1. Introduction
The red swamp crayfish (
Procambarus clarkii), native to the United States and Mexico, was introduced from Japan to Nanjing, China, in the 1930s [
1,
2,
3]. The red swamp crayfish is one of the most aggressive species in the world, with their dredging behavior causing damage to dikes, dams and rice paddies, but due to its high market value, including edible meat, high protein content, low fat content and high nutritional power, it is widely loved and consumed in China and is one of the most economically important aquatic species for aquaculture rather than a destructive invasive species [
4]. Recently it has recently become a popular freshwater aquaculture species in China, especially in the middle and lower reaches of the Yangtze River [
5]. In the past two decades, red swamp crayfish farming in rice fields has become the most commonly farming mode because it can improve ecological conditions and raise the production values of rice fields. However, with the continuous increase in overfishing, the decline in growth performance and disease resistance has seriously restricted the stable and sustainable development of crayfish production [
6]. Therefore, assessment of genetic resources from wild populations is urgent and necessary to breed new strains or varieties with excellent characteristics in the future. In addition, in production practice, integration of breeding and culture in rice fields is the most common production mode at present, which results in a potential risk of inbreeding and loss of genetic diversity [
7]. Therefore, the genetic information of wild and cultured populations is needed to monitor the change of genetic diversity to reveal genetic variation and adaptation ability to the environment in the long-term process [
8].
Genetic diversity refers to the differences in genes and DNA sequences between individuals of a particular species [
9], and it was supposed that the higher the genetic diversity, the greater the ability to adapt to environmental changes because genetic diversity within populations can increase the ability of the population to resist disease and environmental fluctuations [
10]. However, the lack of genetic variation can have some degree of deleterious effects on various commercial traits such as disease resistance and growth [
11,
12]. Several studies on monitoring genetic differences between cultured and wild populations have been reported in various fish species, such as yellow croaker (
Pseudosciaena crocea) [
13], gibel carp (
Carassius gibelio) [
14,
15], striped catfish (
Pangasianodon hypophthalmus) [
16], orange-spotted grouper (
Epinephelus coioides) [
17], Chinese sucker (
Myxocyprinus asiaticus) [
18], turbot (
Scophthalmus maximus) [
19]. However, genetic diversity is often reduced in the breeding process due to the founder effect and inbreeding [
20]. Therefore, it is necessary to take certain measures to ensure the genetic diversity of the breeding population.
Simple sequence repeats (SSRs), also known as microsatellite markers, are motifs with lengths ranging from 1 to 6 bp tandem repeats that are common in most organisms [
21]. SSRs exhibit a high degree of allelic diversity due to the number of repeats and can be easily detected by polymerase chain reaction (PCR) and polyacrylamide gel electrophoresis [
21,
22]. The distinguishing features, including random distribution in the genome, high levels of polymorphism, codominance and reproducibility, make them very effective in assessing genetic diversity, structure and differentiation of different resources and populations [
23,
24]. Microsatellites have been applied for genetic diversity detection in a variety of aquatic species [
14,
15,
18,
19,
25,
26], which verifies SSR as an ideal marker system for genetic diversity analysis and population genetics research. Traditional microsatellite marker development is time-consuming and labor-intensive, and the development of next-generation sequencing (NGS) technology provides a more economical and convenient method. RNA sequencing (RNA-Seq) is a high-throughput NGS-based technology that has great advantages in mining microsatellites and has good applications in numerous species [
27,
28,
29].
In this study, the genetic diversity of red swamp crayfish from wild lake populations and rice field-cultured populations was assessed by SSR markers developed from transcriptome data obtained by RNA-seq technology, which thereby revealed genetic diversity levels within/among populations and explored the genetic differentiation among these populations. It will provide theoretical guidance for genetic resource conservation, parent selection, progeny genetic variation and heterosis prediction and improve breeding efficiency in the future.
4. Discussion
Genetic diversity contributes to a species’ ability to cope with environmental change and is often assessed by molecular markers. A variety of molecular markers are used to detect population genetic diversity and differentiation, including restriction fragment length polymorphism (RFLP), amplified fragment length polymorphism (AFLP), and simple sequence repetition (SSR) [
39,
40,
41]. Microsatellites are widely distributed in the genome and have high levels of polymorphism, codominance and repeatability. They are effective tools for assessing genetic diversity and population structure [
42]. In this study, we screened SSR markers from transcriptome data based on RNA-Seq technology and identified a total of 72,839 SSRs, excluding single nucleotide repeats; dinucleotide repeats were the most abundant type with 21,825, which is consistent with SSRs in many species [
26,
43]. The SSRs obtained in this study provide valuable resources for future studies on genetic variation and genetic breeding in the crayfish.
