Non-destructive Approach for the Prediction of pH in Frozen Fish Meat Using Fluorescence Fingerprints in Tandem with Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Sample Preparation
2.2. Preparation of Buffer and Pure Solutions of Fluorophores
2.3. Settings of Fluorescence Spectrometer for 3D-FFs Measurement
2.4. pH Measurement of Frozen Fish Meat
2.5. Processing of 3D-FFs Data
2.6. Multivariate Analysis of Fish 3D-FFs Data
3. Results and Discussion
3.1. pH Changes in Frozen Horse and Spotted Mackerel Fish Meat
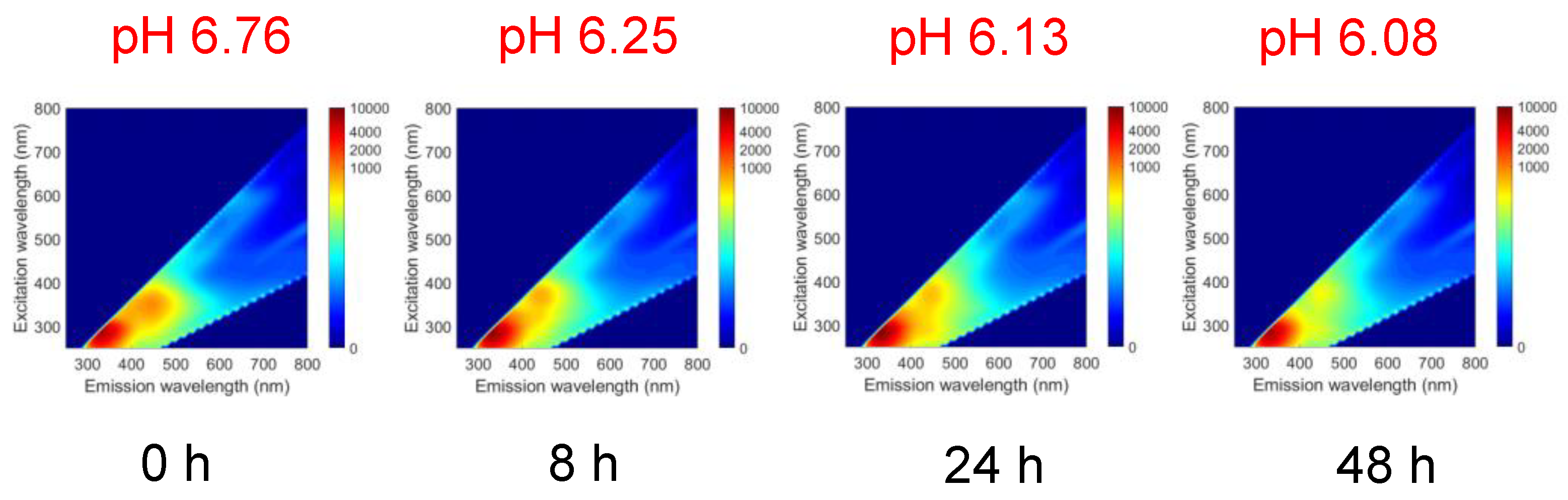
3.2. 3D-FFs of Frozen Fish Meat
3.3. Prediction of pH in Fish Meat by FFs Coupled with PLSR Validation Models
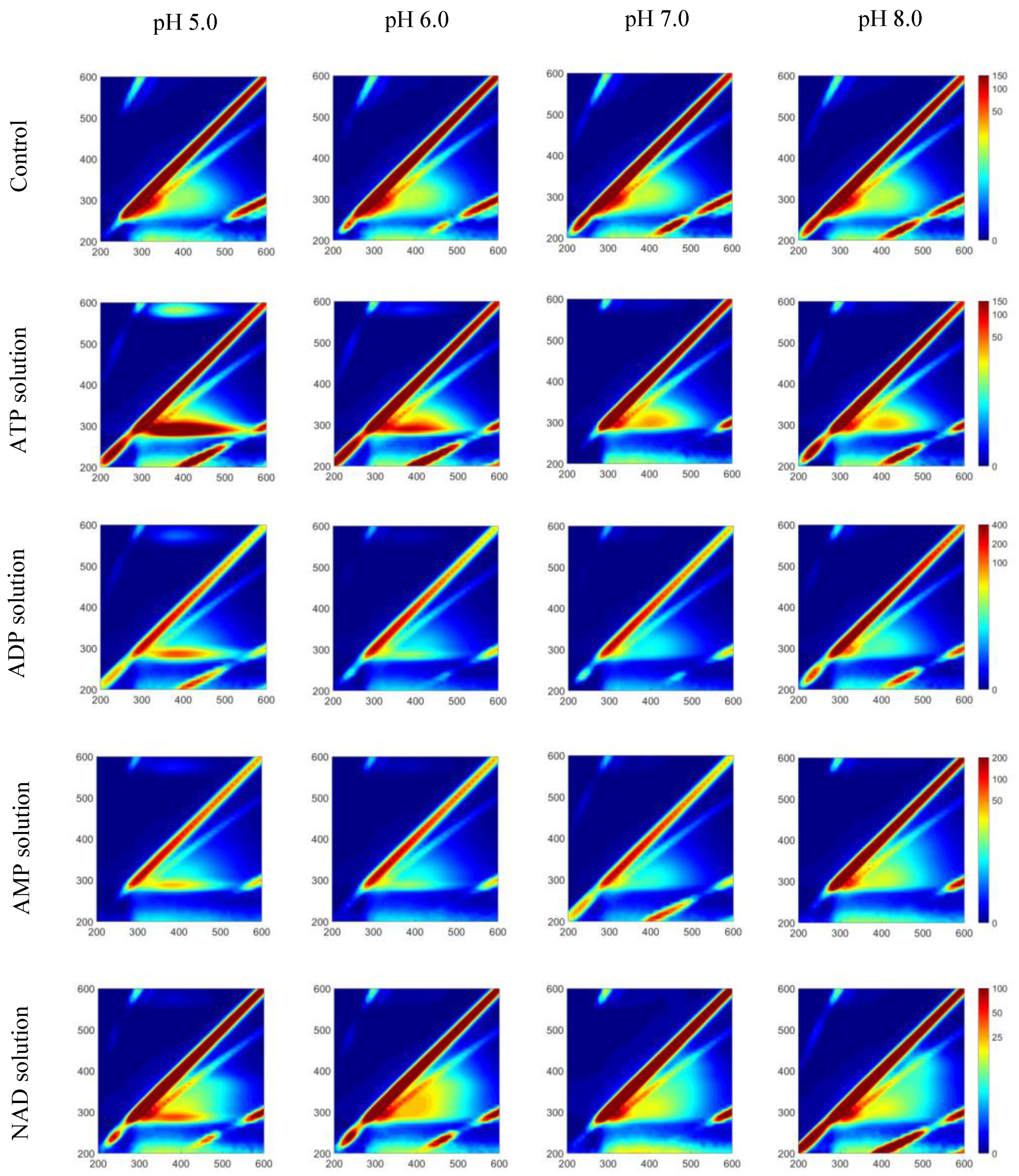
3.4. pH Sensitivity of Pure Fluorophores and Their Relationship to Fish Meat Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lougovois, V.P.; Kyranas, E.R.; Kyrana, V.R. Comparison of selected methods of assessing freshness quality and remaining storage life of iced gilthead sea bream (Sparus aurata). Food Res. Int. 2003, 36, 551–560. [Google Scholar] [CrossRef]
- Liu, D.; Pu, H.; Qu, J.; Sun, D.-W.; Wang, L.; Zeng, X.-A. Combination of spectra and texture data of hyperspectral imaging for prediction of pH in salted meat. Food Chem. 2014, 160, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, N.; Okazaki, E. Recent research on factors influencing the quality of frozen seafood. Fish. Sci. 2020, 86, 231–244. [Google Scholar] [CrossRef]
- Elmasry, G.; Sun, D.-W.; Allen, P. Near-infrared hyperspectral imaging for predicting colour, pH and tenderness of fresh beef. J. Food Eng. 2012, 110, 127–140. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shibata, M.; ElMasry, G.; Nakazawa, N.; Nakauichi, S.; Hagiwara, T.; Osako, K.; Okazaki, E. Expeditious prediction of post-mortem changes in frozen fish meat using three-dimensional fluorescence fingerprints. Biosci. Biotechnol. Biochem. 2019, 83, 901–913. [Google Scholar] [CrossRef] [PubMed]
- User Assistance PerkinElmer Ltd, An Introduction to Fluorescence Spectroscopy; PerkinElmer Inc.: Buckinghamshire, UK, 2000.
