procedure for Regional Lake Water Clarity Assessment Using Landsat Multispectral Data. Remote Sens. Environ. 2002, 82, 38–47. [Google Scholar] [CrossRef]
- Lavery, P.; Pattiaratchi, C.; Wyllie, A.; Hick, P. Water Quality Monitoring in Estuarine Waters Using the Landsat Thematic Mapper. Remote Sens. Environ. 1993, 46, 268–280. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Yan, B.; Yu, J.; Wang, C.; Wang, S.; Shen, Q.; Wu, Y.; Zhang, B. Tracking Historical Chlorophyll- a Change in the Guanting Reservoir, Northern China, Based on Landsat Series Inter-Sensor Normalization. Int. J. Remote Sens. 2021, 42, 3918–3937. [Google Scholar] [CrossRef]
- Patra, P.P.; Dubey, S.K.; Trivedi, R.K.; Sahu, S.K.; Rout, S.K. Estimation of Chlorophyll-a Concentration and Trophic States in Nalban Lake of East Kolkata Wetland, India from Landsat 8 OLI Data. Spat. Inf. Res. 2017, 25, 75–87. [Google Scholar] [CrossRef]
- Lapyga, I.V. Evaluation of Eutrophication the Dnieper River in the City of Kyiv with Use Means Arcgis 10.4.1 and Landsat 8. Ecol. Sci. 2019, 1, 92–97. [Google Scholar] [CrossRef]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Estimation of Chlorophyll- a Concentration in Case II Waters Using MODIS and MERIS Data—Successes and Challenges. Environ. Res. Lett. 2009, 4, 045005. [Google Scholar] [CrossRef]
- Chamberlain, S. Rnoaa: “NOAA” Weather Data from R. R Package, Version 1.2.0. 2020. [Google Scholar]
- Marcon, E.; Hérault, B. Entropart: An R Package to Measure and Partition Diversity. J. Stat. Softw. 2015, 67, 1–26. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Göthe, E.; Riis, T.; O’Hare, M.T. Functional Trait Composition of Aquatic Plants Can Serve to Disentangle Multiple Interacting Stressors in Lowland Streams. Sci. Total Environ. 2016, 543, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; Ter Braak, C.J.F. Combining the Fourth-Corner and the RLQ Methods for Assessing Trait Responses to Environmental Variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kleyer, M.; Dray, S.; Bello, F.; Lepš, J.; Pakeman, R.J.; Strauss, B.; Thuiller, W.; Lavorel, S. Assessing Species and Community Functional Responses to Environmental Gradients: Which Multivariate Methods? J. Veg. Sci. 2012, 23, 805–821. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; Ter Braak, C.J.F.; Champely, S. Matching Species Traits to Environmental Variables: A New Three-Table Ordination Method. Environ. Ecol. Stat. 1996, 3, 143–166. [Google Scholar] [CrossRef]
- Thioulouse, J.; Dray, S.; Dufour, A.-B.; Siberchicot, A.; Jombart, T.; Pavoine, S. Multivariate Analysis of Ecological Data with Ade4; Springer: New York, NY, USA, 2018; ISBN 978-1-4939-8848-8. [Google Scholar]
- ter Braak, C.J.F. Correspondence Analysis of Incidence and Abundance Data: Properties in Terms of a Unimodal Response Model. Biometrics 1985, 41, 859. [Google Scholar] [CrossRef]
- Hill, M.O.; Smith, A.J.E. Principal Component Analysis of Taxonomic Data with Multistate Discrete Characters. Taxon 1976, 25, 249–255. [Google Scholar] [CrossRef]
- Dray, S.; Legendre, P. Testing the Species Traits Environment Relationships: The Fourth-Corner Problem Revisited. Ecology 2008, 89, 3400–3412. [Google Scholar] [CrossRef]
