Abstract
The present study aimed to evaluate the growth performance, batch uniformity, and survival rate of the Amazonian ornamental fish Heros severus and Pterophyllum scalare during the post-larvae growing stage, fed with diets containing levels of Euterpe oleraceae essential oil (EOO) during 30 days. In the first experiment, 160 H. severus post-larvae were distributed in 16 aquariums (1 L), and in the second, 200 P. scalare post-larvae were randomly distributed in 20 aquariums (1 L). The experiments were carried out in a completely randomized design, with four treatments for H. severus (0.0%, 0.50%, 1.00%, and 2.00% of dietary EOO) and five treatments for P. scalare (0.0%, 0.5%, 1.0%, 2.0%, and 4.0% of dietary EOO), both with four replications and dietary EOO being included in replacing soybean oil. A quadratic effect (p < 0.01) was observed for the final length, length gain, specific growth rate for length, final weight, weight gain, specific growth rate for weight, and batch uniformity for weight for both fish species. As for ornamental fish larviculture, survival rate and batch uniformity are the most important parameters to be considered. For P. scalare and H. severus post-larvae, the inclusion of dietary EOO was beneficial in up to 2.48% and 0.88%, respectively.
1. Introduction
The use of plant products has gained importance in the aquaculture sector, especially due to the presence of bioactive molecules that improve growth parameters, stimulate anti-pathogen and antimicrobial organic properties as the expression of genes related to antioxidant defense systems and immunological response of fish and crustaceans [1,2]. Thus, using plants rich in bioactive compounds, such as phenolic compounds and carotenoids, can act as a feeding strategy for reducing oxidative stress in aquatic organisms [2,3,4]. In this context, essential oils (EOs), which are a mixture of hydrophobic liquids from some kinds of plants [5], must be evaluated as a feed additive for fish [6].
The Euterpe oleracea, also known as the Amazon açaí, is an endemic fruit of the Amazon region. In recent years, it has been given considerable attention due to its antioxidant, anti-inflammatory, and nutritional properties [7]. The high antioxidant capacity of this fruit is related to the presence of polyphenols and flavonoids, including a great concentration of anthocyanins in their composition [7,8]. This special lipid is not only responsible for giving the fruit color but also for modulating lipid metabolism, minimizing the damage caused by oxidative stress. Studies involving the administration of dietary Euterpe oleracea essential oil (EOO) for juvenile Oreochromis niloticus demonstrated an improvement in the organic antioxidant responses and the consequent reduction in lipid peroxidation, as well as free radical formation [9] and the mitigation of ammonia-induced histopathological changes in Litopenaeus vannamei [10]. According to the literature, EOO is also important for possessing high contents of palmitic acid (C16:0) and oleic acid (C18:1n9) and for polyunsaturated fatty acids such as linoleic acid (C18:3n3) [11].
Hatchery and larviculture are important phases for commercial aquaculture, responsible for producing fingerlings for stocking and pond culture. However, larviculture can be one of the most critical stages of fish production due to the high mortality rate caused, mainly by the lack of basic knowledge about fish nutrition and food management at this life stage [12]. In this context, post-larvae feeding is one of the critical factors during larviculture [13]. Once the live food supply during the post-larvae production occurs only in the first ten days after opening the mouth [14], it is necessary to administer the inert diet, properly balanced to meet the nutritional requirements of the fish [15,16,17]. The inert diet can be supplemented with feed additives objecting to the least nutritional stress possible during the first dry feed of fish.
The number of fish farmers dedicated to producing Amazonian ornamental fish species is still very small [18,19]. Hence, studies on nutritional management during larviculture become mandatory for generating good quality ornamental fish, better adapted to captive conditions, and with greater market value [20]. Among the different species of ornamental Amazonian fish, the Pterophyllum scalare and Heros severus stand out for presenting exuberant coloration and being much appreciated by the aquarium market [4,21,22,23].
Thus, the objective of the present study was to evaluate the effect of the dietary EOO as a feed additive on growth performance, survival rate, and batch uniformity of the Amazonian ornamental fish P. scalare and H. severus post-larvae.
2. Materials and Methods
2.1. Fish and Culture Conditions
The post-larvae were hatched in the Laboratory of Ornamental Fish, of the Federal University of Pará, in Brazil. Seven days after hatching, the post-larvae of P. scalare and H. severus were fed with 250 artemia nauplii post-larvae−1 day−1, three times a day, for 10 days. After this period, the food transition was carried out, according to [14]. From that, two trials with the same experimental design were carried out in parallel for 30 days. A total of 160 H. severus post-larvae (14.74 ± 0.61 mg and 43.00 ± 3.09 mm) and 200 P. scalare post-larvae (13.65 ± 0.60 mg and 30.69 ± 4.05 mm) were distributed in 16 and 20 translucent circular aquariums (1 L each), respectively. All aquariums were maintained with constant aeration at a photoperiod adjusted to 12 h Light: 12 h dark, using fluorescent lamps (G-Light®, 60 W, Feira de Santana, BA, Brazil). The aquariums were siphoned once a day to remove uneaten food and feces, then replenished with fresh water.
