Molecular Characterization and Expression Analysis of TAK1, TAB1 and TAB2 of Golden Pompano (Trachinotus ovatus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Tissue Samples
2.2. Total RNA Extraction and cDNA Synthesis
2.3. Molecular Cloning of trTAK1, trTAB1 and trTAB2 cDNA
2.4. Sequence Analysis
2.5. RT-qPCR
2.6. Statistical Analysis
3. Results
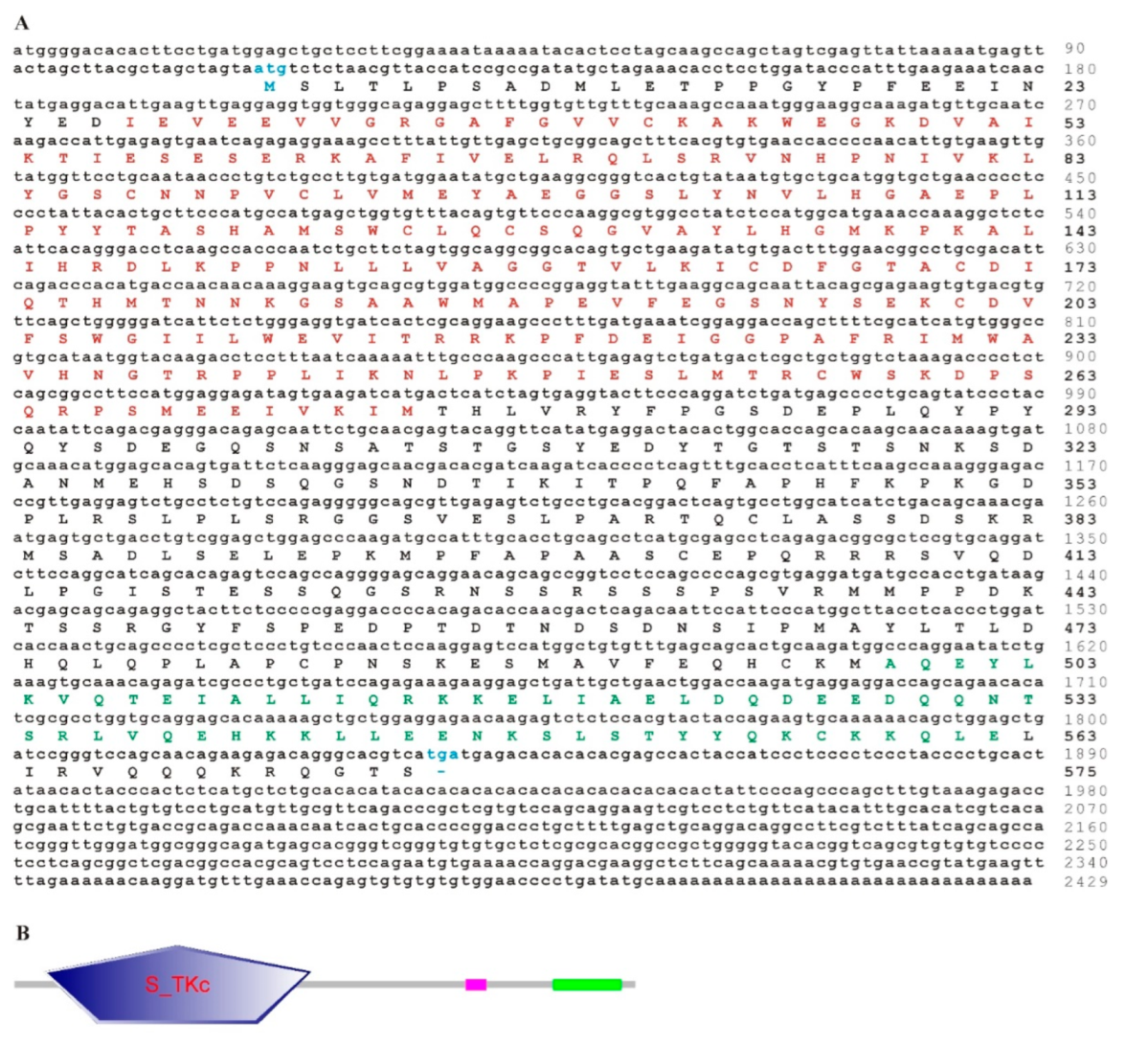
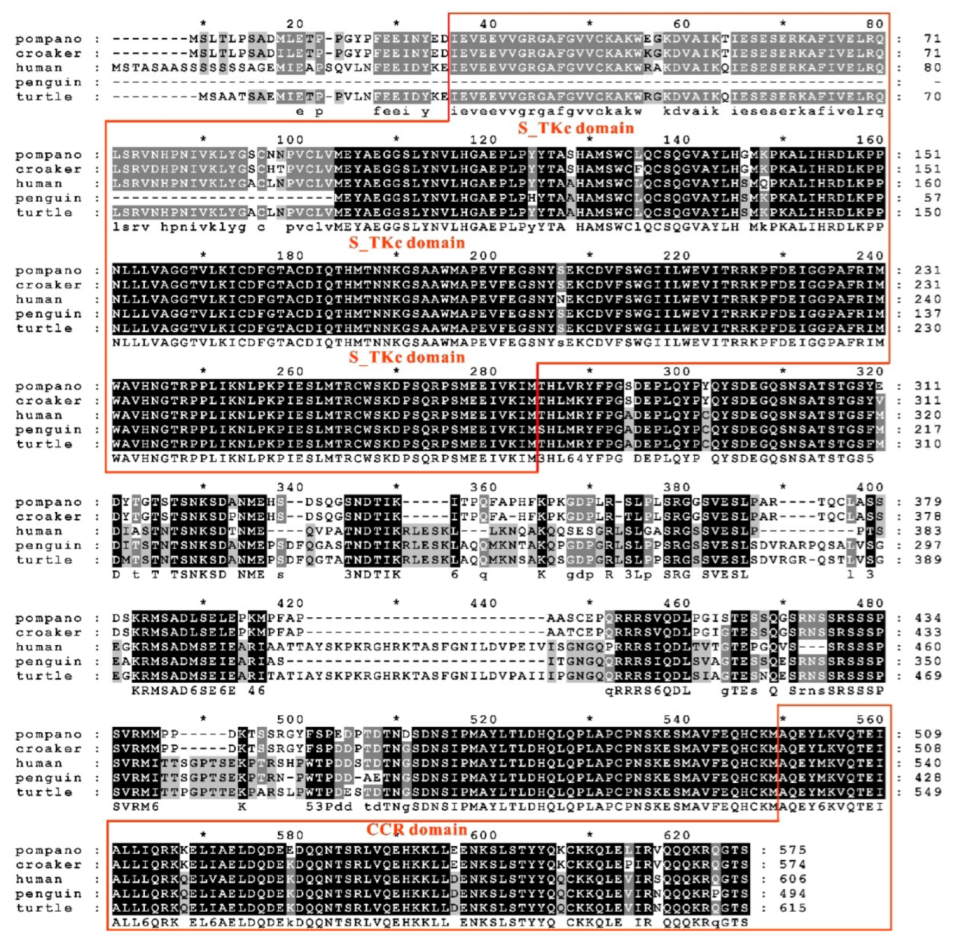
3.1. Sequence Features of trTAK1
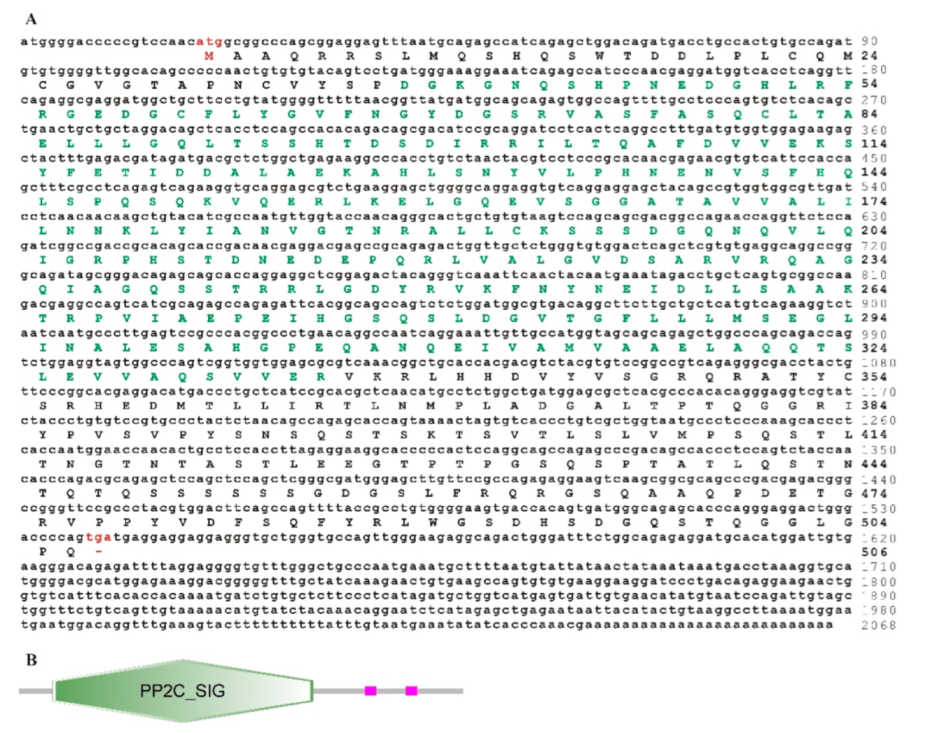
3.2. Sequence Features of trTAB1
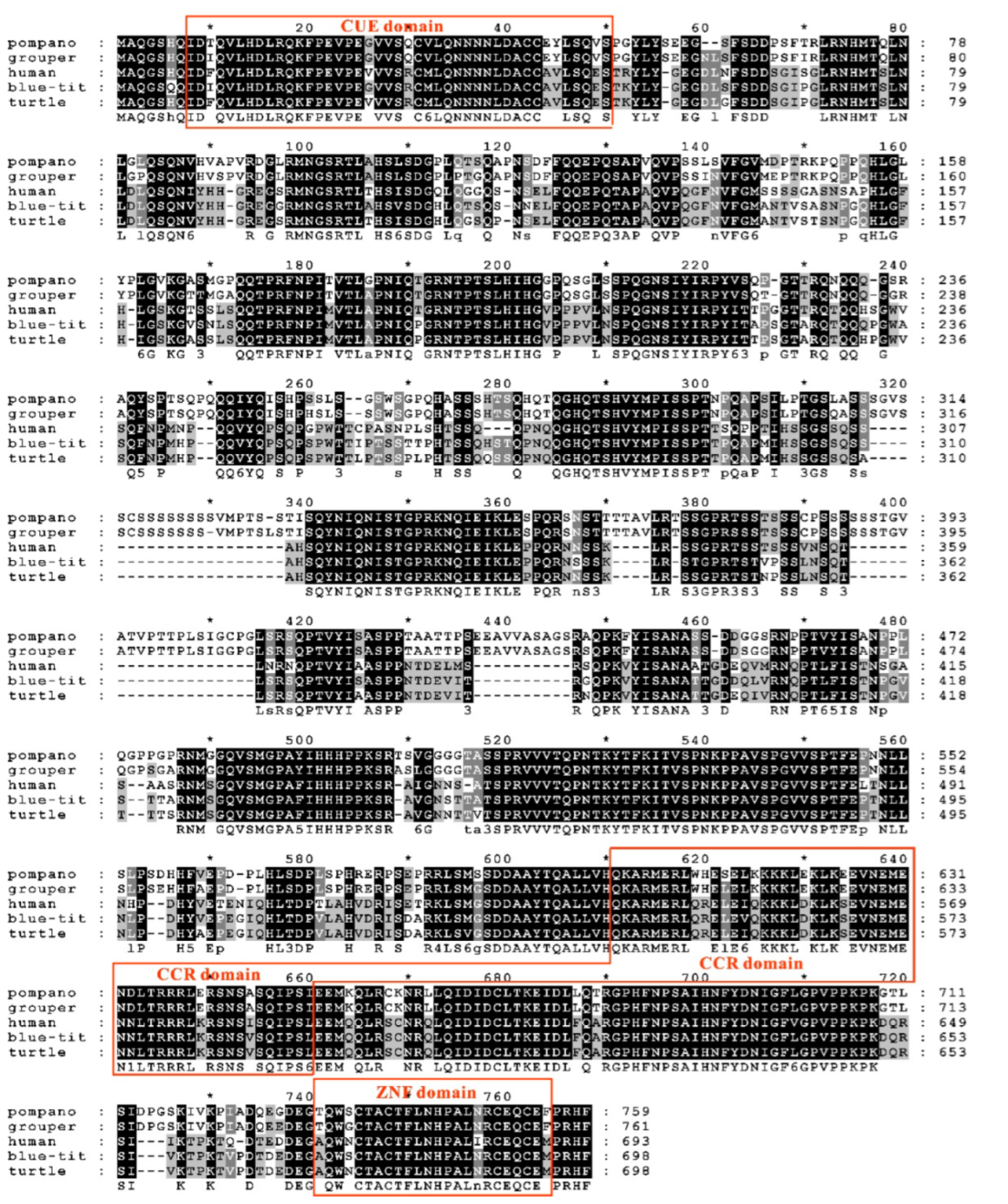
3.3. Sequence Features of trTAB2
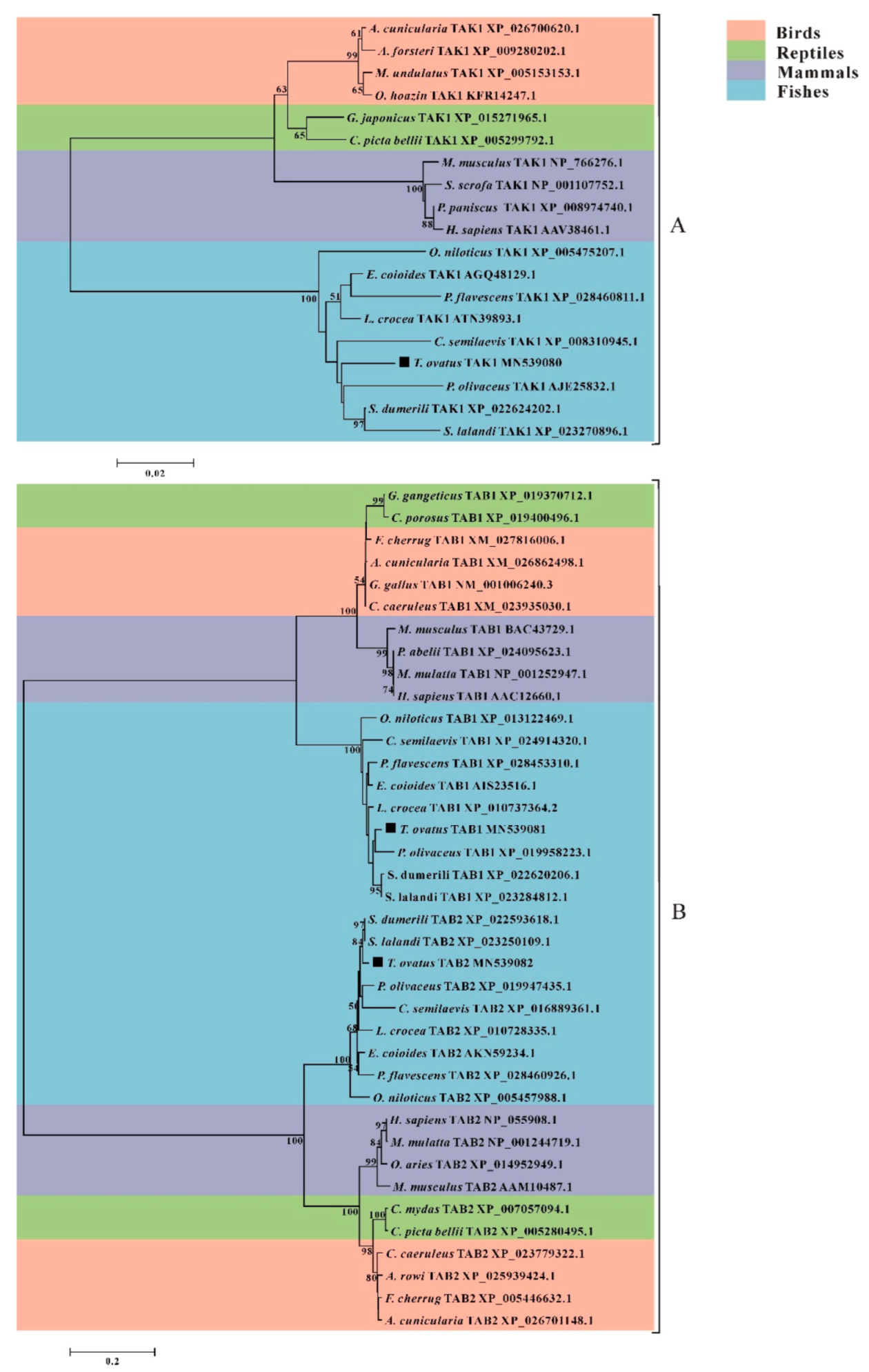
3.4. Phylogenetic Analysis of trTAK1, trTAB1 and trTAB2
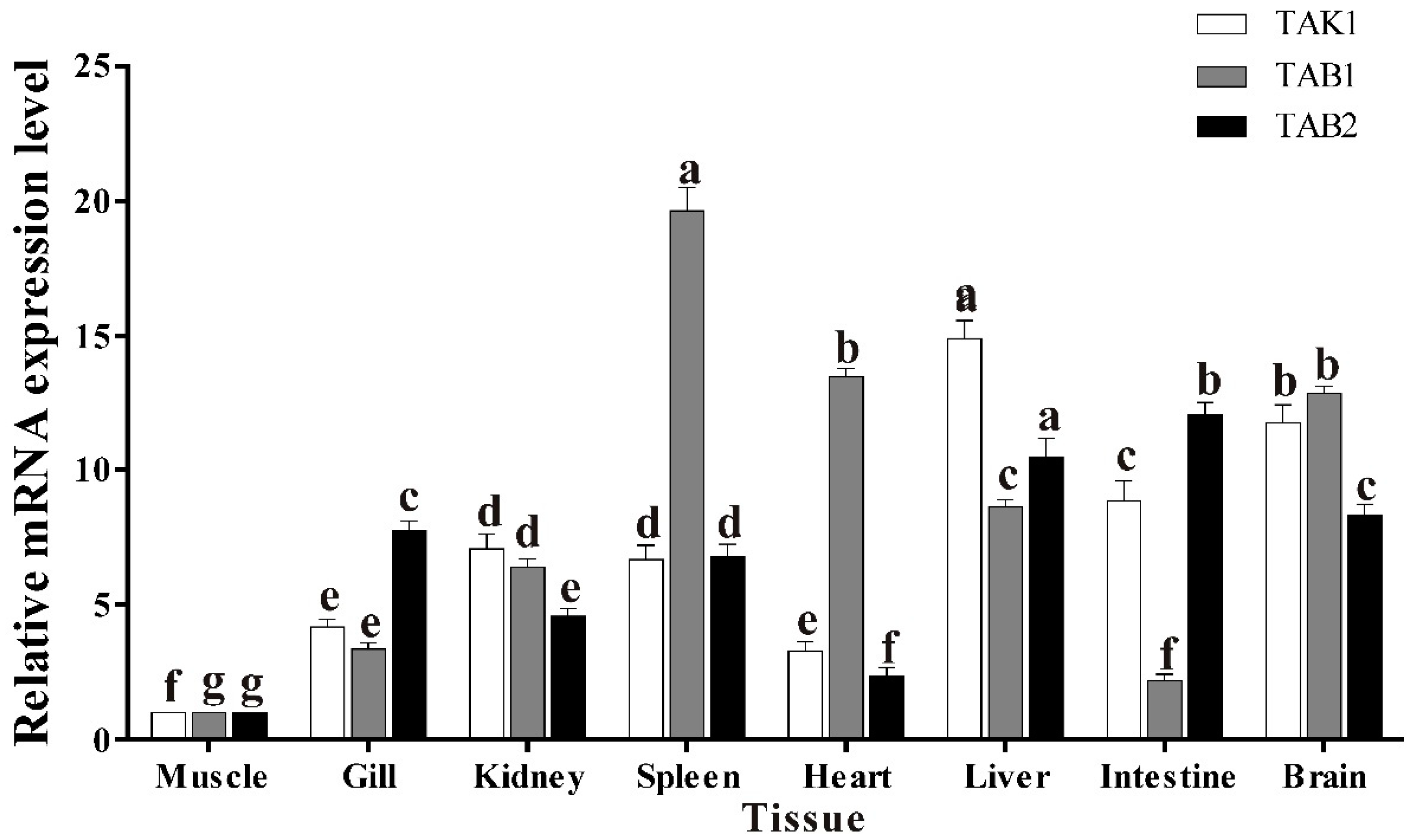
3.5. Basal Tissue Expression of trTAK1, trTAB1 and trTAB2
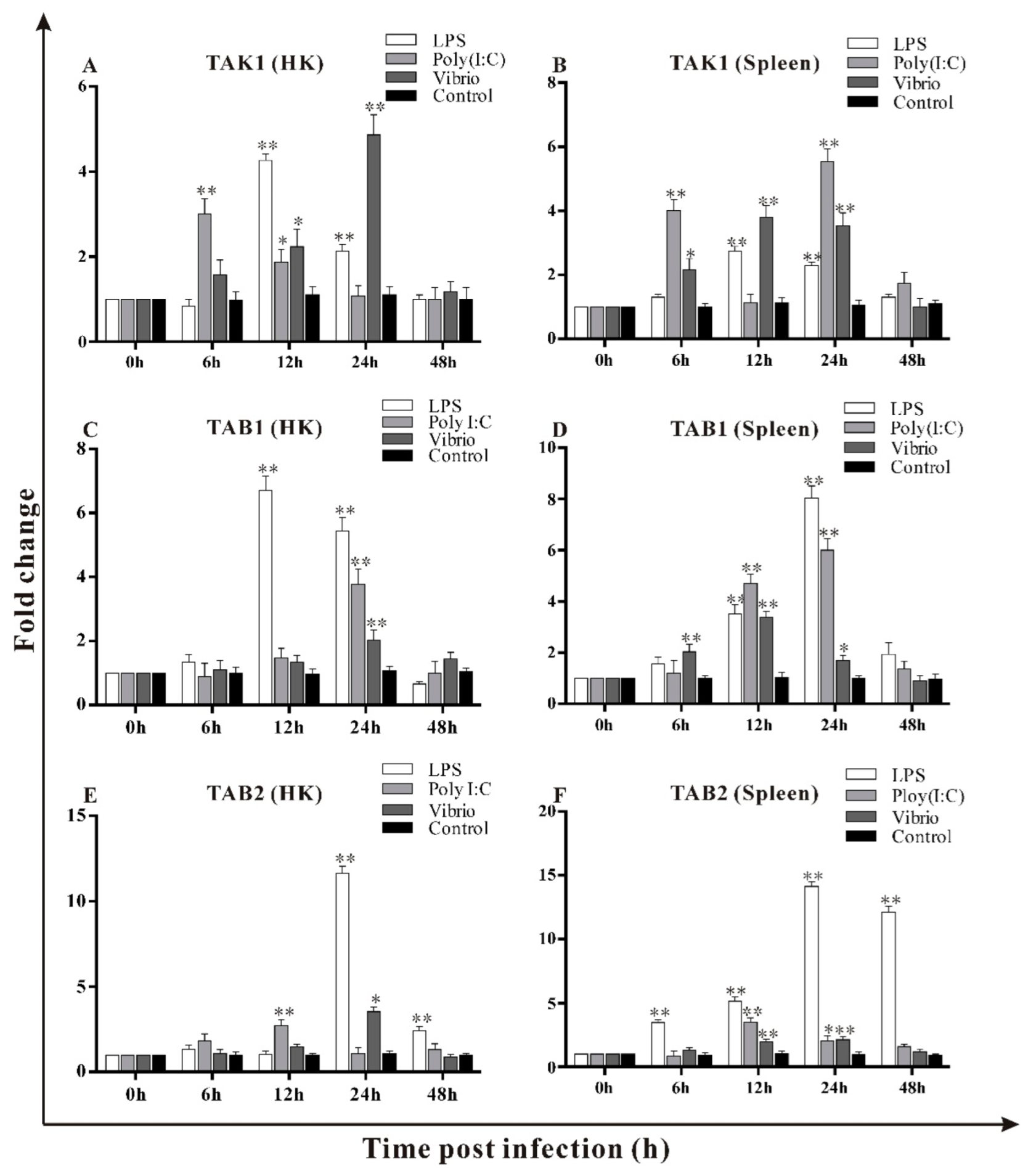
3.6. Transcripts Changes of trTAK1, trTAB1 and trTAB2 in Response to LPS, Poly I:C and V. alginolyticus Challenges
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ajibade, A.A.; Wang, H.Y.; Wang, R.-F. Cell type-specific function of TAK1 in innate immune signaling. Trends. Immunol. 2013, 34, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Fujimoto, W.; Tamaai, T.; Kim, K.H.; Matsuura, H.; Jetten, A.M. TAK1: Molecular cloning and characterization of a new member of the nuclear receptor superfamily. Mol. Endocrinol. 1994, 8, 1667–1680. [Google Scholar] [PubMed] [Green Version]
- Dai, L.; Thu, C.A.; Liu, X.-Y.; Xi, J.; Cheung, P.C.F. TAK1, more than just innate immunity. Iubmb Life 2012, 64, 825–834. [Google Scholar] [CrossRef]
- Shirakabe, K.; Yamaguchi, K.; Shibuya, H.; Irie, K.; Matsuda, S.; Moriguchi, T.; Gotoh, Y.; Matsumoto, K.; Nishida, E. TAK1 mediates the ceramide signaling to stress-activated protein kinase c-Jun N-terminal kinase. J. Biol. Chem. 1997, 272, 8141–8144. [Google Scholar] [CrossRef] [Green Version]
- Takaesu, G.; Surabhi, R.M.; Park, K.J.; Ninomiya-Tsuji, J.; Matsumoto, K.; Gaynor, R.B. TAK1 is critical for I kappa B kinase-mediated activation of the NF-kappa B pathway. J. Mol. Biol. 2003, 326, 105–115. [Google Scholar] [CrossRef]
- Shim, J.H.; Xiao, C.C.; Paschal, A.E.; Bailey, S.T.; Rao, P.; Hayden, M.S.; Lee, K.Y.; Bussey, C.; Steckel, M.; Tanaka, N.; et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005, 19, 2668–2681. [Google Scholar] [CrossRef] [Green Version]
- Delaney, J.R.; Mlodzik, M. TGF-beta activated kinase-1—New insights into the diverse roles of TAK1 in development and immunity. Cell Cycle 2006, 5, 2852–2855. [Google Scholar] [CrossRef] [Green Version]
