Distinct Stocks of the Redtail Scad Decapterus kurroides Bleeker, 1855 (Perciformes: Carangidae) from the Northern Sulu and Southern Sibuyan Seas, Philippines Revealed from Otolith Morphometry and Shape Analysis
Abstract
1. Introduction
2. Materials and Methods
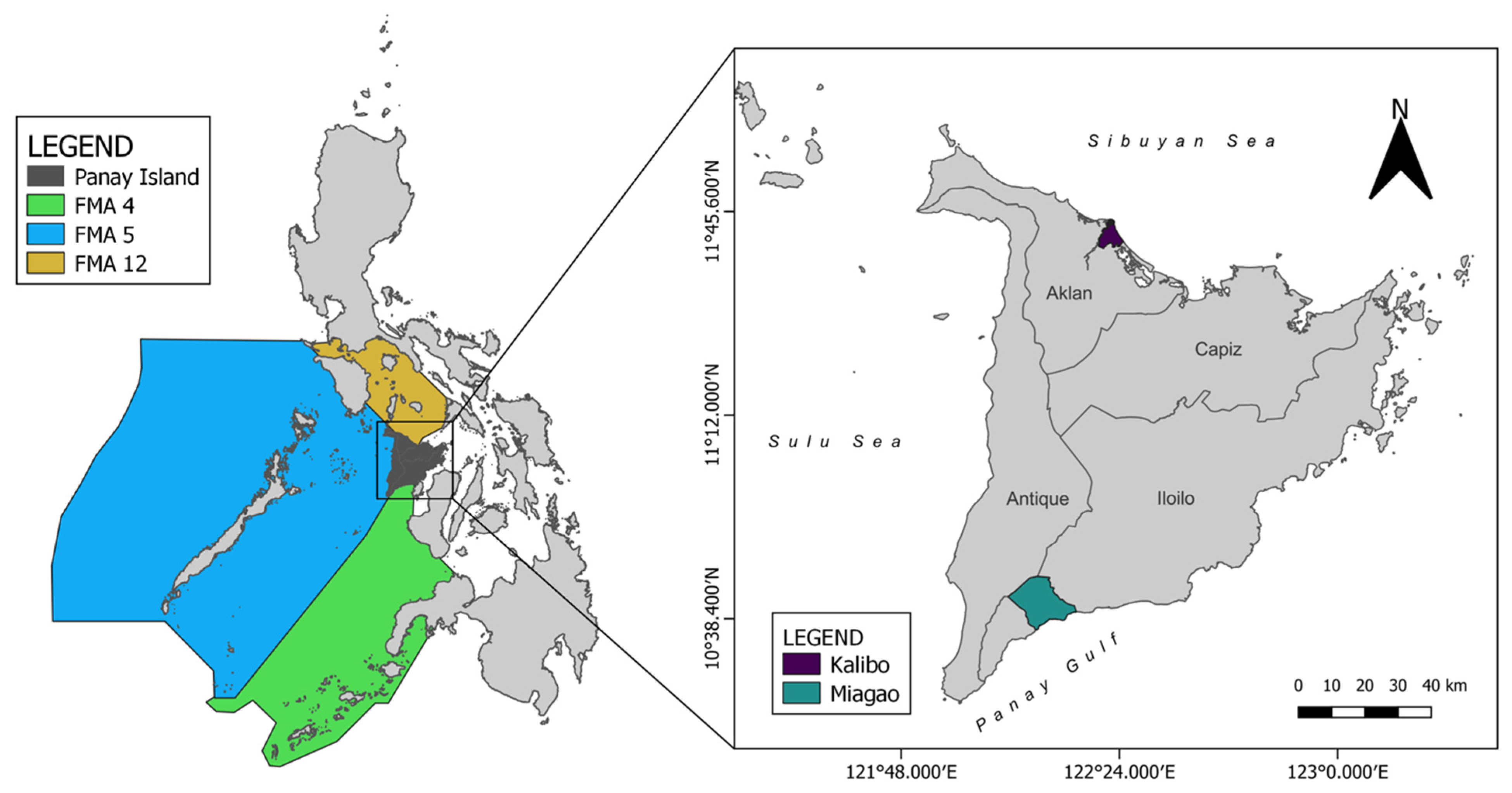
2.1. Sampling and Otolith Collection
2.2. Otolith Morphometrics
2.3. Otolith Shape Analysis
2.4. Allometry Correction
2.5. Independent Samples T-Test
2.6. Principal Component Analysis
3. Results
3.1. Otolith Morphometry
3.2. Principal Component Analysis
3.3. Otolith Shape and Margin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, B.S.; Mapstone, B.D.; Carlos, G.; Begg, G.A. (Eds.) Introduction to otoliths and fisheries in the tropics. In Tropical Fish Otoliths: Information for Assessment, Management, and Ecology; Springer: Dodrecht, The Netherlands, 2009; pp. 1–19. [Google Scholar]
- Nazir, A.; Khan, M.A. Using otoliths for fish stock discrimination: Status and challenges. Acta Ichthyol. Et Piscat. 2021, 51, 199–218. [Google Scholar] [CrossRef]
- Cardinale, M.; Doering-Arjes, P.; Kastowsky, M.; Mosegaard, H. Effects of sex, stock, and environment on the shape of known-age Atlantic cod (Gadus morhua) otoliths. Can. J. Fish. Aquat. Sci. 2004, 61, 158–167. [Google Scholar] [CrossRef]
- Gillanders, B.; Clarke, L.M.; Thorrold, S.R.; Conover, D.O. Population differences in otolith chemistry have a genetic basis in Menidia menidia. Can. J. Fish. Aquat. Sci. 2011, 68, 105–114. [Google Scholar] [CrossRef]
- Berg, F.; Almeland, O.W.; Skadal, J.; Slotte, A.; Andersson, L.; Folkvord, A. Genetic factors have a major effect on growth, number of vertebrae and otolith shape in Atlantic herring (Clupea harengus). PLoS ONE 2018, 13, e0190995. [Google Scholar] [CrossRef]
- Campana, S.E. Photographic Atlas of Fish Otolith of the Northwestern Atlantic Ocean; Canadian Special Publication of Fisheries and Aquatic Sciences 133; NCR Research Press: Ottawa, ON, Canada, 2004; p. 284. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Reichenbacher, B.; Feulner, G.R.; Schulz-Mirbach, T. Geographic variation in otolith morphology among freshwater populations of Aphanius dispar (Teleostei, Cyprinodontiformes) from the southeastern Arabian Peninsula. J. Morphol. 2009, 270, 469–484. [Google Scholar] [CrossRef]
- Tuset, V.M.; Imondi, R.; Aguado, G.; Otero-Ferrer, J.L.; Santschi, L.; Lombarte, A.; Love, M. Otolith patterns of rockfishes from the northeastern pacific. J. Morphol. 2015, 276, 458–469. [Google Scholar] [CrossRef]