- Oto, N.; Oshita, S.; Makino, Y.; Kawagoe, Y.; Sugiyama, J.; Yoshimura, M. Non-destructive evaluation of ATP content and plate count on pork meat surface by fluorescence spectroscopy. Meat Sci. 2013, 93, 579–585. [Google Scholar] [CrossRef]
- Shibata, M.; Elmasry, G.; Moriya, K.; Rahman, M.M.; Miyamoto, Y.; Ito, K.; Nakazawa, N.; Nakauchi, S.; Okazaki, E. Smart technique for accurate monitoring of ATP content in frozen fish fillets using fluorescence fingerprint. LWT-Food Sci. Technol. 2018, 92, 258–264. [Google Scholar] [CrossRef]
- ElMasry, G.; Nagai, H.; Moria, K.; Nakazawa, N.; Tsuta, M.; Sugiyama, J.; Okazaki, E.; Nakauchi, S. Freshness estimation of intact frozen fish using fluorescence spectroscopy and chemometrics of excitation–emission matrix. Talanta 2015, 143, 145–156. [Google Scholar] [CrossRef]
- Bui, M.V.; Rahman, M.M.; Nakazawa, N.; Okazaki, E.; Nakauichi, S. Visualize the quality of frozen fish using fluorescence imaging aided with excitation-emission matrix. Opt. Express 2018, 26, 22954–22964. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shibata, M.; Nakazawa, N.; Hagiwara, T.; Osako, K.; Okazaki, E. Effects of pH on the fluorescence fingerprint of ATP. Trans. JSRAE 2016, 33, 405–410. [Google Scholar]
- Barbin, D.F.; ElMasry, G.; Sun, D.-W.; Allen, P. Predicting quality and sensory attributes of pork using near-infrared hyperspectral imaging. Anal. Chim. Acta. 2012, 719, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Yoon, S.-C.; Zhuang, H.; Wang, W.; Li, C. Prediction of pH of fresh chicken breast fillets by VNIR hyperspectral imaging. J. Food Eng. 2017, 208, 57–65. [Google Scholar] [CrossRef]
- Wencel, D.; Abel, T.; McDonagh, C. Optical Chemical pH Sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Koike, H.; Kimura, I.; Yuan, C. Delaying Post-mortem Changes in the Muscle of Spotted Mackerel Killed by an Instantaneous Way of Neck-breaking and Bleeding. J. Fishsci. 2016, 10, 083–088. [Google Scholar]
- Leygonie, C.; Britz, T.J.; Hofman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, N.; Maeda, T.; Fukushima, H.; Wada, R.; Tanaka, R.; Okazaki, E. Effect of Cooling Conditions on the ATP Content and pH of Chub Mackerel (Scomber japonicus) Meat. Trans. Jpn. Soc. Refrig. Air Cond. Eng. 2019, 36, 49, (In Japanese with English abstract). [Google Scholar]
- Aberoumand, A.; Baesi, F. Effects of vacuum packaging in freezer on oxidative spoilage indexes of fish Lethrinus atkinsoni. Food Sci. Nutr. 2020, 8, 4145–4150. [Google Scholar] [CrossRef]
- Postnikova, G.B.; Shekhovtsova, E.A. Fluorescence Studies on the Interaction of Myoglobin with Mitochondria. Biochemistry 2012, 77, 280–287. [Google Scholar] [CrossRef]
- Bito, M. Changes in NAD and ATP Levels and pH in Frozen-Stored Skipjack Meat, in Relation to Amount of Drip. Bull. Japan Soc. Scient. Fish 1978, 44, 897–902. [Google Scholar] [CrossRef]
- Rinnan, A.; Andersen, C.M. Handling of first-order Rayleigh Scatter in PARAFAC Modelling of Fluorescence Excitation–Emission Data. Chemom. Intell. Lab. Syst. 2005, 76, 91–99. [Google Scholar] [CrossRef]
- Adeyemi, O.T.; Osilesi, O.O.; Onajobi, F.; Adebawo, O.; Afolayan, A.J. Stability study of smoked fish, horse mackerel (Trachurus trachurus) by different methods and storage at room temperature. Afr. Biochem. J. Res. 2013, 7, 98–106. [Google Scholar]
- Maeda, T.; Yuki, A.; Sakurai, H.; Watanabe, K.; Itoh, N.; Inui, E.; Seike, K.; Mizukami, Y.; Fukuda, Y.; Harada, K. Alcohol brine freezing of Japanese horse mackerel (Trachurus japonicus) for raw consumption. Trans. JSRAE 2007, 24, 323–330. [Google Scholar]