- Paquette, N.; Conlon, J.; Sweet, C.; Rus, F.; Wilson, L.; Pereira, A.; Rosadini, C.V.; Goutagny, N.; Weber, A.N.R.; Lane, W.S.; et al. Serine/threonine acetylation of TGF beta-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 12710–12715. [Google Scholar] [CrossRef] [Green Version]
- Boutros, M.; Agaisse, H.; Perrimon, N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev. Cell 2002, 3, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Park, J.M.; Brady, H.; Ruocco, M.G.; Sun, H.Y.; Williams, D.; Lee, S.J.; Kato, T.; Richards, N.; Chan, K.; Mercurio, F.; et al. Targeting of TAK1 by the NF-kappa B protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 2004, 18, 584–594. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, H.; Nagashima, T.; Cascalho, M.I.; Kurosaki, T. TAK1 maintains the survival of immunoglobulin -chain-positive B cells. Genes Cells 2016, 21, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-R.; Lei, C.-Q. TAK1-TABs Complex: A Central Signalosome in Inflammatory Responses. Front. Immunol. 2021, 11, 608976. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Yamaguchi, K.; Shirakabe, K.; Tonegawa, A.; Gotoh, Y.; Ueno, N.; Irie, K.; Nishida, E.; Matsumoto, K. TAB1: An activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science 1996, 272, 1179–1182. [Google Scholar] [CrossRef]
- Ge, B.X.; Gram, H.; Di Padova, F.; Huang, B.; New, L.; Ulevitch, R.J.; Luo, Y.; Han, J.H. MAPKK-independent activation of p38 alpha mediated by TAB1-dependent autophosphorylation of p38 alpha. Science 2002, 295, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-S.; Shinghirunnusorn, P.; Sugishima, Y.; Nishimura, M.; Suzuki, S.; Koizumi, K.; Saiki, I.; Sakurai, H. Cross interference with TNF-alpha-induced TAK1 activation via EGFR-mediated p38 phosphorylation of TAK1-binding protein 1. BBA-Mol. Cell. Res. 2009, 1793, 1156–1164. [Google Scholar]
- Mihaly, S.R.; Ninomiya-Tsuji, J.; Morioka, S. TAK1 control of cell death. Cell Death Differ. 2014, 21, 1667–1676. [Google Scholar] [CrossRef] [Green Version]
- Aashaq, S.; Batool, A.; Andrabi, K.I. TAK1 mediates convergence of cellular signals for death and survival. Apoptosis 2019, 24, 3–20. [Google Scholar] [CrossRef]
- Ono, K.; Ohtomo, T.; Sato, S.; Sugamata, Y.; Suzuki, M.; Hisamoto, N.; Ninomiya-Tsuji, J.; Tsuchiya, M.; Matsumoto, K. An evolutionarily conserved motif in the TAB1 C-terminal region is necessary for interaction with and activation of TAK1 MAPKKK. J. Biol. Chem. 2001, 276, 24396–24400. [Google Scholar] [CrossRef] [Green Version]
- Landstrom, M. The TAK1-TRAF6 signalling pathway. Int. J. Biochem. Cell. 2010, 42, 585–589. [Google Scholar] [CrossRef]
