The Effect of Hormonal Treatment on Selected Sperm Quality Parameters and Sex Steroids in Tropical Cyprinid Bala Shark Balantiocheilos melanopterus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Maintenance
2.2. Hormone Treatments
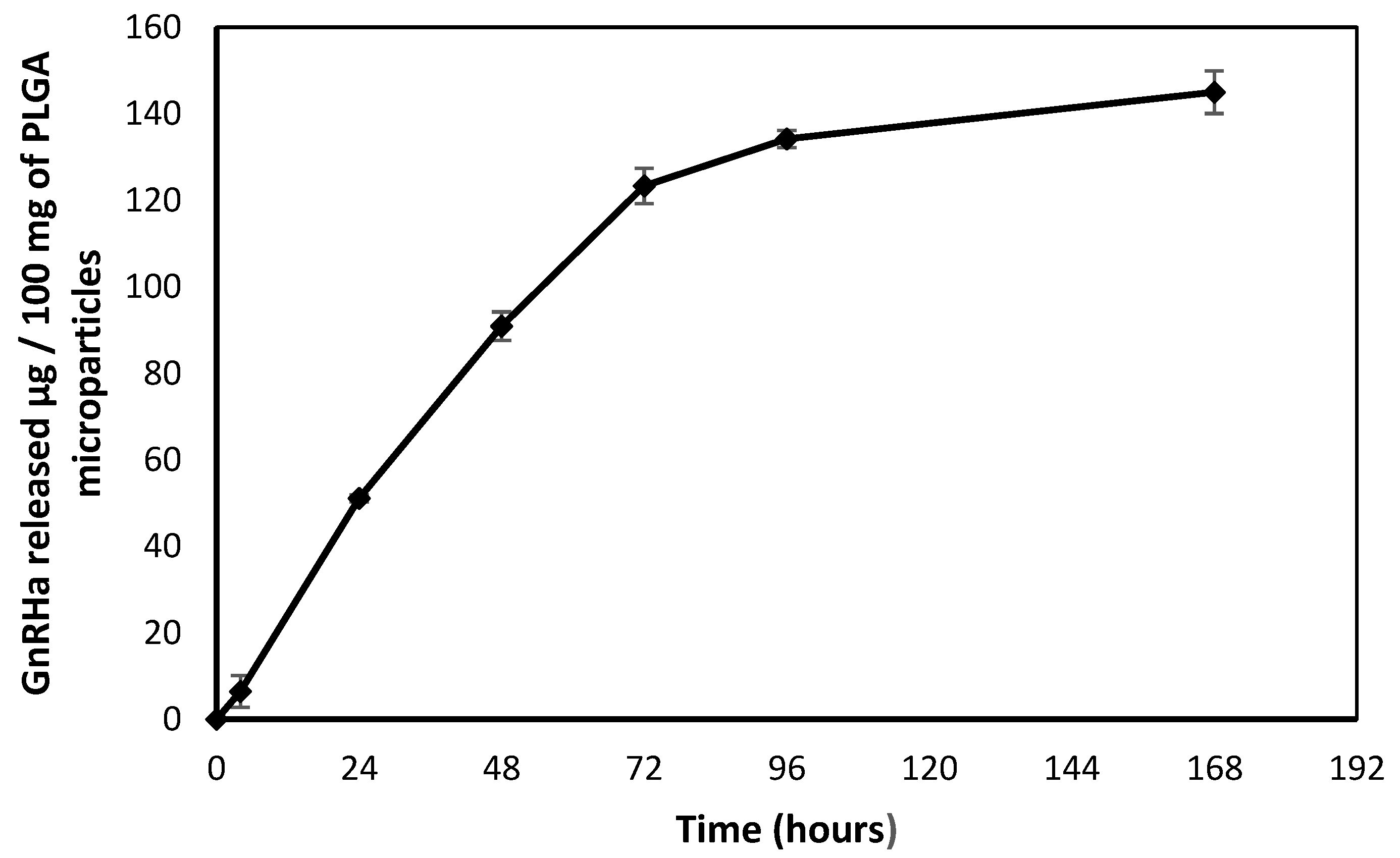
2.2.1. Preparation of PLGA Microparticles with Continuous GnRHa Release
2.2.2. Hormone Treatments
- (1)
- 1 mL/kg BW 0.9% NaCl (Braun Melsungen AG, Melsungen, Germany); control group.