During the experimental period, water quality parameters such as temperature (26.86 ± 0.3 °C) and dissolved oxygen (6.98 ± 0.7 mg L−1) were monitored daily, whereas the pH (6.94 ± 0.3) and total ammonium concentration (0.05 ± 0.03 mg L−1) were monitored every two days. Temperature and dissolved oxygen were measured with a thermometer and a digital oximeter (Lutron DO -5510, São Paulo, SP, Brazil), and total ammonia and pH were measured with a multi-parameter device (HI 3512, Hanna Instruments, Barueri, SP, Brazil).
2.2. Experimental Design and Diets
A completely randomized design with four treatments was used for H. severus (0.0%, 0.50%, 1.00%, and 2.00% of dietary EOO), and five treatments were used for P. scalare (0.0%, 0.5%, 1.0%, 2.0%, and 4.0% of dietary EOO), both treatments with four replicates each. The oil extract was obtained from the Aromatech® industry and made from the fruit at the commercial maturity stage. Right after the obtention of the E. oleraceae pulp, a defatted processes was performed. The solvent removal was accomplished, and the resultant E. oleraceae oil was essentially free of water and solvents. The fatty acid profile was analyzed by reversed-phase high-performance liquid chromatography (HPLC) with a Waters 2695 Alliance system (Waters Corp., Milford, MA, USA) over 60 min (Pacheco-Palencia et al., 2007). The E. oleraceae oil extract fatty acid composition was lauric acid (C12:0; 2.9%), miristic acid (C14:0; 4.6%), palmitic acid (C16:0; 16.1%), estearic acid (C18:0; 3.2%), oleic acid (C18:1; 51.3%), linoleic acid (C18:2; 14.6%), araquidico acid (C20:0; 2.3%), and gondoic acid (20:1; 1.5%).
A basal diet was formulated to contain 42% crude protein and 4200 kcal kg−1 gross energy (Table 1). The diets were then moistened with 25% water at 50 °C, manually mixed and pelleted in an electric meat grinder (G. PANIZ, MCR-22, São Paulo, SP, Brazil), dried in a forced air oven (QUIMIS, São Paulo, SP, Brazil) at 35 °C for 24 h, and stored in a refrigerator, at 4 °C. Before the feeding trial, all diets were crushed in a mill and manually sieved so that the granulometry of the pellets could reach 8 mm, a size that adequately fit into the post-larvae mouth. Both post-larvae fish species were fed to apparent satiation, a moment at which animals showed no interest in the diet, four times daily, at 08:00, 11:00, 14:00, and 17:00 h, for a period of 30 days for each assay.

Table 1.
Values of the percentage and chemical composition of the experimental diet.
2.3. Growth Performance, Batch Uniformity, and Survival Ration
At the end of the feeding trial, all fish were counted, weighed on a precision scale (AG200 Gehaka® 0.0001 g, São Paulo, SP, Brazil). Growth performance was evaluated for the final weight, weight gain (final weight–initial weight), final standard length, standard length gain (final length–initial length), specific growth rate for standard length (SGR(L)), and weight (SGR(W)) being SGR(L) = ((ln final standard length–ln initial length)/number of experiment days) × 100 and (SGR(W)) = ((ln final weight–ln initial weight)/number of experiment days) × 100 (Kestemont and Stalmans, 1992), batch uniformity for standard length (LU) and weight (WU) being LU = (number of fish with standard length varying ±20% from the average in each experimental unit/total number of fish per experimental unit) × 100 and WU = (number of fish with weight varying ±20% from the average in each experimental unit/total number of fish per experimental unit) × 100, and survival rate (SR) SR = (final post-larvae number/initial post-larvae number) × 100.
2.4. Statistical Analysis
For the verification of normality and homogeneity of variances, data were submitted to Lilliefors and Bartlett’s tests, respectively. After assumptions were satisfied, a one-way analysis of variance (ANOVA) at 5% significance was performed. When differences were observed, a regression analysis between dietary EOO level and growth parameters was used. The values of each experimental unit were used to adjust the equations. The significance of the regression coefficients, the magnitude of the coefficients of determination, and the biological response of each variable were considered to choose the most suitable regression model. Statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA).
3. Results
The mean values of growth performance, batch uniformity, and survival ratio of P. scalare and H. severus fed diets with increasing values of dietary EOO replacing soybean oil are shown in Table 2 and Table 3, respectively. There was no effect of the dietary EOO on the parameters of batch uniformity for length and survival rate for both fish species.

Table 2.