- Koitabashi, N.; Danner, T.; Zaiman, A.L.; Pinto, Y.M.; Rowell, J.; Mankowski, J.; Zhang, D.; Nakamura, T.; Takimoto, E.; Kass, D.A. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J. Clin. Investig. 2011, 121, 2301–2312. [Google Scholar] [CrossRef]
- Morioka, S.; Inagaki, M.; Komatsu, Y.; Mishina, Y.; Matsumoto, K.; Ninomiya-Tsuji, J. TAK1 kinase signaling regulates embryonic angiogenesis by modulating endothelial cell survival and migration. Blood 2012, 120, 3846–3857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S.-Y.; Sun, Q.-X.; Yao, C.-L. The interaction of TAK1 and TAB1 enhances LPS-induced cytokine release via modulating NF-kappa B activation (Larimichthys crocea). Fish Shellfish. Immunol. 2018, 74, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, L.; Liu, G.; Wu, C.; Liu, B.; Liu, L.; Lv, Z.; Gong, L.; Song, X. Molecular characterization and expression of TAK-binding proteins (TAB1-3) in Larimichthys crocea infected by Vibrio parahemolyticus and LPS. Dev. Comp. Immunol. 2019, 98, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, Y.-W.; Pan, H.-J.; Wu, S.-Q.; Shi, C.-B.; Luo, X.-C.; Li, A.-X. Grass carp (Ctenopharyngodon idella) TRAF6 and TAK1: Molecular cloning and expression analysis after Ichthyophthirius multifiliis infection. Fish Shellfish. Immunol. 2013, 34, 1514–1523. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.-W.; Pan, H.-J.; Shi, C.-B.; Luo, X.-C.; Li, A.-X.; Wu, S.-Q. TAK1-binding proteins (TAB1 and TAB2) in grass carp (Ctenopharyngodon idella): Identification, characterization, and expression analysis after infection with Ichthyophthirius multifiliis. Fish Shellfish. Immunol. 2014, 38, 389–399. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, X.; Wang, Z.; Mo, Z.Q.; Dan, X.M.; Luo, X.C.; Li, A.X. Orange-spotted grouper Epinephelus coioides Tak1: Molecular identification, expression analysis and functional study. J. Fish. Biol. 2015, 86, 417–430. [Google Scholar] [CrossRef]
- Hu, Y.-Z.; Li, X.; Han, R.; Jiang, B.; Li, Y.-W.; Dan, X.-M.; Li, A.-X. Molecular identification and expression analysis of TAB1 from orange-spotted grouper (Epinephelus coioides). Dev. Comp. Immunol. 2019, 90, 152–156. [Google Scholar] [CrossRef]
- Wang, C.; Peng, J.; Zhou, M.; Liao, G.; Yang, X.; Wu, H.; Xiao, J.; Feng, H. TAK1 of black carp positively regulates IRF7-mediated antiviral signaling in innate immune activation. Fish Shellfish. Immunol. 2019, 84, 83–90. [Google Scholar] [CrossRef]