Genetic diversity within species reflects population size, history, ecology and adaptive capacity [
44]. Genetic diversity of wild
P. clarkii populations in different regions of China has been reported. The highest
He value of 0.72 was revealed in the Nanjing population, and the lowest
He was 0.44 in the Sichuan population [
3]. The
He values of crayfish populations in the Pearl River basin ranged from 0.29 to 0.59 with a mean value of 0.50 [
45]. It was also indicated that the
He ranged from 0.53 to 0.78 with a mean value of 0.62 [
8]. In this study, however, the
He value of wild crayfish was between 0.39 and 0.50, with an average value of 0.45, which was evidently lower than the previous studies in China. [
3,
8,
45]. It is well-known that red swamp crayfish has been widely introduced globally and has been studied from the viewpoint of invasion ecology in Europe [
46,
47] and globally [
48]. Population genetics of native and introduced red swamp crayfish have been compared in Mexico [
49]. As for the non-Chinese populations, the
He value ranging from 0.31 and 0.62, with an average value of 0.51 in the populations from Europe and northern Mexico [
47], were revealed to be higher than that of our populations.
The decline of genetic diversity of wild red swamp crayfish may be related to the bottleneck effect, founder effect and nonrandom mating [
3,
45]. It was revealed that about 100 specimens of red swamp crayfish were carried from New Orleans to Japan in 1927, of which only 20 specimens arrived alive to a pond near Tokyo, then were translocated to Nanjing in 1929, indicating a strong genetic bottleneck with few founders in Chinese populations [
48]. In this study, the second genetic bottleneck was revealed based on the BOTTLENECK’s test. Therefore, we suppose that this might be the consequence of at least two bottlenecks. In addition, inbreeding is likely to occur because red swamp crayfish laid about 300 eggs per individual [
50]. In the past few years, wild crayfish have been seriously overfished, resulting in a sharp decline in wild resources at a rate of 30% each year [
6]. Furthermore, water pollution and habitat destruction may also contribute to the decline of genetic diversity in wild populations [
51]. Therefore, in order to provide better germplasm resources for crayfish in cultured populations, it is necessary to take measures to protect the genetic diversity of wild populations, such as reducing fishing and habitat destruction.
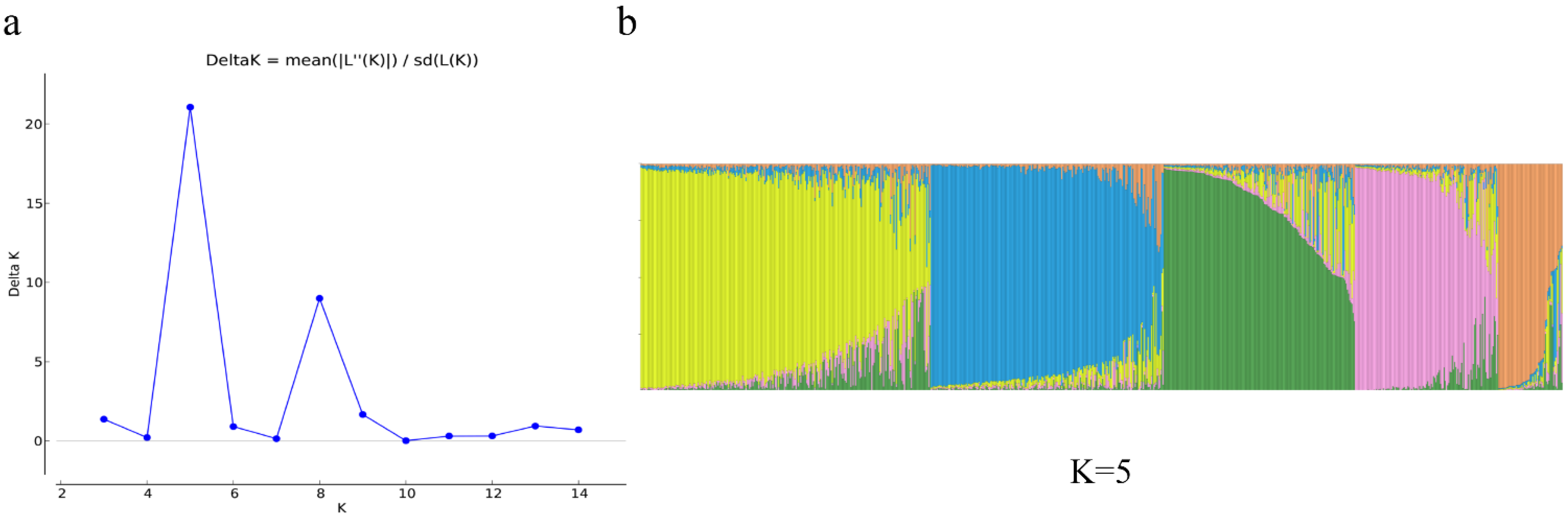
Gene differentiation and gene flow are two important indicators for evaluating the genetic structure of a population [
8].