- Dufour, E.; Frencia, J.P.; Kane, E. Development of a rapid method based on frontface fluorescence spectroscopy for the monitoring of fish freshness. Food Res. Int. 2003, 36, 415–423. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chang, C.C.; Hsiao, T.C. Fluorescence intrinsic characterization of excitation-emission matrix using multi-dimensional ensemble empirical mode decomposition. Int. Mol. J. Sci. 2013, 14, 22436–22448. [Google Scholar] [CrossRef] [PubMed]
- Sádecká, J.; Tóthová, J. Fluorescence spectroscopy and chemometrics in the food classification-a review. Czech J. Food Sci. 2007, 25, 159–173. [Google Scholar] [CrossRef]
- Andersen, C.M.; Mortensen, G. Fluorescence spectroscopy: A rapid tool for analyzing dairy products. J. Agric. Food Chem. 2008, 56, 720–729. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bui, M.V.; Shibata, M.; Nakazawa, N.; Rithu, M.N.A.; Yamashita, H.; Sadayasu, K.; Tsuchiyama, K.; Nakauchi, S.; Hagiwara, T.; et al. Rapid noninvasive monitoring of freshness variation in frozen shrimp using multidimensional fluorescence imaging coupled with chemometrics. Talanta 2021, 224, 121871–121880. [Google Scholar] [CrossRef]
- Mishima, T.; Nonaka, T.; Okamoto, A.; Tsuchimoto, M.; Ishiya, T.; Tachibana, K.; Tsuchimoto, M. Influence of storage temperatures and killing procedures on post-mortem changes in the muscle of horse mackerel caught near Nagasaki Prefecture, Japan. Fish. Sci. 2005, 71, 187–194. [Google Scholar] [CrossRef]
- Leiva, A.D.; Schwartz, S. Relation of pH to Serotonin, Melatonin, and Other Indole Compounds Reacted with o-Phthaldialdehyde. Clin. Chem. 1976, 22, 1999–2005. [Google Scholar] [CrossRef]
- Lepthien, S.; Wiltschi, B.; Bolic, B.; Budisa, N. In Vivo Engineering of Proteins with Nitrogen-containing Tryptophan Analogs. Appl. Microbiol. Biotechnol. 2006, 73, 740–754. [Google Scholar] [CrossRef]






| Frozen Fish Meat with Ice-Storage Condition | Partial Least Square Regression (PLSR) Models Using Wavelength Combinations of Ex. 250–420 nm/EM. 290–650 nm (403 Wavelength Pairs) | ||||||
|---|---|---|---|---|---|---|---|
| Calibration | Validation | Number of Latent Factors | |||||
| R2 | RMSE | Sample Size | R2 | RMSE | Sample Size | LFs | |
| Horse mackerel (0–48 h) | 0.88 | 0.08 | 28 | 0.71 | 0.16 | 14 | 6 |
| Spotted mackerel (0–40 h) | 0.95 | 0.07 | 14 | 0.90 | 0.11 | 7 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Shibata, M.; Nakazawa, N.; Rithu, M.N.A.; Nakauchi, S.; Hagiwara, T.; Osako, K.; Okazaki, E. Non-destructive Approach for the Prediction of pH in Frozen Fish Meat Using Fluorescence Fingerprints in Tandem with Chemometrics. Fishes 2022, 7, 364. https://doi.org/10.3390/fishes7060364
Rahman MM, Shibata M, Nakazawa N, Rithu MNA, Nakauchi S, Hagiwara T, Osako K, Okazaki E. Non-destructive Approach for the Prediction of pH in Frozen Fish Meat Using Fluorescence Fingerprints in Tandem with Chemometrics. Fishes. 2022; 7(6):364. https://doi.org/10.3390/fishes7060364
Chicago/Turabian StyleRahman, Md. Mizanur, Mario Shibata, Naho Nakazawa, Mst. Nazira Akhter Rithu, Shigeki Nakauchi, Tomoaki Hagiwara, Kazufumi Osako, and Emiko Okazaki. 2022. "Non-destructive Approach for the Prediction of pH in Frozen Fish Meat Using Fluorescence Fingerprints in Tandem with Chemometrics" Fishes 7, no. 6: 364. https://doi.org/10.3390/fishes7060364
APA StyleRahman, M. M., Shibata, M., Nakazawa, N., Rithu, M. N. A., Nakauchi, S., Hagiwara, T., Osako, K., & Okazaki, E. (2022). Non-destructive Approach for the Prediction of pH in Frozen Fish Meat Using Fluorescence Fingerprints in Tandem with Chemometrics. Fishes, 7(6), 364. https://doi.org/10.3390/fishes7060364