Growth performance (mean ± standard deviation) of P. scalare post-larvae fed diets containing levels of Euterpe oleracea essential oil.

Table 3.
Growth performance (mean ± standard deviation) of H. severus post-larvae fed diets containing levels of Euterpe oleracea essential oil.
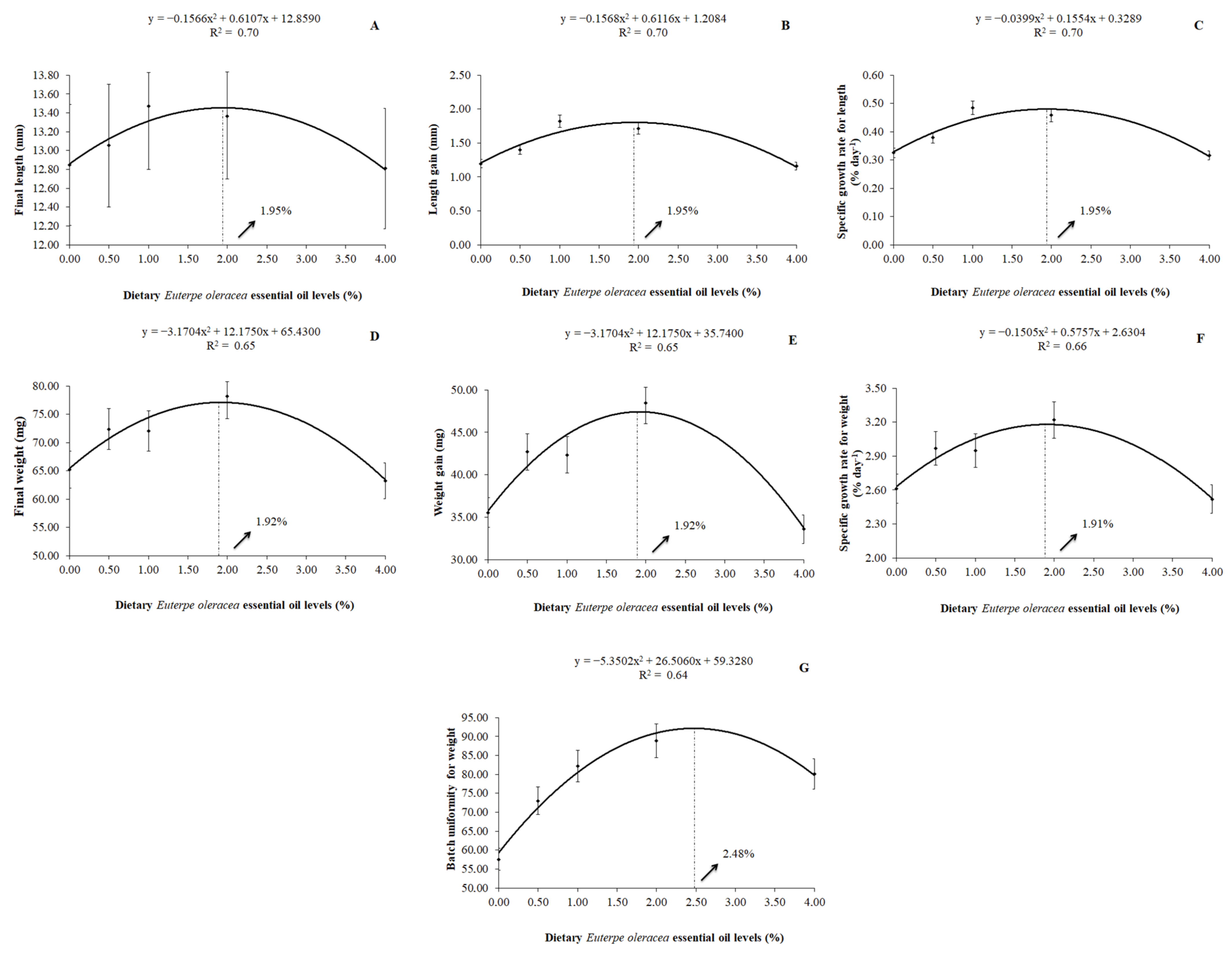
A quadratic effect (p < 0.01) was observed in the parameters of final length, length gain, specific growth rate for length, final weight, weight gain, specific growth rate for weight, and on the batch uniformity for weight by the P. scalare post-larvae with the increasing levels of dietary EOO. The best level of replacement soybean oil by dietary AEO was 1.95% for the final length (y = −0.1566x2 + 0.6107x + 12.8590; R2 = 0.70), length gain (y = −0.1568x2 + 0.6116x + 1.2084; R2 = 0.70), and specific growth rate for length (y = −0.0399x2 + 0.1554x + 0.3289; R2 = 0.70). As for the final weight and weight gain, the best estimated level was 1.92% of dietary EOO, according to the equations y = -3.1704x2 + 12.1750x + 65.4300 (R2 = 0.65) and y = −3.1704x2 + 12.1750x + 35.7400 (R2 = 0.65), respectively. Moreover, for the specific growth rate for weight, the best-estimated value of dietary EOO was 1.91% (y = −0.1505x2 + 0.5757x + 2.6304; R2 = 0.66). The best-estimated value for the batch uniformity for weight was 2.48% of dietary EOO (y = −5.3502x2 + 26.5060x + 59.3280; R2 = 0.64) (Figure 1).