- Agüera, A.; Brophy, D. Use of saggital otolith shape analysis to discriminate northeast Atlantic and western mediterranean stocks of Atlantic saury, Scomberesox saurus saurus (Walbaum). Fish. Res. 2011, 110, 465–471. [Google Scholar] [CrossRef]
- Leguá, J.; Plaza, G.; Pérez, D.; Arkhipkin, A. Otolith shape analysis as a tool for stock identification of the southern blue whiting, Micromesistius australis. Lat. Am. J. Aquat. Res. 2017, 41, 479–489. [Google Scholar] [CrossRef]
- Keating, J.P.; Brophy, D.; Officer, R.A.; Mullins, E. Otolith shape analysis of blue whiting suggests a complex stock structure at their spawning grounds in the northeast Atlantic. Fish. Res. 2014, 157, 1–6. [Google Scholar] [CrossRef]
- Libungan, L.A.; Óskarsson, G.J.; Slotte, A.; Jacobsen, J.A.; Pálsson, S. Otolith shape: A population marker for Atlantic herring Clupea harengus. J. Fish Biol. 2015, 86, 1377–1395. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, A.L.; Hernández-Fraga, K.; Alvarez-Hernández, S. Discrimination analysis of phenotypic stocks comparing fish otolith and scale shapes. Fish. Res. 2017, 185, 6–13. [Google Scholar] [CrossRef]
- Qiao, J.; Zhu, R.; Chen, K.; Zhang, D.; Yan, Y.; He, D. Comparative otolith morphology of two morphs of Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a headwater lake on the Qinghai-Tibet Plateau. Fishes 2022, 7, 99. [Google Scholar] [CrossRef]
- Wujdi, A.; Kim, H.J.; Oh, C.W. Population structure of indian mackerel (Rastrelliger kanagurta) in java and Bali Island, Indonesia inferred from otolith shape. Sains Malays. 2022, 51, 39–50. [Google Scholar] [CrossRef]
- Campana, S.; Casselman, J.M. Stock discrimination using otolith shape analysis. Can. J. Fish. Aquat. Sci. 1992, 50, 1062–1083. [Google Scholar] [CrossRef]
- Nguyen, T.H.D.; Dinh, Q.M. Otolith dimensions and their relationship with the size of Glossogobius sparsipapillus fish along the coastline of Mekong Delta, Vietnam. Egypt. J. Aquat. Biol. Fish. 2020, 24, 525–533. [Google Scholar] [CrossRef]
- Tuset, V.M.; Lozano, I.J.; González, J.A.; Pertusa, J.F.; García-Díaz, M.M. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Hilborn, R.; Walters, C.J. Quantitative fisheries stock assessment. In Choice, Dynamics and Uncertainty; Chapman and Hall: New York, NY, USA, 1992; p. 570. [Google Scholar] [CrossRef]
- Rodgveller, C.J.; Hutchinson, C.E.; Harris, J.P.; Vulstek, S.C.; Iii, C.M.G. Otolith shape variability and associated body growth differences in giant grenadier, Albatrossia pectoralis. PLoS ONE 2017, 12, e0180020. [Google Scholar] [CrossRef]