- Legendre, P.; Galzin, R.; Harmelin-Vivien, M.L. Relating Behavior to Habitat: Solutions to the Fourth-Corner Problem. Ecology 1997, 78, 547. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Wypych, A.; Sulikowska, A.; Ustrnul, Z.; Czekierda, D. Temporal Variability of Summer Temperature Extremes in Poland. Atmosphere 2017, 8, 51. [Google Scholar] [CrossRef]
- Tomczyk, A.M.; Bednorz, E. Heat Waves in Central Europe and Their Circulation Conditions. Int. J. Climatol. 2016, 36, 770–782. [Google Scholar] [CrossRef]
- Lhotka, O.; Kyselý, J. Characterizing Joint Effects of Spatial Extent, Temperature Magnitude and Duration of Heat Waves and Cold Spells over Central Europe. Int. J. Climatol. 2015, 35, 1232–1244. [Google Scholar] [CrossRef]
- Das Sarkar, S.; Sarkar, U.K.; Naskar, M.; Roy, K.; Bose, A.K.; Nag, S.K.; Karnatak, G.; Das, B.K. Effect of Climato-Environmental Parameters on Chlorophyll a Concentration in the Lower Ganga Basin, India. Rev. Biol. Trop. 2020, 69, 60–76. [Google Scholar] [CrossRef]
- Asch, R.G. Climate Change and Decadal Shifts in the Phenology of Larval Fishes in the California Current Ecosystem. Proc. Natl. Acad. Sci. USA 2015, 112, E4065–E4074. [Google Scholar] [CrossRef] [PubMed]
- Petitgas, P.; Rijnsdorp, A.D.; Dickey-Collas, M.; Engelhard, G.H.; Peck, M.A.; Pinnegar, J.K.; Drinkwater, K.; Huret, M.; Nash, R.D.M. Impacts of Climate Change on the Complex Life Cycles of Fish. Fish. Oceanogr. 2013, 22, 121–139. [Google Scholar] [CrossRef]
- Kuczynski, L.; Chevalier, M.; Laffaille, P.; Legrand, M.; Grenouillet, G. Indirect Effect of Temperature on Fish Population Abundances through Phenological Changes. PLoS ONE 2017, 12, e0175735. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Mendes e Mello, M.; van Oosterhout, F.; de Senerpont Domis, L.; Marinho, M.M. Response of Natural Cyanobacteria and Algae Assemblages to a Nutrient Pulse and Elevated Temperature. Front. Microbiol. 2018, 9, 1851. [Google Scholar] [CrossRef]
- Yan, X.; Xu, X.; Wang, M.; Wang, G.; Wu, S.; Li, Z.; Sun, H.; Shi, A.; Yang, Y. Climate Warming and Cyanobacteria Blooms: Looks at Their Relationships from a New Perspective. Water Res. 2017, 125, 449–457. [Google Scholar] [CrossRef]
- Zhezherya, V.A.; Zhezherya, T.P.; Linnik, P.M. Nutrients in the Water of the Reservoirs of the Dnieper Cascade after the Dnieper River Regulation. Hydrobiol. J. 2022, 58, 79–97. [Google Scholar] [CrossRef]
- Poikane, S.; Kelly, M.G.; Salas Herrero, F.; Pitt, J.-A.; Jarvie, H.P.; Claussen, U.; Leujak, W.; Lyche Solheim, A.; Teixeira, H.; Phillips, G. Nutrient Criteria for Surface Waters under the European Water Framework Directive: Current State-of-the-Art, Challenges and Future Outlook. Sci. Total Environ. 2019, 695, 133888. [Google Scholar] [CrossRef] [PubMed]
- Demars, B.O.L.; Edwards, A.C. Tissue Nutrient Concentrations in Freshwater Aquatic Macrophytes: High Inter-Taxon Differences and Low Phenotypic Response to Nutrient Supply. Freshw. Biol. 2007, 52, 2073–2086. [Google Scholar] [CrossRef]
- Zhang, P.; Kuramae, A.; van Leeuwen, C.H.A.; Velthuis, M.; van Donk, E.; Xu, J.; Bakker, E.S. Interactive Effects of Rising Temperature and Nutrient Enrichment on Aquatic Plant Growth, Stoichiometry, and Palatability. Front. Plant Sci. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Global Environmental Impacts of Agricultural Expansion: The Need for Sustainable and Efficient Practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef] [PubMed]