- Zou, Z.; Xie, X.; Li, W.; Song, X.; Tan, Y.; Wu, H.; Xiao, J.; Feng, H. Black carp TAB1 up-regulates TAK1/IRF7/IFN signaling during the antiviral innate immune activation. Fish Shellfish. Immunol. 2019, 89, 736–744. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Y.; Wang, Z.; Zhang, Q.; Yu, H. Molecular Characterization, Expression Profiles, and Immunostimulation Responses of TRAF6 and TAK1 in Japanese Flounder (Paralichthys olivaceus). J. Ocean Univ. China 2019, 18, 165–176. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, H.; Cho, J.H. Molecular cloning and functional characterization of TRAF6 and TAK1 in rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2019, 84, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhu, K.-C.; Guo, L.; Liu, B.-S.; Guo, H.-Y.; Zhang, N.; Yang, J.-W.; Zhang, D.-C. Molecular characterization of GRP94 and HSP90 alpha from Trachinotus ovatus, Linnaeus 1758 and their expression responses to various levels of stocking density stress and Cryptocaryon irritans infection. Aquaculture 2020, 529, 739601. [Google Scholar] [CrossRef]
- Ning, L.; Gao, L.; Zhou, W.; Liu, S.; Chen, X.; Pan, Q. Beneficial effects of dietary mulberry leaf along with multi-enzyme premix on the growth, immune response and disease resistance of golden pompano Trachinotus Ovatus. Aquaculture 2021, 535, 736396. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S.; He, M.; Liu, A.; Long, H.; Guo, W.; Cao, Z.; Xie, Z.; Zhou, Y. Construction and analysis of the immune effect of Vibrio harveyi subunit vaccine and DNA vaccine encoding TssJ antigen. Fish Shellfish. Immunol. 2020, 98, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, Q.; Li, F.; Qin, X.; Dong, D.; Chen, B.; Qin, Q. First identification of the nervous necrosis virus isolated from cultured golden pompano (Trachinotus ovatus) in Guangxi, China. J. Fish. Dis. 2018, 41, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, I.; Hermida, M.; Kamanli, S.A.; Benmansour, B.; Ozak, A.A.; Boxshall, G.A. Caligus madeirensissp. nov. (Copepoda: Caligidae) Parasitic on Pompano, Trachinotus ovatus (Linnaeus, 1758), from Eastern Atlantic Waters, Surrounding the Madeira Archipelago, Portugal. Acta Parasitol. 2021, 66, 361–376. [Google Scholar] [CrossRef]
- Qiao, Y.; Shao, Y.; Pengsakul, T.; Chen, C.; Zheng, S.; Wu, W.; Hardjo, T.B. Morphological and molecular characterization of Ceratomyxa batam n. sp. (Myxozoa: Ceratomyxidae) infecting the gallbladder of the cultured Trachinotus ovatus (Perciformes: Carangidae) in Batam Island, Indonesia. Parasitol. Res. 2019, 118, 1647–1651. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T) (-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Oelze, B.; Elger, K.; Schadzek, P.; Burmeister, L.; Hamm, A.; Laggies, S.; Seiffart, V.; Gross, G.; Hoffmann, A. The inflammatory signalling mediator TAK1 mediates lymphocyte recruitment to lipopolysaccharide-activated murine mesenchymal stem cells through interleukin-6. Mol. Cell. Biochem. 2021, 476, 3655–3670. [Google Scholar] [CrossRef]