- Stransky, C.; Baumann, H.; Fevolden, S.-E.; Harbitz, A.; Høie, H.; Nedreaas, K.H.; Salberg, A.-B.; Skarstein, T.H. Separation of Norwegian coastal cod and Northeast Arctic cod by outer otolith shape analysis. Fish. Res. 2008, 90, 26–35. [Google Scholar] [CrossRef]
- Turan, C. The use of otolith shape and chemistry to determine stock structure of Mediterranean horse mackerel Trachurus mediterraneus (Steindachner). J. Fish Biol. 2006, 69, 165–180. [Google Scholar] [CrossRef]
- Vignon, M. Ontogenetic trajectories of otolith shape during shift in habitat use: Interaction between otolith growth and environment. J. Exp. Mar. Biol. Ecol. 2012, 420–421, 26–32. [Google Scholar] [CrossRef]
- Gagliano, M.; McCormick, M. Feeding history influences otolith shape in tropical fish. Mar. Ecol. Prog. Ser. 2004, 278, 291–296. [Google Scholar] [CrossRef]
- Hüssy, K. Otolith shape in juvenile cod (Gadus morhua): Ontogenetic and environmental effects. J. Exp. Mar. Biol. Ecol. 2008, 364, 35–41. [Google Scholar] [CrossRef]
- Van Neer, W.; Ervynck, A.; Bolle, L.J.; Millner, R.S.; Rijnsdorp, A.D. Fish otoliths and their relevance to archaeology: An analysis of medieval, post-medieval, and recent material of plaice, cod and haddock from the north sea. Environ. Archaeol. 2002, 7, 61–76. [Google Scholar] [CrossRef]
- Catalán, I.A.; Alós, J.; Díaz-Gil, C.; Pérez-Mayol, S.; Basterretxea, G.; Morales-Nin, B.; Palmer, M. Potential fishing-related effects on fish life history revealed by otolith microchemistry. Fish. Res. 2018, 199, 186–195. [Google Scholar] [CrossRef]
- Hoff, N.T.; Dias, J.F.; Zani-Teixeira, M.D.L.; Correia, A.T. Spatiotemporal evaluation of the population structure of the bigtooth corvina Isopisthus parvipinnis from southwest Atlantic Ocean using otolith shape signatures. J. Appl. Ichthyol. 2020, 36, 439–450. [Google Scholar] [CrossRef]
- Neves, J.; Silva, A.A.; Moreno, A.; Veríssimo, A.; Santos, A.M.; Garrido, S. Population structure of the European sardine Sardina pilchardus from Atlantic and Mediterranean waters based on otolith shape analysis. Fish. Res. 2021, 243, 106050. [Google Scholar] [CrossRef]
- Republic Act (RA) 8550. The Philippine Fisheries Code of 1998. Official Gazette. Congress of the Philippines. Manila, 1998. Available online: https://leap.unep.org/countries/ph/national-legislation/philippine-fisheries-code-1998-republic-act-no-8550 (accessed on 7 November 2022).