- Zymaroieva, A.; Zhukov, O.; Fedoniuk, T.; Pinkina, T.; Vlasiuk, V. Edaphoclimatic Factors Determining Sunflower Yields Spatiotemporal Dynamics in Northern Ukraine. OCL 2021, 28, 26. [Google Scholar] [CrossRef]
- Rashleigh, B. Relation of Environmental Characteristics to Fish Assemblages in the Upper French Broad River Basin, North Carolina. Environ. Monit. Assess. 2004, 93, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.; Neal, C.; Jarvie, H.; Doody, D. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Kleinman, P.J.A.; Srinivasan, M.S.; Dell, C.J.; Schmidt, J.P.; Sharpley, A.N.; Bryant, R.B. Role of Rainfall Intensity and Hydrology in Nutrient Transport via Surface Runoff. J. Environ. Qual. 2006, 35, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Dai, Q.; Gao, R.; Gan, Y.; Yi, X. Effects of Rainfall Intensity on Runoff and Nutrient Loss of Gently Sloping Farmland in a Karst Area of SW China. PLoS ONE 2021, 16, e0246505. [Google Scholar] [CrossRef]
- Maurer, V.M.; Zibtsev, S.V.; Savushchyk, M.P.; Borsuk, O.A. Scientific and Practical Recommendations on Environmentally Safe Approaches and Methods of Forest Restoration in the Exclusion Zone; National University of Life and Environmental Sciences of Ukraine: Kyiv, Ukraine, 2015. [Google Scholar]
- Ingole, N.P.; An, K.G. Modifications of Nutrient Regime, Chlorophyll-a, and Trophic State Relations in Daechung Reservoir after the Construction of an Upper Dam. J. Ecol. Environ. 2016, 40, 5. [Google Scholar] [CrossRef][Green Version]
- Maavara, T.; Chen, Q.; Van Meter, K.; Brown, L.E.; Zhang, J.; Ni, J.; Zarfl, C. River Dam Impacts on Biogeochemical Cycling. Nat. Rev. Earth Environ. 2020, 1, 103–116. [Google Scholar] [CrossRef]
- Akimov, M.P.; Berestov, A.I. Comparative Biocenotic Analysis of the Animal Population of the Dnieper Rapids and the Dnieper Reservoir in the First Years of Its Formation. Proc. Biol. Dep. Dnepropetr. State Univ. 1947, XXXII, 161–176. [Google Scholar]
- Feitosa, F.S.; Rezende, C.F. Trophic Ecology of the Fish Leporinus piau (Characiformes: Anostomidae) in an Area Influenced by a Dam in the Parnaíba River. Rev. Biol. Trop. 2020, 68, 426–439. [Google Scholar] [CrossRef]
- Franchi, E.; Carosi, A.; Ghetti, L.; Giannetto, D.; Pedicillo, G.; Pompei, L.; Lorenzoni, M. Changes in the Fish Community of the Upper Tiber River after Construction of a Hydro-Dam. J. Limnol. 2014, 73, 203–210. [Google Scholar] [CrossRef]
- Novitskiy, R.A. Scale, Direction and Consequences of Alien Fish Species Invasions in the Dnieper Reservoirs. Manuscript. Thesis for the Degree of Doctor of Biological Science by Specialty 03.00.10—Ichthyology; Institute of Hydrobiology, National Academy of Sciences of Ukraine: Kyiv, Ukraine, 2019. [Google Scholar]
- Zhang, P.; Grutters, B.M.C.; van Leeuwen, C.H.A.; Xu, J.; Petruzzella, A.; van den Berg, R.F.; Bakker, E.S. Effects of Rising Temperature on the Growth, Stoichiometry, and Palatability of Aquatic Plants. Front. Plant Sci. 2019, 9, 1947. [Google Scholar] [CrossRef]
