Characterization of Fish Assemblages and Standard Length Distributions among Different Sampling Gears Using an Artificial Neural Network
Abstract
1. Introduction
2. Materials and Methods
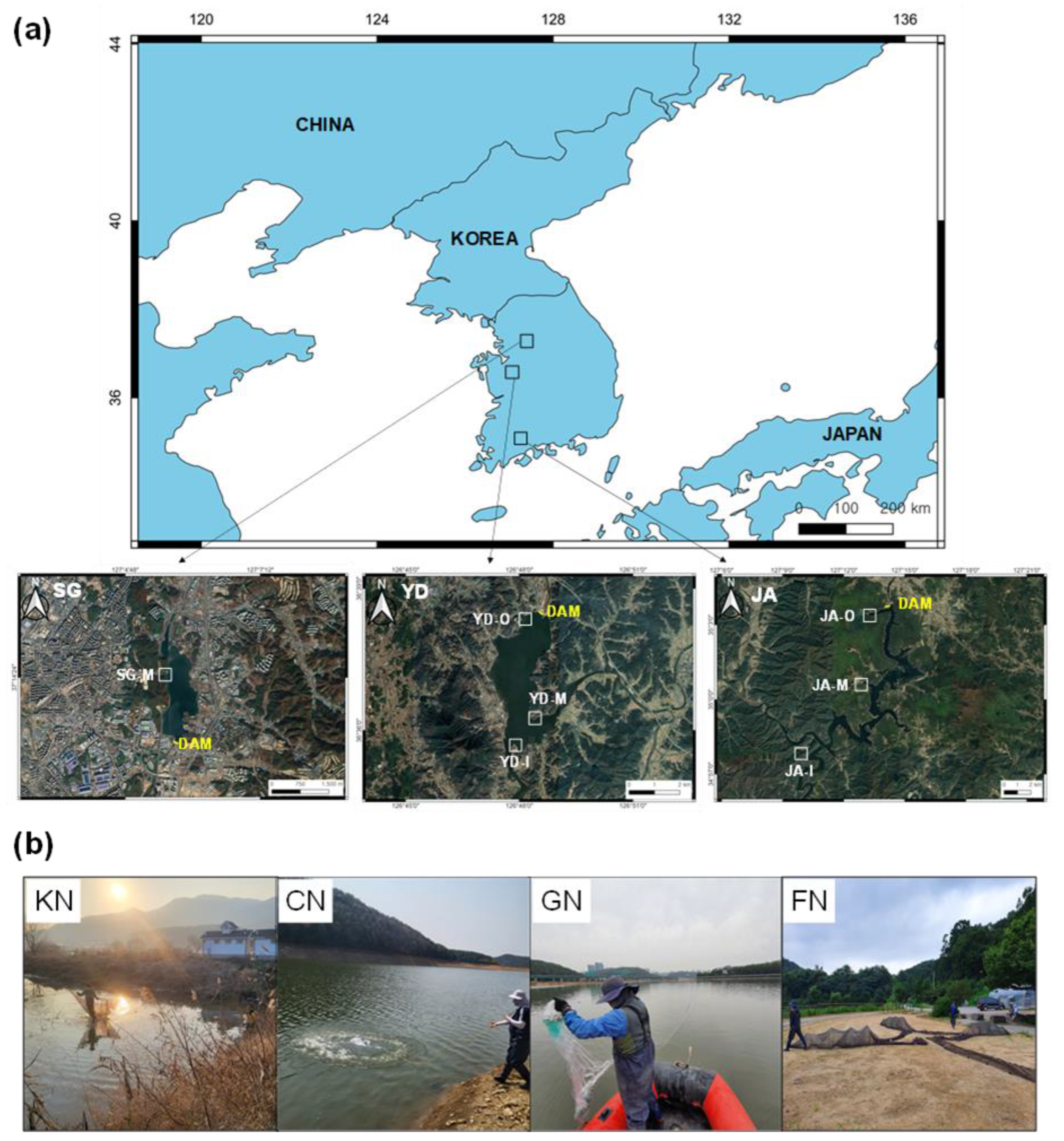
2.1. Study Location
2.2. Fish Collection and Identification
2.3. SOM and Data Analysis
3. Results
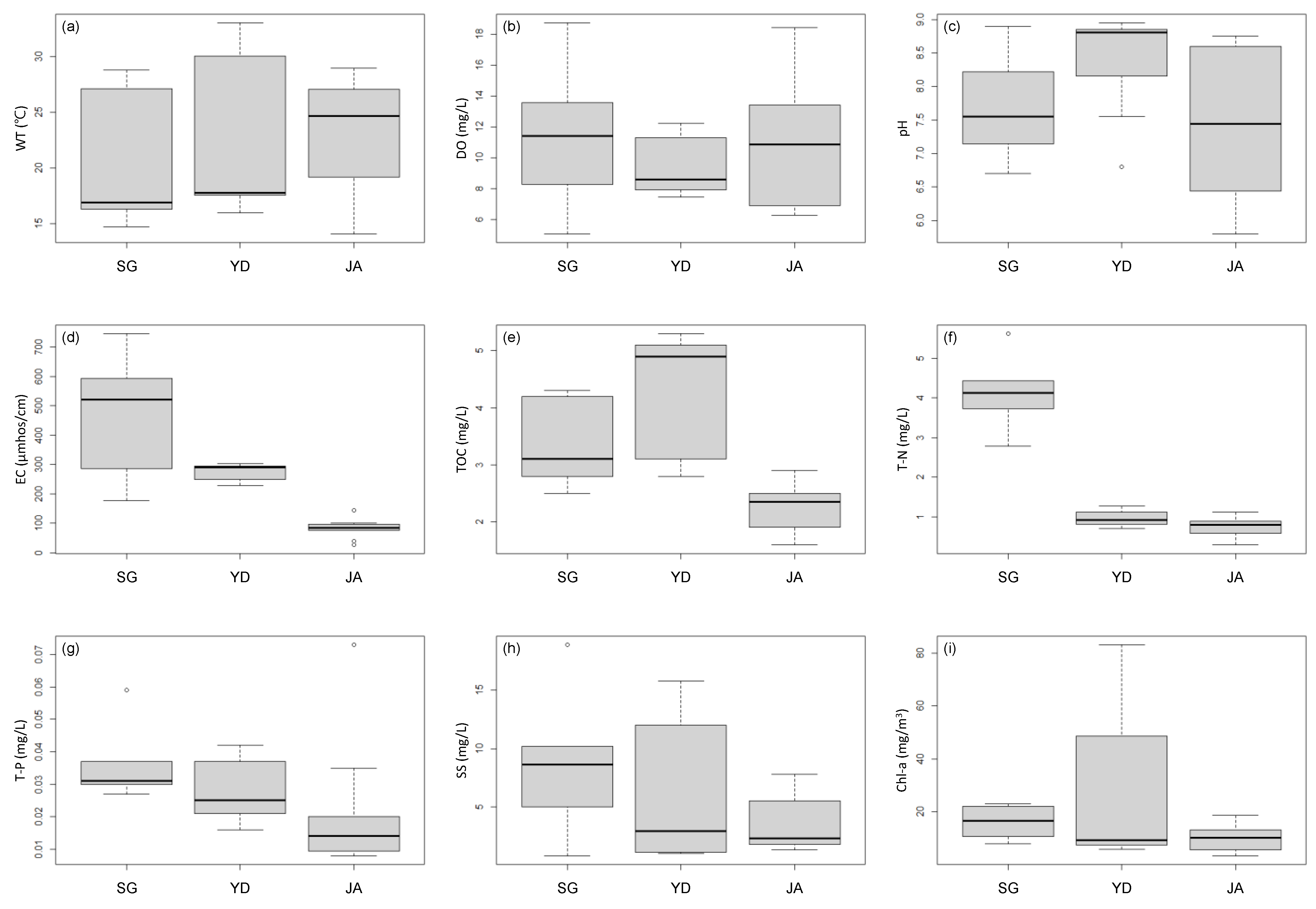
3.1. Water Quality
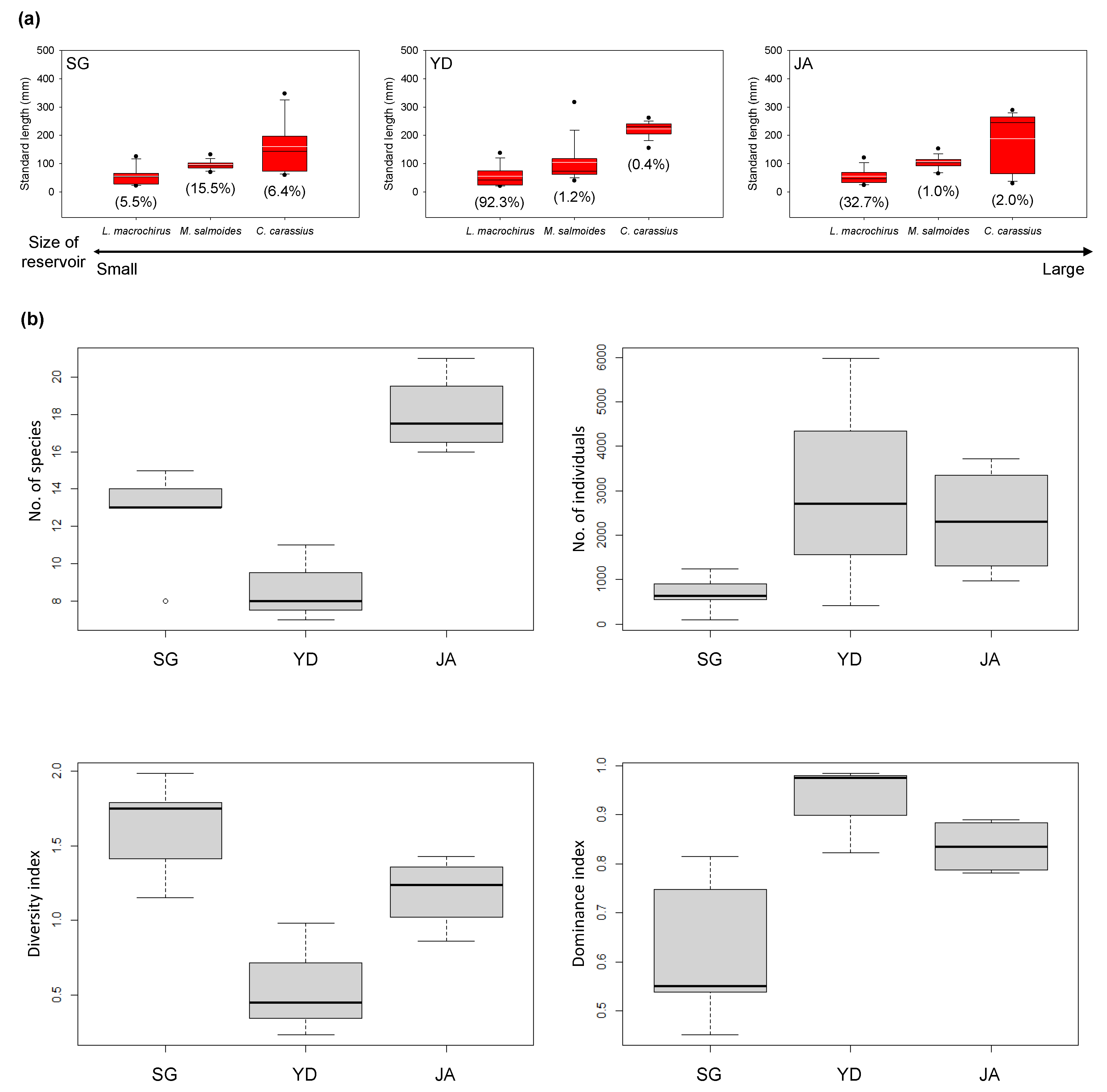
3.2. Fish Community Composition in Each Survey Reservoir
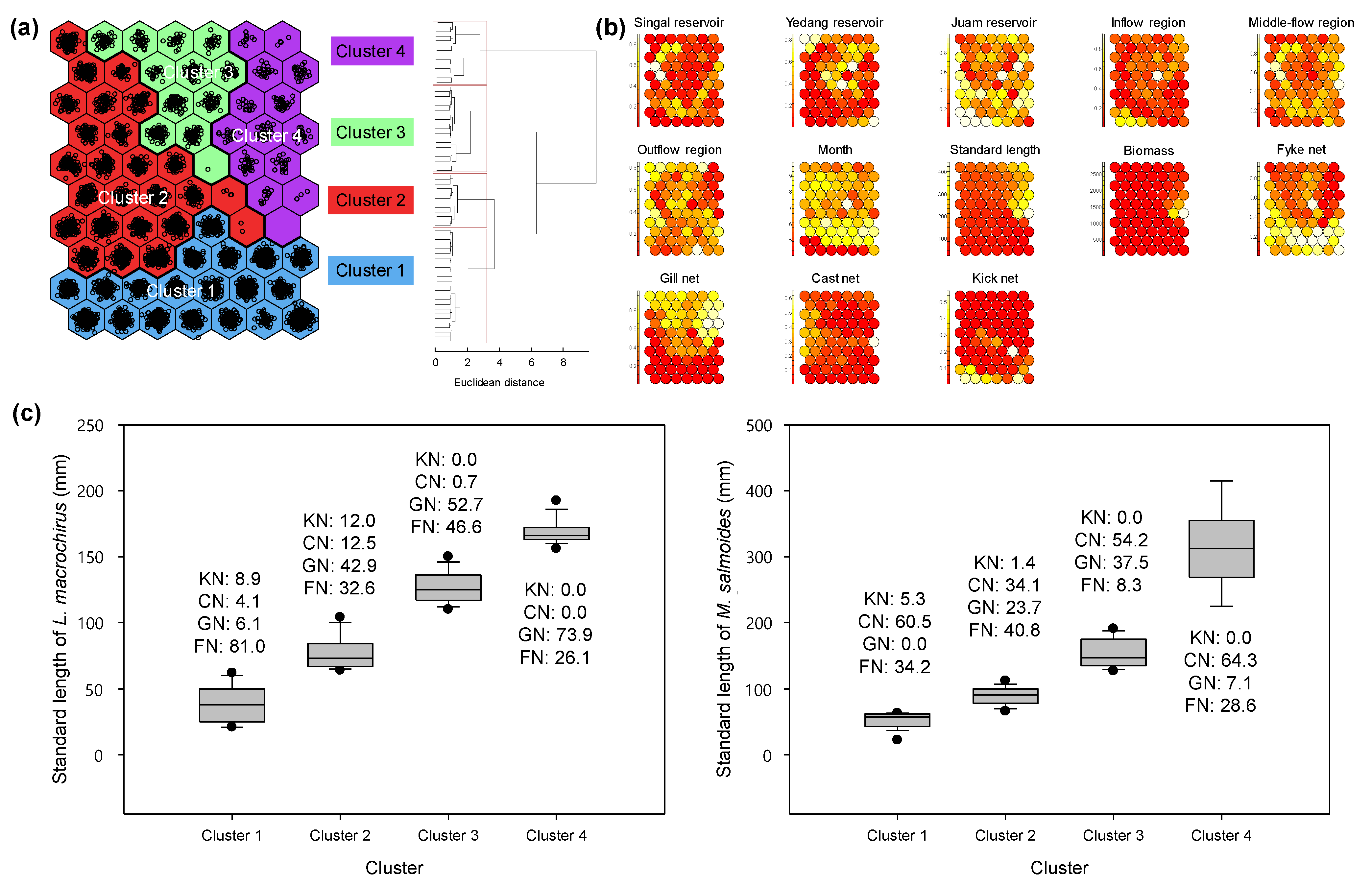
3.3. Extracting Fish Assemblage Using SOM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pennington, M.; Stromme, T. Surveys as a research tool for managing dynamic stocks. Fish. Res. 1998, 37, 97–106. [Google Scholar] [CrossRef]
- Fischer, J.R.; Quist, M.C. Characterizing lentic freshwater fish assemblages using multiple sampling methods. Environ. Monit. Assess. 2014, 186, 4461–4474. [Google Scholar] [CrossRef]