- Anon. Establishment of Fisheries Management Areas (FMA) for the Conservation and Management of Fisheries in Philippine Waters; Bureau of Fisheries and Aquatic Resources, Department of Agriculture: Quezon City, Philippines, 2019; p. 44. [Google Scholar]
- Pinheiro, A.; Teixeira, C.M.; Rego, A.L.; Marques, J.F.; Cabral, H.N. Genetic and morphological variation of Solea lascaris (Risso, 1810) along the Portuguese coast. Fish. Res. 2005, 73, 67–78. [Google Scholar] [CrossRef]
- Svanbäck, R.; Eklöv, P. Genetic variation and phenotypic plasticity: Causes of morphological and dietary variation in Eurasian perch. Evol. Ecol. Res. 2006, 8, 37–49. [Google Scholar]
- Keeley, E.R.; Parkinson, E.A.; Taylor, E.B. The origins of ecotypic variation of rainbow trout: A test of environmental vs. genetically based differences in morphology. J. Evol. Biol. 2007, 20, 725–736. [Google Scholar] [CrossRef]
- Franssen, N.R. Anthropogenic habitat alteration induces rapid morphological divergence in a native stream fish. Evol. Appl. 2011, 4, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Pastoral, P.; Escobar, S.; Lamarca, N.J. Round scad exploration by purse seine in the South China Sea, Area III: Western Philippines. Proc. SEAFDEC Semin. Fish. Resour. South China Sea Area III West. Philippines 2000, 49–64. [Google Scholar]
- Rada, B.; Ramos, E.; Riva, C.; Royo, N. Preliminary study on spawning period and length at maturity of shortfin scad, Decapterus macrosoma, (Bleeker, 1851, Perciformes: Carangidae) from the Coastal Waters of San Fernando, Romblon. Philipp. J. Fish. 2019, 26, 35–43. [Google Scholar] [CrossRef]
- Kimura, S.; Katahira, K.; Kuriiwa, K. The red—Fin decapterus group (Perciformes: Carangidae) with the description of a new species, Decapterus smithvanizi. Ichthyol Res. 2013, 60, 363–379. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species. 2022. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 14 October 2022).