- Dai, X.; Yu, Z.; Yang, G.; Wan, R. Role of Flooding Patterns in the Biomass Production of Vegetation in a Typical Herbaceous Wetland, Poyang Lake Wetland, China. Front. Plant Sci. 2020, 11, 521358. [Google Scholar] [CrossRef]
- Talbot, C.J.; Bennett, E.M.; Cassell, K.; Hanes, D.M.; Minor, E.C.; Paerl, H.; Raymond, P.A.; Vargas, R.; Vidon, P.G.; Wollheim, W.; et al. The Impact of Flooding on Aquatic Ecosystem Services. Biogeochemistry 2018, 141, 439–461. [Google Scholar] [CrossRef]
- Randall, R.G.; Brousseau, C.M.; Hoyle, J.A. Effect of Aquatic Macrophyte Cover and Fetch on Spatial Variability in the Biomass and Growth of Littoral Fishes in Bays of Prince Edward County, Lake Ontario. Aquat. Ecosyst. Health Manag. 2012, 15, 385–396. [Google Scholar] [CrossRef]
- Olson, M.H.; Carpenter, S.R.; Cunningham, P.; Gafny, S.; Herwig, B.R.; Nibbelink, N.P.; Pellett, T.; Storlie, C.; Trebitz, A.S.; Wilson, K.A. Managing Macrophytes to Improve Fish Growth: A Multi-Lake Experiment. Fisheries 1998, 23, 6–12. [Google Scholar] [CrossRef]
- Velle, G.; Skoglund, H.; Barlaup, B.T. Effects of Nuisance Submerged Vegetation on the Fauna in Norwegian Rivers. Hydrobiologia 2022, 849, 539–556. [Google Scholar] [CrossRef]
- Koblitskaya, A.F. Study of Freshwater Fish Spawning; Food Industry: Moscow, Russia, 1966. [Google Scholar]
- Ahas, R.; Aasa, A. The Effects of Climate Change on the Phenology of Selected Estonian Plant, Bird and Fish Populations. Int. J. Biometeorol. 2006, 51, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Brulé, T.; Renán, X.; Colás-Marrufo, T. Potential Impact of Climate Change on Fish Reproductive Phenology: A Case Study in Gonochoric and Hermaphrodite Commercially Important Species from the Southern Gulf of Mexico. Fishes 2022, 7, 156. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Östlund-Nilsson, S. Does Size Matter for Hypoxia Tolerance in Fish? Biol. Rev. 2008, 83, 173–189. [Google Scholar] [CrossRef]
- Holgerson, M.A.; Richardson, D.C.; Roith, J.; Bortolotti, L.E.; Finlay, K.; Hornbach, D.J.; Gurung, K.; Ness, A.; Andersen, M.R.; Bansal, S.; et al. Classifying Mixing Regimes in Ponds and Shallow Lakes. Water Resour. Res. 2022, 58, e2022WR032522. [Google Scholar] [CrossRef]
- Griffiths, J.R.; Kadin, M.; Nascimento, F.J.A.; Tamelander, T.; Törnroos, A.; Bonaglia, S.; Bonsdorff, E.; Brüchert, V.; Gårdmark, A.; Järnström, M.; et al. The Importance of Benthic–Pelagic Coupling for Marine Ecosystem Functioning in a Changing World. Glob. Chang. Biol. 2017, 23, 2179–2196. [Google Scholar] [CrossRef]
- Carpio, A.J.; De Miguel, R.J.; Oteros, J.; Hillström, L.; Tortosa, F.S. Angling as a Source of Non-Native Freshwater Fish: A European Review. Biol. Invasions 2019, 21, 3233–3248. [Google Scholar] [CrossRef]
- Bernery, C.; Bellard, C.; Courchamp, F.; Brosse, S.; Gozlan, R.E.; Jari’c, I.J.; Teletchea, F.; Leroy, B. Freshwater Fish Invasions: A Comprehensive Review. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 427–456. [Google Scholar] [CrossRef]
- Marcon, E.; Zhang, Z.; Hérault, B. The Decomposition of Similarity-Based Diversity and Its Bias Correction. 2022. Available online: https://hal-agroparistech.archives-ouvertes.fr/hal-00989454v2 (accessed on 15 October 2022).