- Kennard, M.J.; Pusey, B.J.; Harch, B.D.; Dore, E.; Arthington, A.H. Estimating local stream fish assemblage attributes: Sampling effort and efficiency at two spatial scales. Mar. Freshw. Res. 2006, 57, 635–653. [Google Scholar] [CrossRef]
- Brandt, A.V. Fish Catching Methods of the World; Fishing News Books: Surrey, UK, 1984; pp. 1–418. [Google Scholar]
- Franco, A.; Perez-Ruzafa, A.; Drouineau, H.; Franzoi, P.; Koutrakis, E.T.; Lepage, M.; Verdiell-Cubedo, D.; Bouchoucha, M.; Lopez-Capel, A.; Riccato, F.; et al. Assessment of fish assemblages in coastal lagoon habitats: Effect of sampling method. Estuar. Coast. Shelf Sci. 2012, 112, 115–125. [Google Scholar] [CrossRef]
- Han, J.H.; An, S.L.; An, K.G. Application of different fish sampling gear in Korean reservoirs and the analysis of sampling efficiencies. J. Asia-Pac. Biodivers. 2019, 12, 528–540. [Google Scholar] [CrossRef]
- Weaver, M.J.; Magnuson, J.J.; Clayton, M.K. Analyses for differentiating littoral fish assemblages with catch data from multiple sampling gears. Trans. Am. Fish. Soc. 1993, 122, 1111–1119. [Google Scholar] [CrossRef]