- Philippine Statistics Authority. Fisheries Situation Report January to December 2021; Philippine Statistics Authority: Quezon City, Philippines, 2022; pp. 1–5. [Google Scholar]
- Delloro, E.S., Jr.; Babaran, R.P.; Gaje, A.C.; Cambronero, P.T.; Alama, U.B.; Motomura, H. First record of slender red scad, Decapterus smithvanizi (Actinopterygii: Perciformes: Carangidae), from the Philippines. Acta Ichthyol. Et Piscat. 2021, 51, 233–239. [Google Scholar] [CrossRef]
- Motomura, H.; Alama, U.B.; Muto, N.; Babaran, R.P.; Ishikawa, S. (Eds.) Commercial and bycatch market fishes of Panay Island, Philippines; The Kagoshima University Museum: Kagoshima, Japan; University of the Philippines Visayas: Iloilo, Philippines; Research Institute for Humanity and Nature: Kyoto, Japan, 2017; p. 244. [Google Scholar]
- Jimenez, C.; Mindanao State University at Naawan; Molina, D.; Garcia, J.; Quiñones, M.; Rosa, H.K.D.; Samson, J.; Paghasian, M. Species composition, abundance, and catch trends of Roundscads decapterus spp. in Iligan Bay, Northern Mindanao, Philippines. J. Environ. Aquat. Resour. 2020, 5, 28–42. [Google Scholar] [CrossRef]
- Lavapie-Gonzales, F. Growth, mortality and recruitment of Decapterus kurroides in Davao Gulf, Philippines. ICLARM Fishbyte 1991, 9, 6–9. [Google Scholar]
- De Guzman, M.F.; Rosario, G.R. Length-weight relationships of marine fishes caught by danish seine in Lingayen gulf. Int. J. Fish. Aquat. Stud. 2020, 8, 16–18. [Google Scholar] [CrossRef]
- Osman, A.; Farrag, M.M.S.; Mehanna, S.; Osman, Y. Use of otolithic morphometrics and ultrastructure for comparing between three goatfish species (family: Mullidae) from the northern Red Sea, Hurghada, Egypt. Iran J. Fish Sci. 2020, 19, 814–832. [Google Scholar] [CrossRef]
- Libungan, L.A.; Palsson, S. ShapeR: An R Package to study otolith shape variation among fish populations. PLoS ONE 2015, 10, e0121102. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package; Version 2.0-7; R Package: Vienna, Austria, 2013; Available online: https://CRAN.R-project.org/package=vegan/ (accessed on 20 August 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.3; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 20 August 2022).
- Lleonart, J.; Salat, J.; Torres, G.J. Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Kamrani, E.; Salarpouri, A.; Sharifian, S. Otolith dimensions (length, width), otolith weight and fish length of Sardinella sindensis (Day, 1878), as index for environmental studies, Persian Gulf, Iran. Mar. Biodivers. Rec. 2016, 9, 44. [Google Scholar] [CrossRef]
- Deepa, K.; Kumar, K.A.; Kottnis, O.; Nikki, R.; Bineesh, K.; Hashim, M.; Saravanane, N.; Sudhakar, M. Population variations of Opal fish, Bembrops caudimacula Steindachner, 1876 from Arabian Sea and Andaman Sea: Evidence from otolith morphometry. Reg. Stud. Mar. Sci. 2018, 25, 100466. [Google Scholar] [CrossRef]
- Tanner, S.E.; Pérez, M.; Presa, P.; Thorrold, S.R.; Cabral, H.N. Integrating microsatellite DNA markers and otolith geochemistry to assess population structure of European hake (Merluccius merluccius). Estuarine Coast. Shelf Sci. 2014, 142, 68–75. [Google Scholar] [CrossRef]