- (2)
- 20 µg/kg BW recombinant hCG (Ovitrelle, Merck Europe B.V., Amsterdam, The Netherland); hCG group.
- (3)
- 25 μg/kg BW [D-Ala6, Pro9, NEt]-GnRH (APExBIO, Houston, TX, USA) combined with 20 mg/kg BW metoclopramide (Met) (Sigma-Aldrich, St. Louis, MO, USA); GnRHa + Met group.
- (4)
- 10 μg/kg BW [D-Ala6, Pro9, NEt]-GnRH in PLGA microparticles; PLGA group.
2.3. Collection and Analysis of Samples
2.3.1. Blood and Sperm Sample Collection
2.3.2. Testosterone and 11-Ketotestosterone Analysis
2.3.3. Sperm Production Indexes
2.3.4. Sperm Motility Analysis
2.4. Statistical Analysis
2.4.1. Kinetic Parameter Analysis
2.4.2. Total Sperm Count
2.4.3. Testosterone and 11-Ketotestosterone Analysis
3. Results
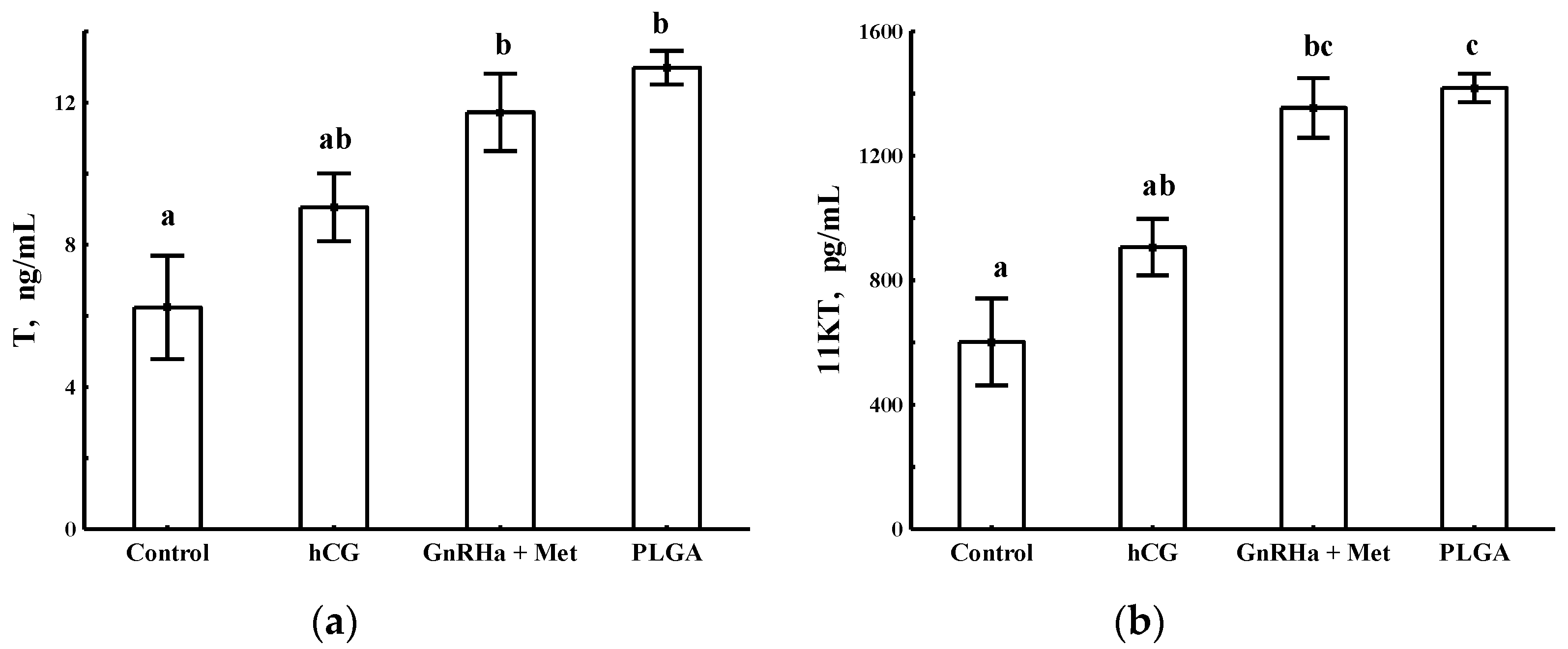
3.1. Plasma Concentration of Testosterone and 11-Ketotestosterone
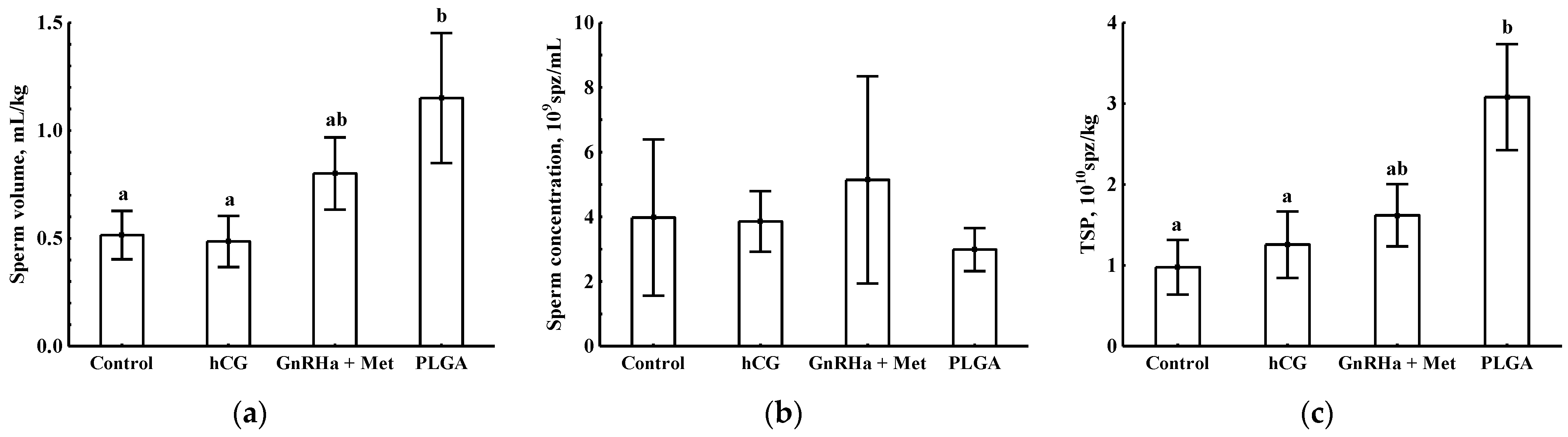
3.2. Sperm Production Indexes
3.3. Sperm Motility Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, H.H.; Kottelat, M. Balantiocheilos ambusticauda, a new and possibly extinct species of cyprinid fish from Indochina (Cypriniformes: Cyprinidae). Zootaxa 2007, 1463, 13–20. [Google Scholar] [CrossRef]
- Roberts, T.R. The Freshwater Fishes of Western Borneo (Kalimantan Barat, Indonesia). Mem. Calif. Acad. Sci. 1989, 14, 20001193319. [Google Scholar]
- Ng, P.K.L.; Tan, H.H. Freshwater fishes of Southeast Asia: Potential for the aquarium fish trade and conservation issues. Aquar. Sci. Conserv. 1997, 1, 79–90. [Google Scholar] [CrossRef]
- Lipscomb, T.N.; Wood, A.L.; DiMaggio, M.A.; Tuckett, Q.M.; Lawson, L.L.; Watson, C.A. Evaluation of spawning aids and administration routes on ovulation success in an ornamental cyprinid. Aquac. Res. 2018, 49, 3926–3929. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Duncan, N.J.; Asturiano, J.F. Hormonal manipulations for the enhancement of sperm production in cultured fish and evaluation of sperm quality. Aquaculture 2017, 472, 21–44. [Google Scholar] [CrossRef]
- Peter, R.E.; Yu, K.L. Neuroendocrine regulation of ovulation in fishes: Basic and applied aspects. Rev. Fish Biol. Fisher. 1997, 7, 173–197. [Google Scholar] [CrossRef]
- Zohar, Y.; Mylonas, C.C. Endocrine manipulations of spawning in cultured fish: From hormones to genes. Aquaculture 2001, 197, 99–136. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Zohar, Y. Use of GnRHa-delivery systems for the control of reproduction in fish. Rev. Fish Biol. Fisher. 2001, 10, 463–491. [Google Scholar] [CrossRef]