- Fechtner, S.; Fox, D.A.; Ahmed, S. Transforming growth factor beta activated kinase 1: A potential therapeutic target for rheumatic diseases. Rheumatology 2017, 56, 1060–1068. [Google Scholar]
- Xu, Y.D.; Zhu, B.; Zhang, R.; Tang, J.Z.; Liu, Y.; Wang, W.J.; Wang, Z.Z.; Mao, Y.; Zeng, G.Q.; Yan, J.P. TAK1 of blunt snout bream promotes NF-kappa B activation via interaction with TAB1 in response to pathogenic bacteria. Fish Shellfish Immunol. 2022, 120, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Shirakabe, T.; Shibuya, H.; Irie, K.; Oishi, I.; Ueno, N.; Taniguchi, T.; Nishida, E.; Matsumoto, K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal-transduction. Science 1995, 270, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.E.; Sakurai, H.; Sugita, T.; Guesdon, F. Alternative splicing and gene structure of the transforming growth factor beta-activated kinase 1. BBA-Gene Struct. Expr. 2000, 1517, 46–52. [Google Scholar] [CrossRef]
- Scholz, R.; Sidler, C.L.; Thali, R.F.; Winssinger, N.; Cheung, P.C.F.; Neumann, D. Autoactivation of Transforming Growth Factor beta-activated Kinase 1 Is a Sequential Bimolecular Process. J. Biol. Chem. 2010, 285, 25753–25766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhirunnusorn, P.; Suzuki, S.; Kawasaki, N.; Saiki, I.; Sakurai, H. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J. Biol. Chem. 2005, 280, 7359–7368. [Google Scholar] [CrossRef] [Green Version]
- Takaesu, G.; Kishida, S.; Hiyama, A.; Yamaguchi, K.; Shibuya, H.; Irie, K.; Ninomiya-Tsuji, J.; Matsumoto, K. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol. Cell 2000, 5, 649–658. [Google Scholar] [CrossRef]
- Fjeld, C.C.; Denu, J.M. Kinetic analysis of human serine threonine protein phosphatase 2C alpha. J. Biol. Chem. 1999, 274, 20336–20343. [Google Scholar] [CrossRef] [Green Version]
- Conner, S.H.; Kular, G.; Peggie, M.; Shepherd, S.; Schuttelkopf, A.W.; Cohen, P.; Van Aalten, D.M.F. TAK1-binding protein 1 is a pseudophosphatase. Biochem. J. 2006, 399, 427–434. [Google Scholar] [CrossRef]
- Brown, K.; Vial, S.C.M.; Dedi, N.; Long, J.M.; Dunster, N.J.; Cheetham, G.M.T. Structural basis for the interaction of TAK1 kinase with its activating protein TAB1. J. Mol. Biol. 2005, 354, 1013–1020. [Google Scholar] [CrossRef]