Fst is an important parameter to measure the genetic differentiation between populations, and the smaller
Fst indicated the lower the differentiation between populations, the closer the genetic distance. In this study, the
Fst values of cultured populations were all less than 0.05, indicating a slight degree of genetic differentiation, while there was a moderate to a large degree of genetic differentiation between wild populations. For example, the
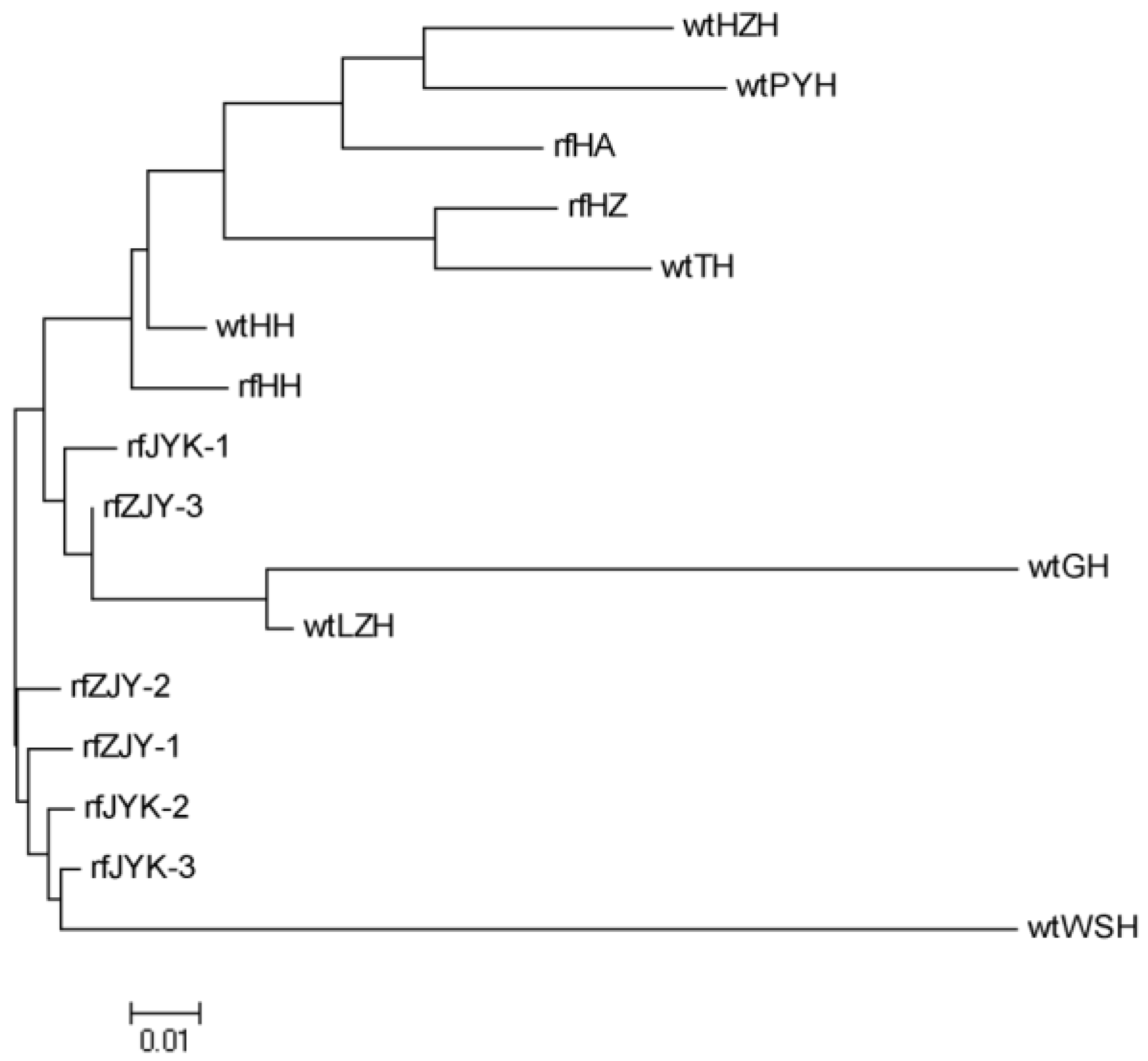
Fst value of 0.024 between wtHH and wtLZH was the smallest, which suggested the genetic differentiation is very small, resulting from close geographical distance, as they were both in the Hubei region. The maximum
Fst value between wtGH and wtWSH was 0.1711, suggesting that there was a large genetic differentiation and fewer gene exchanges between the two populations due to the long geographical distance. The population structure of freshwater crayfish is significantly influenced by geographic position, which limits gene flow and thus facilitates differentiation between populations [
52]. However, the
Fst value was not completely correlated with distance, consistent with the conclusion that the isolation-by-distance pattern was not significant in the previous study [
8], which may be due to human-mediated transmission, genetic drift and population size [
3].