- Forniés, M.A.; Mañanós, E.; Carrillo, M.; Rocha, A.; Laureau, S.; Mylonas, C.C.; Zohar, Y.; Zanuy, S. Spawning induction of individual European sea bass females (Dicentrarchus labrax) using different GnRHa-delivery systems. Aquaculture 2001, 202, 221–234. [Google Scholar] [CrossRef]
- Amini, K.; Siraj, S.S.; Amiri, B.M.; Rostami, S.; Sharr, A.; Hossienzadeh, H. Evaluation of LHRH-a acute release implantation on final maturation and spawning in not-fully matured broodstocks of Persian sturgeon (Acipenser persicus Borodin, 1897). Iran. J. Fish. Sci. 2012, 11, 440–459. [Google Scholar]
- Knowles, J.C.; Vysloužil, J.; Muselík, J.; Stejskal, V.; Kouril, J.; Podhorec, P. Efficacy of poly (lactic-co-glycolic acid) microparticles as a gonadotropin-releasing hormone analogue delivery system to stimulate ovulation of peled Coregonus peled. Czech J. Anim. Sci. 2021, 66, 331–338. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Rainis, S.; Mylonas, C.C.; Kyriakou, Y.; Divanach, P. Enhancement of spermiation in European sea bass (Dicentrarchus labrax) at the end of the reproductive season using GnRHa implants. Aquaculture 2003, 219, 873–890. [Google Scholar] [CrossRef]
- Fakriadis, I.; Mylonas, C.C. Sperm quality of greater amberjack Seriola dumerili throughout the reproductive season and in response to GnRHa treatment with controlled release implants. Fish Physiol. Biochem. 2021, 47, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Fakriadis, I.; Zanatta, E.M.; Fleck, R.P.D.S.; Sena Mateo, D.L.; Papadaki, M.; Mylonas, C.C. Endocrine regulation of long-term enhancement of spermiation in meagre (Argyrosomus regius) with GnRHa controlled-delivery systems. Gen. Comp. Endocr. 2020, 297, 113549. [Google Scholar] [CrossRef]
- Linhart, O.; Peter, R.E.; Rothbard, S.; Zohar, Y.; Kvasnička, P. Spermiation of common tench (Tinca tinca L.) stimulated with injection or implantation of GnRH analogues and injection of carp pituitary extract. Aquaculture 1995, 129, 119–121. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Hatef, A.; Mylonas, C.C.; Gela, D.; Papadaki, M.; Rodina, M.; Kaspar, V.; Psenicka, M.; Podhorec, P.; Linhart, O. Sperm characteristics and androgens in Acipenser ruthenus after induction of spermiation by carp pituitary extract or GnRHa implants. Fish Physiol. Biochem. 2012, 38, 1655–1666. [Google Scholar] [CrossRef]