- Clark, S.J.; Jackson, J.R.; Lochmann, S.E. A comparison of shoreline seines with fyke nets for sampling littoral fish communities in floodplain lakes. N. Am. J. Fish. Manag. 2007, 27, 676–680. [Google Scholar] [CrossRef]
- Jackson, D.A.; Harvey, H.H. Qualitative and quantitative sampling of lake fish communities. Can. J. Fish. Aquat. Sci. 1997, 54, 2807–2813. [Google Scholar] [CrossRef]
- McInerny, M.C.; Cross, T.K. Comparison of Day Electrofishing, Night Electrofishing, and Trap Netting for Sampling in Shore Fish in Minnesota Lakes; Minnesota Department of Natural Resources: Hutchinson, MN, USA, 2004; pp. 1–29. [Google Scholar]
- Mueller, M.; Pander, J.; Knott, J.; Geist, J. Comparison of nine different methods to assess fish communities in lentic flood-plain habitats. J. Fish Biol. 2017, 91, 144–174. [Google Scholar] [CrossRef]
- Chon, T.-S.; Park, Y.S.; Moon, K.H.; Cha, E.Y. Patternizing communities by using an artificial neural network. Ecol. Model. 1996, 90, 69–78. [Google Scholar] [CrossRef]
- Kohonen, T. The self-organizing map. Proc. IEEE 1990, 78, 1464–1480. [Google Scholar] [CrossRef]
- Kohonen, T. The SOM methodology. In Visual Explorations in Finance; Springer: London, UK, 1998; pp. 159–167. [Google Scholar] [CrossRef]
- Park, Y.S.; Cereghino, R.; Compin, A.; Lek, S. Applications of artificial neural networks for patterning and predicting aquatic insect species richness in running waters. Ecol. Model. 2003, 160, 265–280. [Google Scholar] [CrossRef]
- Kohonen, T. Self-organized formation of topologically correct feature maps. Biol. Cybern. 1982, 43, 59–69. [Google Scholar] [CrossRef]
- Kohonen, T. Learning vector quantization. In Self-Organizing Maps; Springer: Berlin/Heidelberg, Germany, 2001; pp. 245–261. [Google Scholar] [CrossRef]
- Rauber, A.; Merkl, D.; Dittenbach, M. The growing hierarchical self-organizing map: Exploratory analysis of high-dimensional data. IEEE Trans. Neural Netw. 2002, 13, 1331–1341. [Google Scholar] [CrossRef]
- Ajemian, M.J.; Wetz, J.J.; Shipley-Lozano, B.; Shively, J.D.; Stunz, G.W. An analysis of artificial reef fish community structure along the northwestern Gulf of Mexico shelf: Potential impacts of “Rigs-to-reefs” programs. PLoS ONE 2015, 10, e0126354. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J.; Tonkin, J.D.; Alexander, M.E.; Dalu, M.T.B.; Motitsoe, S.N.; Manungo, K.I.; Bepe, O.; Dube, T. Assessing drivers of benthic macroinvertebrate community structure in African highland streams: An exploration using multivariate analysis. Sci. Total Environ. 2017, 601, 1340–1348. [Google Scholar] [CrossRef]
- Soininen, J.; Korhonen, J.J.; Karhu, J.; Vetterli, A. Disentangling the spatial patterns in community composition of prokaryotic and eukaryotic lake plankton. Limnol. Oceanogr. 2011, 56, 508–520. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Kim, S.; Mun, J.; Kwon, D.; Kang, B.; Song, N.; Kim, S. Biomonitoring Survey and Assessment Manual; National Institute of Environmental Research: Incheon, Korea, 2016.
