Abstract
Fishery by-products (FBPs) have been increasingly investigated for the extraction and production of a vast array of active molecules. The aim of this study was to produce phenazine compounds from FBPs via microbial fermentation and assess their novel antinematode effect. Among various FBPs, squid pen powder (SPP) was discovered as the most suitable substrate for phenazine production by Pseudomonas aeruginosa TUN03 fermentation. Various small-scale experiments conducted in flasks for phenazine production indicated that the most suitable was the newly designed liquid medium which included 1% SPP, 0.05% MgSO4, and 0.1% Ca3(PO4)2 (initial pH 7). Phenazines were further studied for scale-up bioproduction in a 14 L bioreactor system resulting in a high yield (22.73 µg/mL) in a much shorter cultivation time (12 h). In the fermented culture broth, hemi-pyocyanin (HPC) was detected as a major phenazine compound with an area percentage of 11.28% in the crude sample. In the bioactivity tests, crude phenazines and HPC demonstrate novel potential nematicidal activity against black pepper nematodes, inhibiting both juveniles (J2) nematodes and egg hatching. The results of this work suggest a novel use of SPP for cost-effective bioproduction of HPC, a novel potential nematodes inhibitor. Moreover, the combination of MgSO4 and Ca3(PO4)2 was also found to be a novel salt composition that significantly enhanced phenazine yield by P. aeruginosa fermentation in this work.
1. Introduction
Fishery by-products (FBPs) are obtained from the industrial fish processing, with annual output reaching 27.85 million tons per year, most of which are discarded directly into the environment or utilized into low-value-added products such as fertilizers and animal feed [1,2]. Recently, among FBPs, research on recycling marine discards, including squid pens, shrimp shells, shrimp heads, and crab shells to produce high-value products cost-effectively via microbial fermentation is a topic of great interest [3,4,5,6]. Therein, squid pen powder (SPP) is also an abundant source, being the third most vital seafood product, ranking third only after shrimp and fish [7]. During the processing, SPP accounts for as much as 32.2% of the original materials. Furthermore, SPP is rich in protein, carbohydrates, lipids, and minerals [8]. This fishery-processing by-product has been extensively studied for the biosynthesis of chitooligomers, antioxidants, exopolysaccharides, enzymes, biosorbents, and pigments [9,10,11,12,13,14,15]. In this study, SPP was discovered as a carbon/nitrogen (C/N) source for cost-effective biosynthesis of the bioactive phenazines via bacterial fermentation.
Phenazines are a group of nitrogenous heterocyclic compounds and possess several potential bioactivities for use in many fields such as medicine, agriculture, industry, and technical applications [16,17]. Among phenazine compounds, pyocyanin has been extensively studied for its biosynthesis and applications [18]. In nature, phenazines are obtained exclusively from bacteria; most of that being from Pseudomonas [16]. Of these, Pseudomonas aeruginosa has been used as a phenazine-producing strain in numerous studies [16,17,19]. However, almost all the previous studies used commercial substrates for fermentation, such as tryptone, peptone, King’s A, King’s B, nutrient broth, LB, and glycerol/D-alanine/peptone, and fermentation was also conducted in the minor scale, in flaks [20,21,22,23,24]. Recently, for lower-cost production of phenazines, some studies tended to use several agroproducts as fermentation substrates, such as corn, sweet potato, watermelon seeds, peanut, and cottonseed [25,26]. Some organic wastes, including wastes of cheese whey, frying oil, sugar beet molasses, craft beer waste, and some household wastes, were also used for the bioproduction of phenazines [27,28]. However, only a few studies have attempted to use some major industrial fish-processing wastes as substrates for fermentation to obtain phenazines on large scale for their potential applications.
In our previous study [29], P. aeruginosa TUN03, a rhizobacterial strain isolated in the Central Highland of Vietnam, was newly found as a potential phenazine-producing strain and demonstrated potential antinematodes in vitro and in greenhouse tests. The goal of this study was to produce phenazine compounds via P. aeruginosa TUN03 conversion and assess their novel antinematode effect. Considering cost-effective production and environmental issues, we evaluated some industrial-processing by-products for phenazine production via microbial fermentation in this work. Phenazine compounds were also studied for scale-up production, then the crude phenazines and the major purified phenazine compound (hemi-pyocyanin) were further evaluated for their potential novel antinematode property in this study.