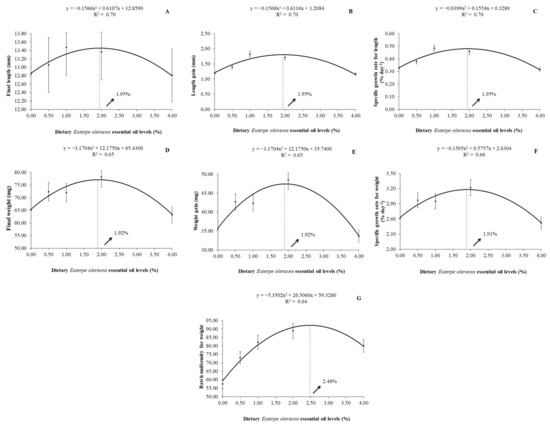
Figure 1.
Final length (A), length gain (B), specific growth rate for length (C), final weight (D), weight gain (E), specific growth rate for weight (F), and batch uniformity for weight (G) of P. scalare post-larvae fed diets containing levels of dietary Euterpe oleracea essential oil.
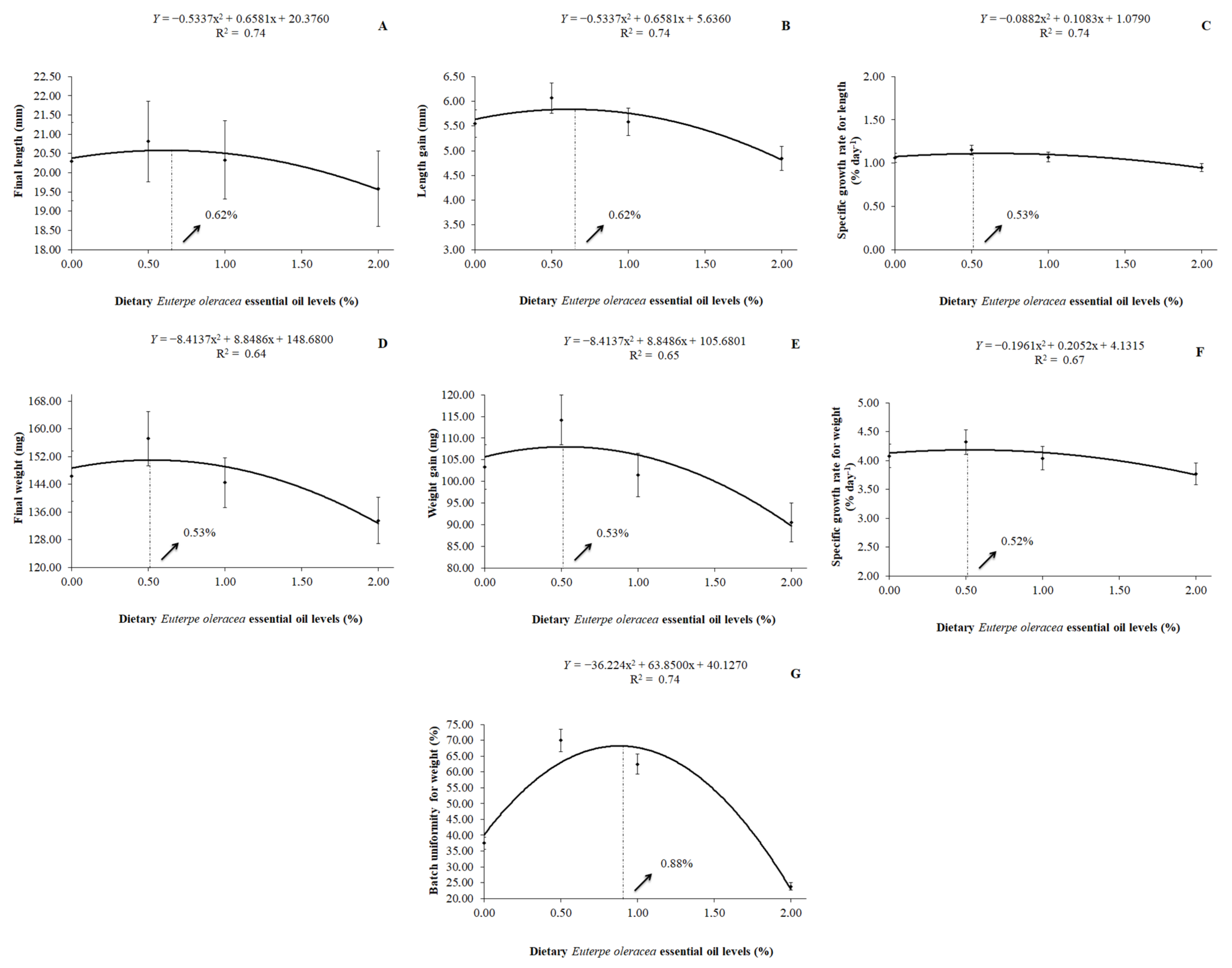
It was also observed that a quadratic effect (p < 0.01) of dietary EOO on the final length, length gain, specific growth rate for length, final weight, weight gain, specific growth rate for weight, and on the batch uniformity for weight by the H. severus post-larvae fed with levels of dietary EOO. The best replacement level of soybean oil by dietary EOO was 0.62% for the final length (y = −0.5337x2 + 0.6581x + 20.3760; R2 = 0.74) and length gain (y = −0.5337x2 + 0.6581x + 5.6360; R2 = 0.74). For the specific growth rate for length, the best-estimated value was 0.53% of dietary EOO (y = −0.0882x2 + 0.1083x + 1.0790; R2 = 0.74). For the final weight and weight gain, the best-estimated value was 0.53% of dietary EOO, according to the equations y = −8.4137x2 + 8.8486x + 148.6800 (R2 = 0.64) and y = −8.4137x2 + 8.8486x + 105.6800 (R2 = 0.65), respectively. As for the specific growth rate for weight, the best-estimated value was 0.52% of dietary EOO (y = −0.1961x2 + 0.2052x + 4.1315; R2 = 0.67) and, for the batch uniformity for weight, the better-estimated value was 0.88% of dietary EOO (y = −36.2240x2 + 63.8500x + 40.1270; R2 = 0.74) (Figure 2).
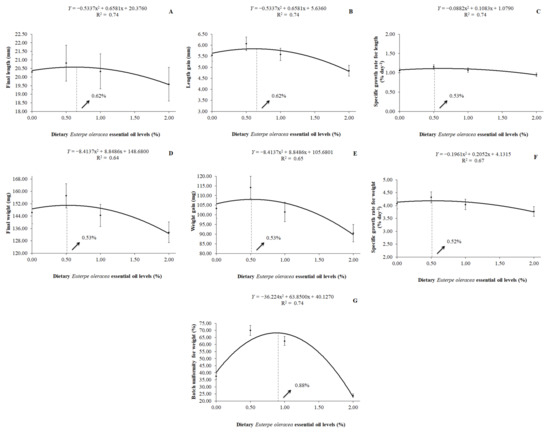
Figure 2.
Final length (A), length gain (B), specific growth rate for length (C), final weight (D), weight gain (E), specific growth rate for weight (F), and batch uniformity for weight (G) of H. severus post-larvae fed diets containing levels of dietary Euterpe oleracea essential oil.
4. Discussion
For the ornamental fish industry, the survival rate and the uniformity of the batch throughout the larviculture phase are extremely important parameters. As ornamental fish are sold individually, small losses during cultivation can considerably reduce the sector’s profitability due to the high market value of the ornamental fish unit. Furthermore, an irregular batch can cause production