- Park, J.-Y.; Jung, W. Measurement Theory development of suspended solid concentration using glass fiber membrane module. Membr. J. 2009, 19, 268–276. [Google Scholar]
- Kim, I.S.; Choi, Y.; Lee, C.; Lee, Y.; Kim, B.; Kim, J. Illustrated Book of Korean Fishes; Kyo-Hak Publishing Co.: Seoul, Korea, 2005; pp. 1–615. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 25 February 2022).
- Kim, B.; Lee, S.; An, J. Fish species of Korea. National List of Species of Korea: Vertebrates; National Institution of Biological Resources, Ed.; National Institution of Biological Resources: Incheon, Korea, 2011; pp. 86–87.
- Shannon, C.E.; Weaver, W. ProQuest. The Mathematical Theory of Communication, 1st ed.; University of Illinois Press: Urbana, IL, USA, 1949; 117p. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Ron Wehrens, J.K. Flexible Self-Organizing Maps in kohonen 3.0. J. Stat. Softw. 2018, 87, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.; Simpson, G.; Solymos, P. Vegan: Community Ecology Package (Version 2.5-6); The Comprehensive R Archive Network: Wien, Austria, 2019. [Google Scholar]
- Tran, C.P.; Bode, R.W.; Smith, A.J.; Kleppel, G.S. Land-use proximity as a basis for assessing stream water quality in New York State (USA). Ecol. Indic. 2010, 10, 727–733. [Google Scholar] [CrossRef]
- Cross, W.F.; Benstead, J.P.; Rosemond, A.D.; Wallace, J.B. Consumer-resource stoichiometry in detritus-based streams. Ecol. Lett. 2003, 6, 721–732. [Google Scholar] [CrossRef]
- Frost, P.C.; Tank, S.E.; Turner, M.A.; Elser, J.J. Elemental composition of littoral invertebrates from oligotrophic and eutrophic Canadian lakes. J. N. Am. Benthol. Soc. 2003, 22, 51–62. [Google Scholar] [CrossRef]
- Stelzer, R.S.; Lamberti, G.A. Effects of N: P ratio and total nutrient concentration on stream periphyton community structure, biomass, and elemental composition. Limnol. Oceanogr. 2001, 46, 356–367. [Google Scholar] [CrossRef]
- Guildford, S.J.; Hecky, R.E. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol. Oceanogr. 2000, 45, 1213–1223. [Google Scholar] [CrossRef]
- Wang, H.J.; Liang, X.M.; Jiang, P.H.; Wang, J.; Wu, S.K.; Wang, H.Z. TN: TP ratio and planktivorous fish do not affect nutrient-chlorophyll relationships in shallow lakes. Freshw. Biol. 2008, 53, 935–944. [Google Scholar] [CrossRef]
- Byon, H.K.; Jeon, S.R. Feeding habit of Bluegill, Lepomis macrochirus introduced in Korea. Korean J. Envrion. Biol. 1997, 15, 165–174. [Google Scholar]
- Choe, S.; Park, H.; Lee, D.; Kang, Y.; Jeon, H.K.; Eom, K.S. Infections with digenean trematode metacercariae in two invasive alien fish, Micropterus salmoides and Lepomis macrochirus, in two rivers in Chungcheongbuk-do, Republic of Korea. Korean J. Parasitol. 2018, 56, 509–513. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Lee, S.-J.; An, K.-G. Physical habitat and chemical water quality characteristics on the distribution patterns of ecologically disturbing fish (Largemouth bass and Bluegill) in Dongjin-River Watershed. Korean J. Environ. Biol. 2019, 37, 177–188. [Google Scholar] [CrossRef]