- Kanayama, A.; Seth, R.B.; Sun, L.J.; Ea, C.K.; Hong, M.; Shaito, A.; Chiu, Y.H.; Deng, L.; Chen, Z.J. TAB2 and TAB3 activate the NF-kappa B pathway through binding to polyubiquitin chains. Mol. Cell 2004, 15, 535–548. [Google Scholar] [CrossRef]
- Kishida, S.; Sanjo, H.; Akira, S.; Matsumoto, K.; Ninomiya-Tsuji, J. TAK1-binding protein 2 facilitates ubiquitination of TRAF6 and assembly of TRAF6 with IKK in the IL-1 signaling pathway. Genes Cells 2005, 10, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kulathu, Y.; Akutsu, M.; Bremm, A.; Hofmann, K.; Komander, D. Two-sided ubiquitin binding explains specificity of the TAB2 NZF domain. Nat. Struct. Mol. Biol. 2009, 16, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cao, M.; Ni, Y.; Han, L.; Dai, A.; Chen, R.; Ning, X.; Liu, X.; Ke, K. Up-regulation of TAB3 is involved in neuronal apoptosis after intracerebral hemorrhage. Cell. Mol. Neurobiol. 2017, 37, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.F.; Cao, S.L.; Liu, X.Y.; Zhu, X.C.; Zhou, X.H.; Li, J.N. Grass carp (Ctenopharyngodon idella) TAK1 promotes NF-kappa B activation via interaction with TAB1 in response to potential vaccine antigen of Vibrio mimicus. Aquaculture 2020, 529, 735712. [Google Scholar] [CrossRef]
- Chen, Z.J.; Bhoj, V.; Seth, R.B. Ubiquitin, TAK1 and IKK: Is there a connection? Cell Death Differ. 2006, 13, 687–692. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, Y.; Wang, X.; Wang, S.; Zhang, Q.; Wang, Z.; Gao, Q. TLR13, TLR22, TRAF6, and TAK1 in the soiny mullet (Liza haematocheila): Molecular characterization and expression profiling analysis. Dev. Comp. Immunol. 2020, 112, 103774. [Google Scholar] [CrossRef]
- Komatsu, Y.; Shibuya, H.; Takeda, N.; Ninomiya-Tsuji, J.; Yasui, T.; Miyado, K.; Sekimoto, T.; Ueno, N.; Matsumoto, K.; Yamada, G. Targeted disruption of the Tab1 gene causes embryonic lethality and defects in cardiovascular and lung morphogenesis. Mech. Develop. 2002, 119, 239–249. [Google Scholar] [CrossRef]
- Sanjo, H.; Takeda, K.; Tsujimura, T.; Ninomiya-Tsuji, J.; Matsumoto, K.; Akira, S. TAB2 is essential for prevention of apoptosis in fetal liver but not for interleukin-1 signaling. Mol. Cell. Biol. 2003, 23, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Morioka, S.; Matsumoto, K.; Ninomiya-Tsuji, J. TAK1 binding protein 2 is essential for liver protection from stressors. PLoS ONE 2014, 9, e88037. [Google Scholar] [CrossRef]
- Deng, F.; Xu, G.; Cheng, Z.; Huang, Y.; Ma, C.; Luo, C.; Yu, C.; Wang, J.; Xu, X.; Liu, S.; et al. Hepatitis B surface antigen suppresses the activation of nuclear factor kappa B pathway via interaction with the TAK1-TAB2 complex. Front. Immunol. 2021, 12, 618196. [Google Scholar] [CrossRef]