scheduling problems, increase competition between individuals, and generates a stressor factor that can inhibit food consumption [24]. Thus, according to our results, the dietary EOO as a feed additive was beneficial in up to 2.48% and 0.88% for both species studied, P. scalare and H. severus post-larvae, respectively, as both values resulted in a greater batch uniformity for weight. However, it is important to highlight that although an irregular batch for weight was observed, the post-larvae batch uniformity for length and survival rate was not influenced by the dietary EOO.
Fish post-larvae present high metabolic rates with a gradual increase production of reactive oxygen species [25]. Additionally, both species present an altricial post-larvae type which corresponds to digestive and immune systems still in development and, therefore, lacking digestive enzymes and antioxidant molecules. In this context, it is possible that the growth performance results observed in the present study are a response to the antioxidant characteristic of dietary EOO well determined by [26] on the typical oxidative stress of post-larvae metabolism, as observed by [27]. Previous works have reported a positive role of dietary EOO in modulating the production of reactive oxygen species and in the expression of antioxidant genes in the liver of rats [28]. Furthermore, according to [29], the antioxidant activity of dietary EOO can effectively protect Caenorhabditis elegans against oxidative stress and reduce the levels of endogenous reactive oxygen species. The authors also found that EOO performed its antioxidant activity not only by scavenging the radicals but also by modulating the expression of stress-response genes. Similar results were also observed by [9] in O. niloticus juveniles with fed diets containing different levels of inclusion of dietary EOO in place of soybean oil, which concluded that the addition of dietary EOO stimulated the antioxidant responses of the fish. However, as opposed to our observations, no effect of dietary EOO on the growth performance of O. niloticus was observed. Nevertheless, according to these authors, the exact mechanism for the absence of effects remains unknown but appears linked to a dose-dependent effect.