- Meador, M.; Kelso, W. Physiological responses of largemouth bass, Micropterus salmoides, exposed to salinity. Can. J. Fish. Aquat. Sci. 1990, 47, 2358–2363. [Google Scholar] [CrossRef]
- Stevens, P.W.; Montague, C.L.; Sulak, K.J. Patterns of fish use and piscivore abundance within a reconnected saltmarsh impoundment in the northern Indian River Lagoon, Florida. Wetl. Ecol. Manag. 2006, 14, 147–166. [Google Scholar] [CrossRef]
- Emmanuel, B.E.; Chukwu, L.O.; Azeez, L.O. Cast net design characteristics, catch composition and selectivity in tropical open lagoon. Afr. J. Biotechnol. 2008, 7, 2081–2089. [Google Scholar] [CrossRef]
- Hamley, J.M. Review of gillnet selectivity. J. Fish. Res. Board Can. 1975, 32, 1943–1969. [Google Scholar] [CrossRef]
- Carol, J.; Garcia-Berthou, E. Gillnet selectivity and its relationship with body shape for eight freshwater fish species. J. Appl. Ichthyol. 2007, 23, 654–660. [Google Scholar] [CrossRef]
- Reis, E.G.; Pawson, M.G. Fish morphology and estimating selectivity by gillnets. Fish. Res. 1999, 39, 263–273. [Google Scholar] [CrossRef]
- Rulifson, R.A. Finfish utilization of man-initiated and adjacent natural creeks of South Creek estuary, North Carolina using multiple gear types. Estuaries 1991, 14, 447–464. [Google Scholar] [CrossRef]
- Fischer, J.R.; Johnson, N.P.; Schultz, R.D.; Quist, M.C. A comparison of modified fyke nets for evaluating fish assemblages and population structure. J. Freshw. Ecol. 2010, 25, 555–563. [Google Scholar] [CrossRef]
- Casselman, J.M.; Penczak, T.; Carl, L.; Mann, R.H.; Holcik, J.; Woitowich, W.A. An evaluation of fish sampling methodologies for large river systems. Pol. Arch. Hydrobiol. 1990, 37, 521–551. [Google Scholar]
- Lapointe, N.W.R.; Corkum, L.D.; Mandrak, N.E. A comparison of methods for sampling fish diversity in shallow offshore waters of large rivers. N. Am. J. Fish. Manag. 2006, 26, 503–513. [Google Scholar] [CrossRef]
- Poesch, M.S. Developing standardized methods for sampling freshwater fishes with multiple gears: Effects of sampling order versus sampling method. Trans. Am. Fish. Soc. 2014, 143, 353–362. [Google Scholar] [CrossRef]
- Poos, M.S.; Mandrak, N.E.; McLaughlin, R.L. The effectiveness of two common sampling methods for assessing imperilled freshwater fishes. J. Fish Biol. 2007, 70, 691–708. [Google Scholar] [CrossRef]
- Radinger, J.; Britton, J.R.; Carlson, S.M.; Magurran, A.E.; Alcaraz-Hernandez, J.D.; Almodovar, A.; Benejam, L.; Fernandez-Delgado, C.; Nicola, G.G.; Oliva-Paterna, F.J.; et al. Effective monitoring of freshwater fish. Fish Fish. 2019, 20, 729–747. [Google Scholar] [CrossRef]
- Booth, A.J.; Potts, W.M. Estimating gill-net selectivity for Labeo umbratus (Pisces: Cyprinidae), and an evaluation of using fyke-nets as a non-destructive sampling gear in small reservoirs. Fish. Res. 2006, 79, 202–209. [Google Scholar] [CrossRef]