- Miyan, K.; Khan, M.A.; Patel, D.K.; Khan, S.; Ansari, N.G. Truss morphometry and otolith microchemistry reveal stock discrimination in Clarias batrachus (Linnaeus, 1758) inhabiting the Gangetic river system. Fish. Res. 2016, 173, 294–302. [Google Scholar] [CrossRef]
- Avigliano, E.; Carvalho, B.; Velasco, G.; Tripodi, P.; Volpedo, A.V. Inter-annual variability in otolith chemistry of catfish Genidens barbus from south-western Atlantic estuaries. J. Mar. Biol. Assoc. United Kingd. 2018, 98, 855–865. [Google Scholar] [CrossRef]
- Avigliano, E.; De Carvalho, B.M.; Leisen, M.; Romero, R.; Velasco, G.; Vianna, M.; Barra, F.; Volpedo, A.V. Otolith edge fingerprints as approach for stock identification of Genidens barbus. Estuar. Coast. Shelf Sci. 2017, 194, 92–96. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Sial, A.; Caeiro, A.; Vaz-Pires, P.; Correia, A. Population structure of the blue jack mackerel (Trachurus picturatus) in the NE Atlantic inferred from otolith microchemistry. Fish. Res. 2018, 197, 113–122. [Google Scholar] [CrossRef]
- Artetxe-Arrate, I.; Fraile, I.; Crook, D.A.; Zudaire, I.; Arrizabalaga, H.; Greig, A.; Murua, H. Otolith microchemistry: A useful tool for investigating stock structure of yellowfin tuna (Thunnus albacares) in the Indian Ocean. Mar. Freshw. Res. 2019, 70, 1708–1721. [Google Scholar] [CrossRef]
- Maciel, T.R.; Vianna, M.; de Carvalho, B.M.; Miller, N.; Avigliano, E. Integrated use of otolith shape and microchemistry to assess Genidens barbus fish stock structure. Estuar. Coast. Shelf Sci. 2021, 261, 107560. [Google Scholar] [CrossRef]
- Lombarte, A.; Lleonart, J. Otolith size changes related with body growth, habitat depth and temperature. Environ. Biol. Fishes 1993, 37, 297–306. [Google Scholar] [CrossRef]
- Bose, A.P.H.; McCallum, E.S.; Raymond, K.; Marentette, J.R.; Balshine, S. Growth and otolith morphology vary with alternative reproductive tactics and contaminant exposure in the round goby Neogobius melanostomus. J. Fish Biol. 2018, 93, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Więcaszek, B.; Nowosielski, A.; Dąbrowski, J.; Górecka, K.; Keszka, S.; Strzelczak, A. Fish size effect on sagittal otolith outer shape variability in round goby Neogobius melanostomus (Pallas 1814). J. Fish Biol. 2020, 97, 1520–1541. [Google Scholar] [CrossRef] [PubMed]
- Manginsela, F.B.; Rompas, R.M.; Mamuaya, G.E.; Lumingas, L.J.L. Otolith size and shape index of mackerel scad Decapterus macarellus (Cuvier, 1833) from Manado Bay and Kema Bay, North Sulawesi, Indonesia. Aquac. Aquar. Conserv. Legis 2020, 13, 1723–1734. [Google Scholar]
- Chanthran, S.S.D.; Lim, P.E.; Poong, S.-W.; Du, J.; Loh, K.-H. Relationships between sagittal otolith size and body size of Terapon jarbua (Teleostei, Terapontidae) in Malaysian waters. J. Oceanol. Limnol. 2020, 39, 372–381. [Google Scholar] [CrossRef]
- Qamar, N.; Panhwar, S.K. Otolith dimensions versus fish lengths estimated for five carangids (Pisces) in Pakistan. Pak. J. Zool. 2019, 51, 1963–1965. [Google Scholar] [CrossRef]
- Libungan, L.A.; Slotte, A.; Otis, E.O.; Pálsson, S. Otolith variation in Pacific herring (Clupea pallasii) reflects mitogenomic variation rather than the subspecies classification. Polar Biol. 2016, 39, 1571–1579. [Google Scholar] [CrossRef]
- Vignon, M.; Morat, F. Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish. Mar. Ecol. Prog. Ser. 2010, 411, 231–241. [Google Scholar] [CrossRef]