- Egan, C.E.; Sukhumavasi, W.; Butcher, B.A.; Denkers, E.Y. Functional aspects of Toll-like receptor/MyD88 signalling during protozoan infection: Focus on Toxoplasma gondii. Clin. Exp. Immunol. 2009, 156, 17–24. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.; Korbel, D.S.; Barakat, F.M.; Choudhry, N.; Petry, F. Innate immune responses against Cryptosporidium parvum infection. Parasite Immunol. 2013, 35, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sasai, M.; Yamamoto, M. Pathogen recognition receptors: Ligands and signaling pathways by Toll-Like receptors. Int. Rev. Immunol. 2013, 32, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Yarovinsky, F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef]
- Yin, D.; Li, W.; Fu, M.; Chen, L.; Ma, F.; Jin, P. Identification and characterization of a TAB1 gene involved in innate immunity of amphioxus (Branchiostoma belcheri). Gene 2016, 575, 294–302. [Google Scholar] [CrossRef]
- Cao, Y.; Jin, N.; Fan, M.; Lv, C.; Song, X.; Jin, P.; Ma, F. Phylogenetically conserved TAK1 participates in Branchiostoma belcheri innate immune response to LPS stimulus. Fish Shellfish Immunol. 2019, 94, 264–270. [Google Scholar] [CrossRef]
- Mu, Y.; Li, M.; Ding, F.; Ding, Y.; Ao, J. De Novo Characterization of the spleen transcriptome of the large yellow croaker (Pseudosciaena crocea) and analysis of the immune relevant genes and pathways involved in the antiviral response. PLoS ONE 2014, 9, e101069. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Cui, J.; Xu, T.; Sun, Y. microRNA-128 inhibits the inflammatory responses by targeting TAB2 in miiuy croaker, Miichthys miiuy. Dev. Comp. Immunol. 2021, 117, 103976. [Google Scholar] [CrossRef]
- Liu, S.L.; Zhou, A.G.; Xie, S.L.; Sun, D.; Zhang, Y.; Sun, Z.L.; Chen, Y.F.; Zou, J.X. Immune-related genes expression analysis of Western mosquitofish (Gambusia affinis) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2020, 102, 92–100. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′ to 3′) | Application |
|---|---|---|
| PCR | ||
| TrTAK1F1 | ATGGRAAGGCAAAGATGTYGCAAT | TrTAK1 cDNA cloning |
| TrTAK1F2 | TTGTGATGGAATATGCTGAAGGCG | |
| TrTAK1R1 | CTGTCTCTTCTGTTGCTGGACCCG | |
| TrTAK1R2 | NAGACTCTTGTTCTCCTCCAGCAGCT | |
| TrTAB1F1 | ATCARAGCTGGACAGATGACCTGCC | TrTAB1 cDNA cloning |
| TrTAB1F2 | GCCTTTGATGTGGTGGAGAAGAGCT | |
| TrTAB1R1 | AGCTCTGCGTCTGGGTGTTGGTR | |
| TrTAB1R2 | YARGGTGGAGGCMGTGTTGGTTC | |
| TrTAB2F1 | CAGGGAAGCCACCAGATTGACAYTC | TrTAB2 cDNA cloning |
| TrTAB2F2 | GCCAGAAGTTCCCTGAAGTCCCA | |
| TrTAB2R1 | TGCACTGGGATTAAAGTGTGGTCCT | |