The fish growth performance improvement observed in the present study could also be in response to the high content of palmitic acid (C16:0) in EOO composition [8,11]. According to [30], in mammals, of the 20–30 g daily intake of palmitic acid, 20–30% is beta-oxidized [31], and about 60–70% is incorporated into phospholipids [32], both enzymatic pathways highly activated in post-larvae metabolism. However, it is important to highlight that these observations were made only in juvenile fish, leaving doubt about the dietary palmitic acid effect of fish with different physiological conditions and higher metabolic rates.
Another important result observed was the difference between the effect of the dietary EOO on the growth performance of each fish species. While H. severus post-larvae required an average of 0.52% in dietary EOO to achieve its highest growth rate, P. scalare post-larvae required more than triple to achieve the same result. Although H. severus nutrient requirement is similar to species of the same family (Cichlidae) as P. scalare and O. niloticus [33,34,35,36], it is possible that H. severus post-larvae are more responsive to dietary EOO than P. scalare, which suggest specific studies on the lipid source requirement for both fish species. As for ornamental fish larviculture, survival rate and batch uniformity are the most important parameters to be evaluated. According to the present study, for P. scalare post-larvae and H. severus post-larvae, the inclusion of dietary EOO in replacing soybean oil was beneficial in up to 2.48% and 0.88%, respectively.
Author Contributions
Conceptualization, L.B.d.M., A.L.S., L.A.L.B. and D.A.V.C.; Data curation, L.B.d.M., A.L.S., G.C.V. and D.A.V.C.; Formal analysis, L.B.d.M., J.G.C., I.C.e.S. and A.L.S.; Funding acquisition, L.B.d.M., E.T.d.S.N., L.A.L.B. and D.A.V.C.; Investigation, J.G.C., E.T.d.S.N., I.C.e.S. and D.A.V.C.; Methodology, L.B.d.M., J.G.C., E.T.d.S.N., I.C.e.S., G.C.V. and D.A.V.C.; Project administration, L.B.d.M. and D.A.V.C.; Resources, L.B.d.M., A.L.S. and L.A.L.B.; Supervision, L.B.d.M. and D.A.V.C.; Visualization, L.B.d.M. and D.A.V.C.; Writing—original draft, L.B.d.M., A.L.S., L.A.L.B., G.C.V. and D.A.V.C.; Writing—review and editing, L.B.d.M. and D.A.V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The experiment was approved by the Ethics Committee on the Use of Animals of the Federal University of Pará, CEUA/UFPA (protocol number CEUA 1432240621) and conducted at the Laboratory of Ornamental Fish of the Federal University of Pará, Bragança—PA, Brazil.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasília, DF, Brazil and the Pró-reitoria de Pesquisa e Pós-graduação of Universidade Federal do Pará (PROPESP/UFPA), Belém, PA, Brazil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dawood, M.A.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.; Alagawany, M.; Kari, Z.A.; Razab, M.K.A.A.; Hamid, N.K.A.; Moonmanee, T.; Doan, H.V. Exploring the roles of dietary herbal essential oils in aquaculture: A review. Animals 2022, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Dawood, M.A.; Moghadam, M.S.; Sheikhzadeh, N.; Hoseinifar, S.H.; Musthafa, M.S. Modulation of immune parameters and antioxidant defense in zebrafish (Danio rerio) using dietary apple cider vinegar. Aquaculture 2019, 513, 734412. [Google Scholar] [CrossRef]
- De Souza, M.O.; Silva, M.; Silva, M.E.; De Paula, O.R.; Pedrosa, M.L. Diet supplementation with acai (Euterpe oleracea Mart.) pulp improves biomarkers of oxidative stress and the serum lipid profile in rats. Nutrition 2010, 26, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Deon, M.P.P.; Souza, P.H.A.A.; Arauco, L.R.R.; Lima, B.S.L. Influência da densidade de estocagem no desempenho produtivo do acará-bandeira (Pterophyllum scalare) cultivado em gaiolas. Bol. Ind. Anim. 2017, 74, 156–161. [Google Scholar] [CrossRef][Green Version]