- Skora, K.; Olenin, S.; Gollasch, S. Neogobius Melanostomus (Pallas, 1811). In Case Histories on Introduced Species: Their General Biology, Distribution, Range Expansion and Impact; Gollasch, S., Michin, D., Rosenthal, H., Voight, M., Eds.; Logos Verlag: Berlin, Germany, 1999; pp. 69–73. [Google Scholar]
- Osipov, V.V.; Kiyashko, V.I. Specific Features of the Reproduction of Clupeonella cultrivensis (Clupeiformes, Clupeoidei) at the Introduction into Freshwater Water Bodies. J. Ichthyol. 2006, 46, 552–554. [Google Scholar] [CrossRef]
- Ridley, M.; Rechten, C. Female Sticklebacks Prefer to Spawn with Males Whose Nests Contain Eggs. Behaviour 1981, 76, 152–161. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Maurice Kottelat: Cornol, Switzerland, 2007; ISBN 9782839902984. [Google Scholar]
- Gammon, D.B.; Li, W.; Scott, A.P.; Zielinski, B.S.; Corkum, L.D. Behavioural Responses of Female Neogobius melanostomus to Odours of Conspecifics. J. Fish Biol. 2005, 67, 615–626. [Google Scholar] [CrossRef]
- Shevchenko, P.G.; Shcherbukha, A.Y.; Pilipenko, Y.V.; Martsenyuk, N.O.; Khalturin, M.B.; Cherednichenko, I.S. Identification Key of Fish of Continental Water Bodies and Streams of Ukraine; Oldi Plus: Kherson, Ukraine, 2020. [Google Scholar]
- Silva, K.; Monteiro, N.M.; Vieira, M.N.; Almada, V.C. Reproductive Behaviour of the Black-Striped Pipefish Syngnathus sbaster (Pisces; Syngnathidae). J. Fish Biol. 2006, 69, 1860–1869. [Google Scholar] [CrossRef]



| Variable | Mean ± st. Error | Minimum | Maximum | PC1, λ = 5.0, 62.5% | PC2, λ = 1.7, 21.8% |
|---|---|---|---|---|---|
| Chlorophyll-a *, µg/L | 81.47 ± 0.99 | 39.94 | 136.25 | 0.50 | 0.81 |
| Water temperature*, °C | 30.43 ± 0.09 | 25.28 | 35.26 | 0.59 | 0.74 |
| kt × 10−3 ** | −1.57 ± 0.011 | −1.96 | −1.03 | −0.84 | – |
| Date of maximum air temperature | 197.1 ± 0.13 | 190 | 204 | −0.79 | −0.29 |
| Maximum of the air temperature trend | 22.47 ± 0.042 | 19.73 | 24.03 | 0.90 | −0.30 |
| R2 of the air temperature trend | 0.58 ± 0.004 | 0.43 | 0.70 | 0.91 | −0.22 |
| Precipitation intensity (kp) | 1.12 ± 0.014 | 0.43 | 1.99 | −0.83 | 0.42 |
| R2 of the precipitation trend | 0.92 ± 0.001 | 0.85 | 0.98 | −0.83 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zymaroieva, A.; Bondarev, D.; Kunakh, O.; Svenning, J.-C.; Zhukov, O. Which Fish Benefit from the Combined Influence of Eutrophication and Warming in the Dnipro River (Ukraine)? Fishes 2023, 8, 14. https://doi.org/10.3390/fishes8010014
Zymaroieva A, Bondarev D, Kunakh O, Svenning J-C, Zhukov O. Which Fish Benefit from the Combined Influence of Eutrophication and Warming in the Dnipro River (Ukraine)? Fishes. 2023; 8(1):14. https://doi.org/10.3390/fishes8010014
Chicago/Turabian StyleZymaroieva, Anastasiia, Dmytro Bondarev, Olga Kunakh, Jens-Christian Svenning, and Oleksandr Zhukov. 2023. "Which Fish Benefit from the Combined Influence of Eutrophication and Warming in the Dnipro River (Ukraine)?" Fishes 8, no. 1: 14. https://doi.org/10.3390/fishes8010014
APA StyleZymaroieva, A., Bondarev, D., Kunakh, O., Svenning, J.-C., & Zhukov, O. (2023). Which Fish Benefit from the Combined Influence of Eutrophication and Warming in the Dnipro River (Ukraine)? Fishes, 8(1), 14. https://doi.org/10.3390/fishes8010014