Characterization of the genetic divergence between the brood stocks and wild population could be helpful for defining better management strategies, such as establishment and maintenance of nursery populations, conservation of genetic diversity and promoting sustainable production of crayfish [
53]. Intensive cultured populations usually have lower genetic diversity than wild populations due to inbreeding, the founder effect and the increase in genetic drift caused by the use of fewer parents [
17,
54,
55,
56]. In this study, we compared the genetic diversity between seven wild populations and nine cultured populations, and lower genetic differentiation and a high level of gene flow between the populations were detected. However, contrary to expectations, the genetic diversity of the cultured population was higher than that of the wild population, which could be that the cultured populations were produced from a sufficient number of parents with high levels of polymorphism. Therefore, we recommend that wild individuals continue to be introduced as hatchery stock and minimize inbreeding to maintain a high level of genetic diversity in the cultured population. In addition, due to their higher genetic variation of the native populations from the southeastern United States or Mexico, new genetic material should be brought in from the native range to increase genetic variation and reverse any inbreeding.
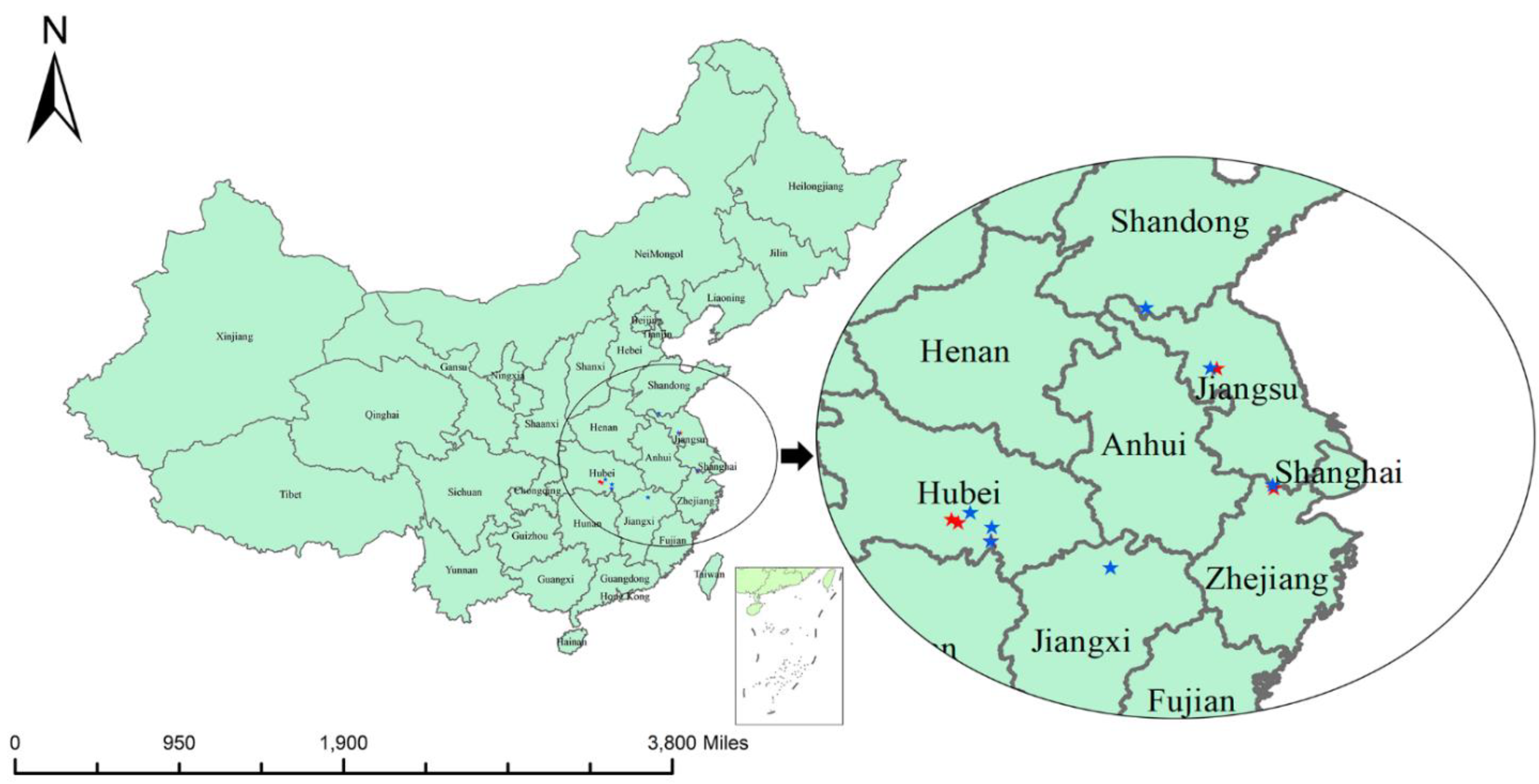
Qianjiang City of the Hubei province has been recognized as the hometown of red swamp crayfish in China by the Ministry of Agriculture of the People’s Republic of China [
57]. The artificial breeding and culture model of crayfish is considered to be a popular model of crayfish production [
5]. A population breeding strategy was applied, and several generations have been performed to obtain good characteristics, such as growth, disease resistance and suitability [
58,
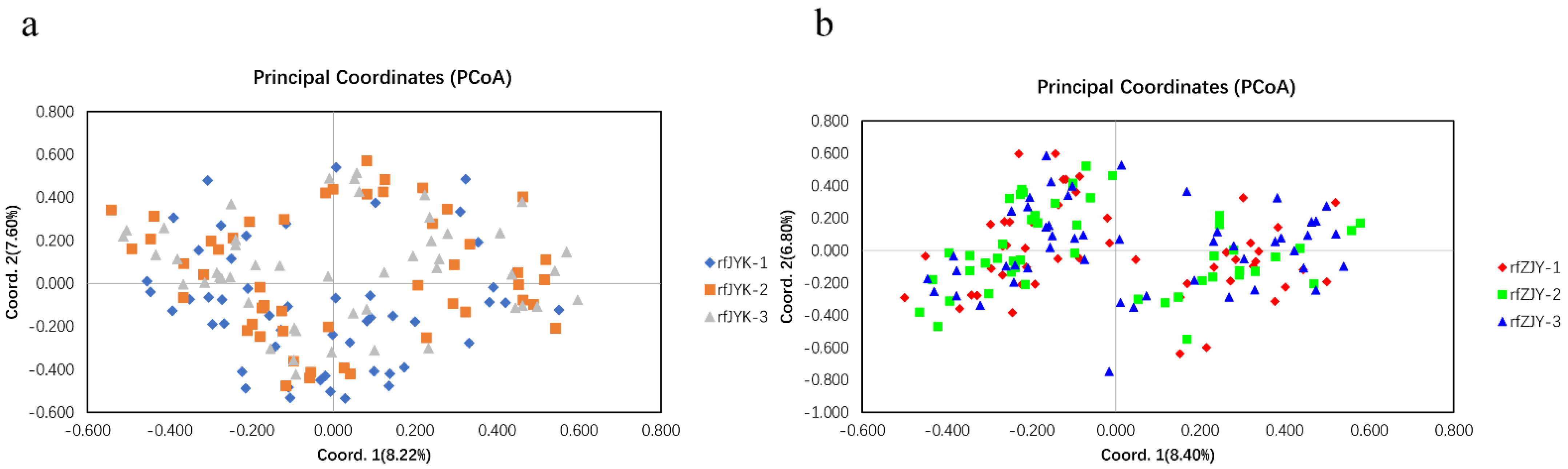
59]. To assess the effect of genetic breeding on the genetic diversity of the populations, we evaluated the genetic diversity of three consecutive generations from ZJY and JYK in Qianjiang City. No apparent differentiation of genetic diversity was observed, indicating that the two populations are of significant selection breeding potential because their aquaculture traits are better than other cultured populations.