- Máthé, A.K.O.S. Essential oils-biochemistry, production and utilization. In Phytogenics in Animal Nutrition. Natural Concepts to Optimize Gut Health and Performance; Steiner, T., Ed.; Nottingham University Press: Nottingham, UK, 2009; pp. 1–18. [Google Scholar]
- Campelo, D.A.V.; Gonçalves, I.S.; Silva, I.C.; De Moura, L.B.; Brabo, M.F.; Barbas, L.A.L.; Veras, G.C. Dietary garlic essential oil on development parameters of severum post-larvae. Rev. Bras. Saúde Prod. Anim. 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Schauss, A.G. Advances in the study of the health benefits and mechanisms of action of the pulp and seed of the Amazonian palm fruit, Euterpe oleracea Mart., known as “Açai”. In Fruits, Vegetables, and Herbs; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 179–220. [Google Scholar] [CrossRef]
- Cedrim, P.C.A.S.; Barros, E.M.A.; Nascimento, T.G.D. Propriedades antioxidantes do açaí (Euterpe oleracea) na síndrome metabólica. Braz. J. Food Technol. 2018, 21, 2017092. [Google Scholar] [CrossRef]
- Leite, T.C.; Picoli, F.; Lopes, D.A.; Baldissera, M.D.; Souza, C.F.; Baldisserotto, B.; Costa, A.P.O.; Nora, L.; Marcelino, A.H.; Da Silva, A.S. The effects of açaí oil addition in tilapia diets on performance, hepatic energy metabolism enzymes and antioxidant responses. Aquac. Res. 2021, 52, 395–402. [Google Scholar] [CrossRef]
- Colombo, G.M.; dos Santos Simião, C.; Schmitz, M.J.; Pedrosa, V.F.; Romano, L.A.; Tesser, M.B.; Ramos, P.B.; Wasielesky, W.; Monserrat, J.M. The role of açaí (Euterpe oleracea Mart. 1824) as a chemoprotective agent in the evaluation of antioxidant defence, oxidative damage and histology of juvenile shrimp Litopenaeus vannamei (Boone, 1931) exposed to ammonia. Aquac. Res. 2020, 51, 1551–1566. [Google Scholar] [CrossRef]
- Minighin, E.C.; Anastácio, L.R.; Melo, J.O.F.; Labanca, R.A. Açaí (Euterpe oleracea) e suas contribuições para alcance da ingestão diária aceitável de ácidos graxos essenciais. Res. Soc. Dev. 2020, 9, 760986116. [Google Scholar] [CrossRef]
- Hill, M.; Pernetta, A.; Crooks, N. Size matters: A review of live feeds used in the culture of marine ornamental fish. Asian Fish. Sci. 2020, 33, 161–174. [Google Scholar] [CrossRef]
- Urbinati, E.D.; Gonçalves, F.D. Pacu (Piaractus mesopotamicus). In Espécies Nativas para a Piscicultura no Brasil; Baldisserotto, B., Gomes, L.C., Eds.; Editora UFSM: Santa Maria, Brazil, 2005; pp. 225–255.mesopotamicus). In Espécies Nativas para a Piscicultura no Brasil; Baldisserotto, B., Gomes, L.C., Eds.; Editora UFSM: Santa Maria, Brazil, 2005; pp. 225–255. [Google Scholar]
- Campelo, D.A.V.; Silva, I.C.; Marques, M.H.C.; Eiras, B.J.C.F.; Brabo, M.F.; De Moura, L.B.; Veras, G.C. Estratégias alimentares na larvicultura do peixe ornamental amazônico acará-severo (Heros severus) (Heckel, 1840). Arq. Bras. Med. Vet. Zootec. 2019, 71, 1601–1608. [Google Scholar] [CrossRef]
- Fosse, P.J.; Mattos, D.C.; Cardoso, L.D.; Radael, M.C.; Filho, J.C.F.; Vidal, M.V.V., Jr. Duration of co-feeding on the Nishikigoi Cyprinus carpio larvae during weaning from live to inert food in na indoor system. Ciênc. Rural. 2018, 48, 1–9. [Google Scholar] [CrossRef]
- Palma-Cancino, D.J.; Álvarez-González, C.A.; Vega-Villasante, F.; Vargas-Ceballos, M.; Pena-Marín, E.; Castillo-Arias, E.A.; Martínez-Garcia, R. Cost-benefit analysis of the production of juvenile tropical Gar ‘’pejelagarto’’ (Atractosteus tropicus Gill): Comparing four feeding schemes. Agro Productividad. 2021, 2, 1–9. [Google Scholar] [CrossRef]
- Veras, G.C.; Soares, L.M.O.; Brabo, M.F.; Paixão, D.J.M.R.; Dias, B.C.B.; Alves, A.X.; Murgas, L.D.S.; Campelo, D.A.V. Fotoperíodo e frequência alimentar na larvicultura do acará-bandeira Pterophyllum scalare. Arch. Zootec. 2016, 65, 581–584. [Google Scholar] [CrossRef]
- Junk, W.J.; Soares, M.G.M.; Bayley, P.B. Freshwater fishes of the Amazon River basin: Their biodiversity, fisheries, and habitats. Aquat. Ecosyst. Health Manag. 2007, 10, 153–173. [Google Scholar] [CrossRef]