- Podhorec, P.; Kouril, J. Induction of final oocyte maturation in Cyprinidae fish by hypothalamic factors: A review. Vet. Med. 2009, 54, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Cejko, B.I.; Kucharczyk, D. Application of dopaminergic antagonist: Metoclopramide, in reproduction of crucian carp Carassius carassius (L.) under controlled conditions. Anim. Reprod. Sci. 2015, 160, 74–81. [Google Scholar] [CrossRef]
- Brzuska, E. Reproduction effectiveness of carp (Cyprinus carpio L.) from the Hungarian W breeding line after stimulating ovulation with spawning inducing agents of natural (CPH, hCG, PMSG) and/or synthetic origin (Ovopel, Dagin, Ovaprim, mGnRH-a). Aquaculture 2021, 532, 736023. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Nowosad, J.; Kucharczyk, D.J.; Kupren, K.; Targońska, K.; Wyszomirska, E.; Kujawa, R. Out-of-season artificial reproduction of common dace (Leuciscus leuciscus L.) under controlled conditions. Anim. Reprod. Sci. 2019, 202, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, D.; Nowosad, J.; Wyszomirska, E.; Cejko, B.I.; Arciuch-Rutkowska, M.; Juchno, D.; Boroń, A. Comparison of artificial spawning effectiveness of hCG, CPH and GnRHa in combination with dopamine inhibitors in a wild strain of ide Leuciscus idus (L.) in hatchery conditions. Anim. Reprod. Sci. 2020, 221, 106543. [Google Scholar] [CrossRef]
- Montero, R.; Chan, J.T.H.; Köllner, B.; Kuchta, R.; Vysloužil, J.; Podhorec, P.; Holzer, A.S.; Korytář, T. The Acute Immune Responses of the Common Carp Cyprinus carpio to PLGA Microparticles—The Interactions of a Teleost Fish with a Foreign Material. Biomolecules 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Rodina, M.; Cosson, J.; Gela, D.; Linhart, O. Kurokura solution as immobilizing medium for spermatozoa of tench (Tinca tinca L.). Aquac. Int. 2004, 12, 119–131. [Google Scholar] [CrossRef]
- Vermeirssen, E.L.M.; de Quero, C.M.; Shields, R.J.; Norberg, B.; Kime, D.E.; Scott, A.P. Fertility and motility of sperm from Atlantic halibut (Hippoglossus hippoglossus) in relation to dose and timing of gonadotrophin-releasing hormone agonist implant. Aquaculture 2004, 230, 547–567. [Google Scholar] [CrossRef]
- Zohar, Y.; Goren, A.; Tosky, M.; Pagelson, G.; Leibovitz, D.; Koch, Y. The bioactivity of gonadotropin-releasing hormones and its regulation in the gilthead seabream, Sparus aurata in vivo and in vitro studies. Fish Physiol. Biochem. 1989, 7, 59–67. [Google Scholar] [CrossRef]