- Kraft, C.E.; Johnson, B.L. Fyke-net and gill-net size selectivities for yellow perch in Green Bay, Lake Michigan. N. Am. J. Fish. Manag. 1992, 12, 230–236. [Google Scholar] [CrossRef]
| Fish Species | Reservoirs | Total | * R.A. (%) | ||
|---|---|---|---|---|---|
| Singal | Yedang | Juam | |||
| Cyprinus carpio | 55 | 3 | 7 | 65 | 0.3 |
| Carassius carassius | 179 | 35 | 184 | 398 | 2.0 |
| Carassius cuvieri | 41 | 12 | 21 | 74 | 0.4 |
| Channa argus | 1 | 1 | 2 | <0.1 | |
| Acheilognathus yamatsutae | 6 | 6 | <0.1 | ||
| Acanthorhodeus chankaensis | 1 | 106 | 107 | 0.5 | |
| Tanakia lanceolata | 1 | 1 | <0.1 | ||
| Hemibarbus labeo | 55 | 55 | 0.3 | ||
| Hemibarbus longirostris | 4 | 4 | <0.1 | ||
| Pseudogobio esocinus | 7 | 1 | 8 | <0.1 | |
| Pseudorasbora parva | 933 | 48 | 15 | 996 | 4.9 |
| Squalidus chankaensis tsuchigae | 113 | 113 | 0.6 | ||
| Squalidus japonicus coreanus | 12 | 12 | 0.1 | ||
| Pungtungia herzi | 23 | 23 | 0.1 | ||
| Microphysogobio yaluensis | 27 | 27 | 0.1 | ||
| Hemiculter leucisculus | 7 | 464 | 596 | 1067 | 5.3 |
| Zacco platypus | 92 | 1 | 2400 | 2493 | 12.3 |
| Opsariichthys uncirostris amurensis | 121 | 121 | 0.6 | ||
| Nipponocypris temminckii | 2 | 2 | <0.1 | ||
| Cobitis lutheri | 1 | 1 | <0.1 | ||
| Cobitis tetralineata | 12 | 12 | 0.1 | ||
| Misgurnus anguillicaudatus | 2 | 2 | <0.1 | ||
| Silurus asotus | 2 | 4 | 9 | 15 | 0.1 |
| Tachysurus fulvidraco | 7 | 7 | <0.1 | ||
| Hypomesus olidus | 2421 | 2421 | 11.9 | ||
| Odontobutis interrupta | 1 | 1 | 2 | <0.1 | |
| Rhinogobius brunneus | 44 | 12 | 26 | 82 | 0.4 |
| Rhinogobius giurinus | 1 | 1 | <0.1 | ||
| Siniperca scherzeri | 1 | 1 | <0.1 | ||
| Micropterus salmoides | 377 | 109 | 97 | 583 | 2.9 |
| Lepomis macrochirus | 143 | 8410 | 3040 | 11,593 | 57.1 |
| Number of individuals | 1887 | 9113 | 9294 | 20,294 | |
| Number of species | 13 | 15 | 27 | 31 | |
| Biomass (g) | 78,993.3 | 82,013.8 | 176,169.9 | 337,177.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.-S.; Ji, C.W.; Park, Y.-S.; Han, K.-H.; Kwak, I.-S. Characterization of Fish Assemblages and Standard Length Distributions among Different Sampling Gears Using an Artificial Neural Network. Fishes 2022, 7, 275. https://doi.org/10.3390/fishes7050275
Yu T-S, Ji CW, Park Y-S, Han K-H, Kwak I-S. Characterization of Fish Assemblages and Standard Length Distributions among Different Sampling Gears Using an Artificial Neural Network. Fishes. 2022; 7(5):275. https://doi.org/10.3390/fishes7050275
Chicago/Turabian StyleYu, Tae-Sik, Chang Woo Ji, Young-Seuk Park, Kyeong-Ho Han, and Ihn-Sil Kwak. 2022. "Characterization of Fish Assemblages and Standard Length Distributions among Different Sampling Gears Using an Artificial Neural Network" Fishes 7, no. 5: 275. https://doi.org/10.3390/fishes7050275
APA StyleYu, T.-S., Ji, C. W., Park, Y.-S., Han, K.-H., & Kwak, I.-S. (2022). Characterization of Fish Assemblages and Standard Length Distributions among Different Sampling Gears Using an Artificial Neural Network. Fishes, 7(5), 275. https://doi.org/10.3390/fishes7050275