- Araújo, J.G.; Santos, M.A.S.; Rebello, F.K.; Isaac, V.J. Cadeia comercial de peixes ornamentais do Rio Xingu, Pará, Brasil. Bol. Inst. Pesca 2017, 43, 297–307. [Google Scholar] [CrossRef]
- Valenti, W.C.; Barros, H.P.; Moraes-Valenti, P.; Bueno, G.W.; Cavalli, R.O. Aquaculture in Brazil: Past, present and future. Aquac. Rep. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Favero, J.M.; Pompeu, P.S.; Prado-Valladares, A.C. Biologia reprodutiva de Heros efasciatus Heckel, 1840 (Pisces, Cichlidae) na Reserva de Desenvolvimento Sustentável Amanã-AM, visando seu manejo sustentável. Acta Amazon. 2010, 40, 373–380. [Google Scholar] [CrossRef]
- Alishahi, M.; Karamifar, M.; Mesbah, M.; Zarei, M. Hemato-immunological responses of Heros severus fed diets supplemented with different levels of Dunaliella salina. Fish Physiol. Biochem. 2014, 40, 57–65. [Google Scholar] [CrossRef]
- Navarro, R.D.; Navarro, F.K.S.P.; Filho, J.T.S.; Filho, O.P.R. Nutrição e alimentação de reprodutores de peixes. Rev. Augustus 2010, 30, 108–118. [Google Scholar]
- Gonçalves, L.P., Jr.; Pereira, S.L.; Matielo, M.D.; Mendonça, P.P. Efeito da densidade de estocagem no desenvolvimento inicial do acará-bandeira (Pterophyllum scalare). Arq. Bras. Med. Vet. Zootec. 2013, 65, 1176–1182. [Google Scholar] [CrossRef]
- Kadomura, K.; Naruse, S.; Sugihara, S.; Yamaguchi, K.; Oda, T. Production of Reactive Oxygen Species (ROS) by Various Marine Fish Species during the Larval Stage. Biosci. Biotechnol. Biochem. 2007, 71, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.V.; Ferreira Ferreira da Silveira, T.; Mattietto RD, A.; Padilha de Oliveira MD, S.; Godoy, H.T. Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2017, 97, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Zeppenfeld, C.C.; Velho, M.C.; Klein, B.; Abbad, L.B.; Ourique, A.F.; Wagner, R.; Da Silva, A.S.; Baldisserotto, B. Dietary supplementation with nerolidol nanospheres improves growth, antioxidant status and fillet fatty acid profiles in Nile tilapia: Benefits of nanotechnology for fish health and meat quality. Aquaculture 2020, 516, 734635. [Google Scholar] [CrossRef]
- Guerra, F.Q.S.; Mendes, J.M.; Sousa, J.P.D.; Morais-Braga, M.F.; Santos, B.H.C.; Melo Coutinho, H.D.; Lima, E.D.O. Increasing antibiotic activity against a multidrug-resistant Acinetobacter spp. by essential oils of Citrus limon and Cinnamomum zeylanicum. Nat. Prod. Res. 2012, 26, 2235–2238. [Google Scholar] [CrossRef]
- Peixoto, H.; Roxo, M.; Krstin, S.; Roöhrig, T.; Richling, E.; Wink, M. An Anthocyanin-Rich Extract of Acai (Euterpe precatoria M. art.) Increases Stress Resistance and Retards Aging-Related Markers in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 1283–1290. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Burdge, G.C. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot. Essent. Fatty Acids. 2006, 75, 161–168. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Lisai, S.; Sirigu, A.; Piras, A.; Collu, M.; Batetta, B.; Gambelli, L.; Banni, S. Dietary triacylglycerols with palmitic acid in the sn-2 position modulate levels of N-acylethanolamides in rat tissues. PLoS ONE 2015, 10, 0120424. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Ahmad, M.H.; Khattab, Y.A.; Shalaby, A.M. Effect of dietary protein level, initial body weight, and their interaction on the growth, feed utilization, and physiological alterations of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 2010, 298, 267–274. [Google Scholar] [CrossRef]
- Ribeiro, F.A.S.; Preto, B.L.; Fernandes, J.B.K. Sistemas de criação para o acará bandeira Pterophyllum scalare. Acta Sci. 2008, 30, 459–466. [Google Scholar] [CrossRef][Green Version]
- Zuanon, J.A.S.; Salaro, A.L.; Furuya, W.M. Produção e nutrição de peixes ornamentais. Rev. Bras. Zootec. 2011, 40, 165–174. [Google Scholar]
- Kestemont, P.; Stalmans, J.M. Initial feeding of European minnow larvae, Phoxinus phoxinus L. 1. Influence of diet and feeding level. Aquaculture 1992, 104, 327–340. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).