- Clemens, H.P.; Grant, F.B. The Seminal Thinning Response of Carp (Cyprinus carpio) and Rainbow Trout (Salmo gairdnerii) after Injections of Pituitary Extracts. Copeia 1965, 1965, 174–177. [Google Scholar] [CrossRef]
- Zadmajid, V. Comparative effects of human chorionic gonadotropin (hCG) and Ovaprim™ (sGnRHa+domperidone) on the reproductive characteristics of wild-caught male Longspine scraper, Capoeta trutta (Heckel, 1843). Aquaculture 2016, 463, 7–15. [Google Scholar] [CrossRef]
- Zadmajid, V.; Bashiri, S.; Sharafi, N.; Butts, I.A.E. Effect of hCG and Ovaprim™ on reproductive characteristics of male Levantine scraper, Capoeta damascina (Valenciennes, 1842). Theriogenology 2018, 115, 45–56. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.-J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocr. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Yamauchi, K.; Takahashi, H.; Nagahama, Y. The role of hormones in the acquisition of sperm motility in salmonid fish. J. Exp. Zool. 1992, 261, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y. Endocrine regulation of gametogenesis in fish. Int. J. Dev. Biol. 1994, 38, 217–229. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podhorec, P.; Knowles, J.; Vysloužil, J.; Boryshpolets, S.; Sotnikov, A.; Holická, M.; Kouřil, J.; Dzyuba, B. The Effect of Hormonal Treatment on Selected Sperm Quality Parameters and Sex Steroids in Tropical Cyprinid Bala Shark Balantiocheilos melanopterus. Fishes 2022, 7, 122. https://doi.org/10.3390/fishes7030122
Podhorec P, Knowles J, Vysloužil J, Boryshpolets S, Sotnikov A, Holická M, Kouřil J, Dzyuba B. The Effect of Hormonal Treatment on Selected Sperm Quality Parameters and Sex Steroids in Tropical Cyprinid Bala Shark Balantiocheilos melanopterus. Fishes. 2022; 7(3):122. https://doi.org/10.3390/fishes7030122
Chicago/Turabian StylePodhorec, Peter, Jindřiška Knowles, Jakub Vysloužil, Sergii Boryshpolets, Anatolii Sotnikov, Martina Holická, Jan Kouřil, and Borys Dzyuba. 2022. "The Effect of Hormonal Treatment on Selected Sperm Quality Parameters and Sex Steroids in Tropical Cyprinid Bala Shark Balantiocheilos melanopterus" Fishes 7, no. 3: 122. https://doi.org/10.3390/fishes7030122
APA StylePodhorec, P., Knowles, J., Vysloužil, J., Boryshpolets, S., Sotnikov, A., Holická, M., Kouřil, J., & Dzyuba, B. (2022). The Effect of Hormonal Treatment on Selected Sperm Quality Parameters and Sex Steroids in Tropical Cyprinid Bala Shark Balantiocheilos melanopterus. Fishes, 7(3), 122. https://doi.org/10.3390/fishes7030122