| TrTAB2R2 | GGAGATCAATTTCTTTGGTGAGGCA | |
| RACE-PCR | ||
| TrTAK1-3F1 | GCTCCCTGTCCCAACTCCAARNAG | TrTAK1-3′ RACE cloning |
| TrTAK1-3F2 | AGRAGGACCAGCAGAACACATCGC | |
| TrTAK1-5R1 | GCTCATGGCATGGGAAGCAGTGTA | TrTAK1-5′ RACE cloning |
| TrTAK1-5R2 | GGTTCAGCACCATGCAGCACATTA | |
| TrTAB1-3F1 | AGGACATGACCCTGCTCATCCG | TrTAB1-3′ RACE cloning |
| TrTAB1-3F2 | ACCCTCACCAATGGAACCAACACT | |
| TrTAB1-5R1 | TAGCTCCTCCTGACACCTCCTGCY | TrTAB1-5′ RACE cloning |
| TrTAB1-5R2 | TCGTCTCAAAGTAGCTCTTCTCCACC | |
| TrTAB2-3F1 | CATGGAGAGGCTGTGGCATGAGTY | TrTAB2-3′ RACE cloning |
| TrTAB2-3F2 | GAGAATGACCTGACCAGAAGACGGC | |
| TrTAB2-5R1 | ATTTCGGAGTCTGGTGAAGCTGGG | TrTAB2-5′ RACE cloning |
| TrTAB2-5R2 | TGGGACAAGTATTCACAGCAGGCG | |
| UPM-L | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT | RACE random primer |
| UPM-S | CTAATACGACTCACTATAGGGC | |
| NUP | AAGCAGTGGTATCAACGCAGAGT | |
| qPCR | ||
| TrTAK1QF | ATGTGACTTTGGAACGGCCT | TrTAK1 qPCR |
| TrTAK1QR | TGATGCGAAAAGCTGGTCCT | |
| TrTAB1QF | CTAGTGTCACCCTGTCGCTG | TrTAB1 qPCR |
| TrTAB1QR | GACTTCCTCTCTGGCGGAAC | |
| TrTAB2QF | CCCACCCAAACCCAAAGGTA | TrTAB2 qPCR |
| TrTAB2QR | CGGGAATTCGCACTGTTCAC | |
| β-actin-F | GCTACGTCGCCCTGGACTTC | β-actin qPCR |
| β-actin-R | CTCATGGATTCCGCAGGACTC |
| T. ovatus | H. sapiens | M. musculus | S. dumerili | S. lalandi | L. crocea | P. flavescens | E. coioides | P. olivaceus | O. niloticus | C. semilaevis |
|---|---|---|---|---|---|---|---|---|---|---|
| TAK1 | 80.5 | 81.1 | 97.9 | 96.2 | 97.0 | 95.1 | 96.9 | 96.3 | 95.9 | 95.5 |
| TAB1 | 65.0 | 64.8 | 96.4 | 96.4 | 95.5 | 94.5 | 96.0 | 94.6 | 92.9 | 92.1 |
| TAB2 | 69.7 | 68.7 | 97.9 | 98.1 | 94.6 | 93.8 | 95.6 | 95.1 | 89.4 | 86.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Lei, K.; He, J.; Wei, Y. Molecular Characterization and Expression Analysis of TAK1, TAB1 and TAB2 of Golden Pompano (Trachinotus ovatus). Fishes 2022, 7, 173. https://doi.org/10.3390/fishes7040173
Xie Y, Lei K, He J, Wei Y. Molecular Characterization and Expression Analysis of TAK1, TAB1 and TAB2 of Golden Pompano (Trachinotus ovatus). Fishes. 2022; 7(4):173. https://doi.org/10.3390/fishes7040173
Chicago/Turabian StyleXie, Yushuai, Kun Lei, Jinquan He, and Youchuan Wei. 2022. "Molecular Characterization and Expression Analysis of TAK1, TAB1 and TAB2 of Golden Pompano (Trachinotus ovatus)" Fishes 7, no. 4: 173. https://doi.org/10.3390/fishes7040173
APA StyleXie, Y., Lei, K., He, J., & Wei, Y. (2022). Molecular Characterization and Expression Analysis of TAK1, TAB1 and TAB2 of Golden Pompano (Trachinotus ovatus). Fishes, 7(4), 173. https://doi.org/10.3390/fishes7040173