- Sparre, P.; Venema, S.C. Introduction to tropical fish stock assessment. In Part I: Manual. FAO Series Technical Paper 306/1 Rev. 2; FAO: Rome, Italy, 1998; p. 423. [Google Scholar]
- Pedrosa-Gerasmio, I.R.; Agmata, A.B.; Santos, M.D. Genetic diversity, population genetic structure, and demographic history of Auxis thazard (Perciformes), Selar crumenophthalmus (Perciformes), Rastrelliger kanagurta (Perciformes) and Sardinella lemuru (Clupeiformes) in Sulu-Celebes Sea inferred by mitochondrial DNA sequences. Fish. Res. 2015, 162, 64–74. [Google Scholar] [CrossRef]
- National Aeronautics and Space Administration (NASA). Jet Propulsion Laboratory (JPL)/Ocean Biology Processing Group (OBPG)/Rosentiel School of Marine and Atmospheric Science (RSMAS); Moderate-Resolution Imaging Spectroradiometer (MODIS) Aqua L2P Swath SST Data Set. Ver. 2019.0; National Aeronautics and Space Administration (NASA): Washington, DC, USA, 2020. [Google Scholar]
- Meñez, L.A.B.; Villanoy, C.L.; David, L.T. Movement of water across passages connecting Philippine inland sea basins. Sci. Diliman 2006, 18, 10–17. [Google Scholar]
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith analyses highlight morpho-functional differences of three species of Mullet (Mugilidae) from transitional water. Sustainability 2021, 14, 398. [Google Scholar] [CrossRef]
- Couillard, C.M.; Maltais, D.; Lazartigues, A.; Sirois, P. Combined use of otolith morphometry and microchemistry to study the origin of spring-spawning Atlantic herring in the St. Lawrence estuary and the gulf of St. Lawrence. Mar. Coast. Fish. 2022, 14, 10189. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Gillanders, B. Interactive effects of temperature and salinity on otolith chemistry: Challenges for determining environmental histories of fish. Can. J. Fish. Aquat. Sci. 2002, 59, 1796–1808. [Google Scholar] [CrossRef]
- Umezawa, A.; Tsukamoto, K. Factors influencing otolith increment formation in Japanese eel, Anguilla japonica T. & S., elvers. J. Fish Biol. 1991, 39, 211–223. [Google Scholar] [CrossRef]
- Clark, F.J.K.; Lima, C.S.D.S.; Pessanha, A.L.M. Otolith shape analysis of the Brazilian silverside in two northeastern Brazilian estuaries with distinct salinity ranges. Fish. Res. 2021, 243, 106094. [Google Scholar] [CrossRef]





| Size Parameters | Shape Indices | Equation |
|---|---|---|
| otolith length, OL | rectangularity, RE | |
| otolith height, OH | squareness, SQ | |
| otolith perimeter, OP | ellipticity, EL | |
| otolith area, OA | roundness, RO | |
| otolith weight, OW | aspect ratio, AR | |
| form factor, FF | ||
| compactness, CO | ||
| circularity, CI |
| n | Fish Length (cm) | Fish Weight (g) | |
|---|---|---|---|
| Sulu Sea | 83 | 17.9 ± 0.3 | 71.6 ± 3.8 |
| Sibuyan Sea | 87 | 19.5 ± 0.1 | 85.2 ± 1.1 |
| Variable | Pearson Correlation | p-Value | Relationship to Fish Length | |
|---|---|---|---|---|
| Direct otolith descriptors | OL | 0.915 ** | <0.001 | Positive |
| OH | 0.847 ** | <0.001 | Positive | |
| OW | 0.873 ** | <0.001 | Positive | |
| OA | 0.917 ** | <0.001 | Positive | |
| OP | 0.911 ** | <0.001 | Positive | |
| Derived otolith descriptors | RE | –0.408 ** | <0.001 | Negative |
| SQ | –0.832 ** | <0.001 | Negative | |
| EL | 0.340 ** | <0.001 | Positive | |
| AR | 0.347 ** | <0.001 | Positive | |
| RO | –0.496 ** | <0.001 | Negative | |
| CI | –0.879 ** | <0.001 | Negative | |
| CO | 0.503 ** | <0.001 | Positive | |
| FF | –0.496 ** | <0.001 | Negative | |
| Variable | t | df | Sulu | Sibuyan | p-Value | |
|---|---|---|---|---|---|---|
| Direct otolith descriptors | OL | –3.89 | 168 | 0.497 | 0.528 | <0.001 ** |
| OH | –0.39 | 168 | 0.2217 | 0.2228 | 0.695 | |
| OW | –2.14 | 168 | 0.0046 | 0.005 | 0.034 * | |
| OA | –2.57 | 168 | 0.0716 | 0.0766 | 0.011 * | |
| OP | –4.30 | 168 | 1.191 | 1.273 | <0.001 ** | |
| Derived otolith descriptors | RE | –1.88 | 168 | 0.645 | 0.650 | 0.062 |
| SQ | 4.55 | 168 | 32.67 | 29.37 | <0.001 ** | |
| EL | –7.55 | 168 | 0.380 | 0.410 | <0.001 ** | |
| AR | –7.32 | 168 | 2.240 | 2.370 | <0.001 ** | |
| RO | 5.97 | 168 | 0.369 | 0.350 | <0.001 ** | |
| CI | 3.70 | 168 | 246.82 | 221.6 | <0.001 ** | |
| CO | –7.02 | 168 | 19.940 | 21.210 | <0.001 ** | |
| FF | 7.42 | 168 | 0.633 | 0.594 | <0.001 ** |
| Variable | Component | ||
|---|---|---|---|
| 1 | 2 | ||
| Direct otolith descriptors | OL | 0.882 | 0.438 |
| OH | 0.990 | - | |
| OW | 0.949 | - | |
| OA | 0.958 | - | |
| OP | 0.883 | 0.417 | |
| Derived otolith descriptors | RE | −0.538 | - |
| SQ | −0.855 | −0.317 | |
| EL | - | 0.948 | |
| AR | - | 0.946 | |
| RO | - | −0.878 | |
| CI | −0.919 | - | |
| CO | 0.305 | 0.790 | |
| FF | −0.300 | −0.795 | |
| Component | Total | % of Variance | Cumulative % |
|---|---|---|---|
| 1 | 8.023 | 61.71 | 61.71 |
| 2 | 2.860 | 22.0 | 83.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnuevo, K.D.E.; Morales, C.J.C.; Calizo, J.K.S.; Delloro, E.S., Jr.; Añasco, C.P.; Babaran, R.P.; Lumayno, S.D.P. Distinct Stocks of the Redtail Scad Decapterus kurroides Bleeker, 1855 (Perciformes: Carangidae) from the Northern Sulu and Southern Sibuyan Seas, Philippines Revealed from Otolith Morphometry and Shape Analysis. Fishes 2023, 8, 12. https://doi.org/10.3390/fishes8010012
Barnuevo KDE, Morales CJC, Calizo JKS, Delloro ES Jr., Añasco CP, Babaran RP, Lumayno SDP. Distinct Stocks of the Redtail Scad Decapterus kurroides Bleeker, 1855 (Perciformes: Carangidae) from the Northern Sulu and Southern Sibuyan Seas, Philippines Revealed from Otolith Morphometry and Shape Analysis. Fishes. 2023; 8(1):12. https://doi.org/10.3390/fishes8010012
Chicago/Turabian StyleBarnuevo, Kyle Dominic E., Christian James C. Morales, Jenylle Kate S. Calizo, Emmanuel S. Delloro, Jr., Cherry Pilapil Añasco, Ricardo P. Babaran, and Sanny David P. Lumayno. 2023. "Distinct Stocks of the Redtail Scad Decapterus kurroides Bleeker, 1855 (Perciformes: Carangidae) from the Northern Sulu and Southern Sibuyan Seas, Philippines Revealed from Otolith Morphometry and Shape Analysis" Fishes 8, no. 1: 12. https://doi.org/10.3390/fishes8010012
APA StyleBarnuevo, K. D. E., Morales, C. J. C., Calizo, J. K. S., Delloro, E. S., Jr., Añasco, C. P., Babaran, R. P., & Lumayno, S. D. P. (2023). Distinct Stocks of the Redtail Scad Decapterus kurroides Bleeker, 1855 (Perciformes: Carangidae) from the Northern Sulu and Southern Sibuyan Seas, Philippines Revealed from Otolith Morphometry and Shape Analysis. Fishes, 8(1), 12. https://doi.org/10.3390/fishes8010012