2. Materials and Methods
2.1. Materials
The bacterial strain P. aeruginosa TUN03 was obtained from our earlier study [29]. Nematode eggs were isolated from the roots of sick black peppers in Buon Ma Thuot City, Vietnam. The shrimp shells, crab shells, and squid pens were procured from Shin-Ma Frozen Food Co. (I-Lan, Taiwan). Crab shells and shrimp shells were demineralized for obtaining demineralized shrimp shell powder (de-SSP) and demineralized crab shell powder (de-CSP) per the method presented in our previous study [30]. Silica gel (Geduran® Si 60, size: 0.040–0.063 mm) was purchased from Merck Sigma Chemical Co. (St. Louis City, MO, USA) Yeast extract, peptone, gelatin, and casein were from Creative Life Science Co., Taipei, Taiwan, and some solvents used in this work were from Sigma Aldrich (St. Louis City, MO, USA).
2.2. Methods
2.2.1. Phenazine Production via Microbial Fermentation Experiments in Small Flasks
Preparation of bacterial strains: The P. aeruginosa TUN03 strain was activated in a trypticase soy broth (TSB, Sigma Aldrich) medium according to the method presented in the previous report [29]. The medium was sterilized in an autoclave for 30 min at 121 °C. Proceed with fermentation with shaking speed (150 rpm) at condition 28 °C for 48 h.
Screening of suitable marine chitinous by-products for fermentation: For choosing the most suitable substrate source for fermentation, marine by-products and commercial free protein were added independently into the media or combined according to the designed experiments as in Table 1. Three marine by-products, including SPP, de-SSP, de-CSP, and four commercial material media, including yeast extract, peptone, gelatin, and casein, were used as the sole C/N source for fermentation. These three marine by-products (each material) were also mixed with free protein (peptone) at the marine by-product/peptone ratio of 7/3 and used C/N source for fermentation to compare the phenazine production. The culture medium (30 mL with the initial pH 6.8 in a 100-mL flask) containing 1% C/N source, MgSO4 0.05%, and K2HPO4 0.1% was fermented by P. aeruginosa TUN03 at 28 °C with shaking speed of 150 rpm for 3 d (*) [31]. The supernatant was harvested by centrifugation at 10,000× g for 15 min then used for estimation of phenazine content.

Table 1.
Experiments for screening the most suitable phenazine-producing substrate.
The effect of SPP concentration on phenazine production: Various concentrations (0.5, 1, 1.5, 2, and 2.5%) of SPP were added into the culture medium containing 0.05% MgSO4 and K2HPO4 (initial pH 6.8). The fermentation was performed according to the above protocol (*). 1% SPP was found as the most suitable concentration for fermentation and was thus used for all the following experiments.
The effect of salt composition on phenazine productivity by fermentation:
The effect of chloride salt on phenazine production: some concentrations of NaCl (0.5, 1, 2, and 3%), and a specific concentration of combined salts (0.05% MgSO4 and 0.1% K2HPO4) were used in the previous study [31] were added into the media containing 1% SPP then fermented as per the above-mentioned protocol (*).
The effect of sulfate salts for phenazine production: five types of sulfate (ZnSO4, FeSO4, MnSO4, (NH4)2SO4, MgSO4) at 0.05% concentration in combination with 0.1%K2HPO4 were added into the media containing 1% SPP and then fermented as per the above-mentioned protocol (*).
The effect of phosphate salts on phenazine production: Phosphate salts including K2HPO4, KH2PO4, Ca3(PO4)2, NaH2PO4, Na2HPO4 at 0.1% concentration in combination with 0.05% MgSO4 were added to the media containing 1% SPP then fermentation using the above protocol (*).
Determination of some culture parameters: These tests were designed based on some earlier reports [21,32,33]. The culture parameters, including pH value and temperature, were examined. The different mediums in pH value (range of 6, 6.5, 7, 7.5, 8, 8.5, and 9), culture temperature (20, 25, 30, 35, and 40 °C), and the time courses of fermentation (1, 2, 3, 4, and 5 d) were performed with culture ingredients of medium collected from the above tests and fermentation as per the above-mentioned protocol (*).
2.2.2. Scale-Up Production of Phenazines via Using a 14 L Bioreactor System
Phenazines were produced in mass using a 14 L bioreactor system. The pre-cultivation of Pseudomonas aeruginosa TUN03 seed was conducted in several 500 mL flasks at 30 °C for 1.5 d then injected into the bioreactor system containing 4.5 L of a novel liquid medium (1% SPP, 0.05% MgSO4, 0.1% Ca3(PO4)2 at initial pH 7). The condition parameters for fermentation were set up with a shaking speed of 250 rpm, culture temperature at 30 °C, a dissolved oxygen content of 1.2 vvm, and a fermentation period of 14 h. The phenazine yield was determined per 2 h.
2.2.3. Quantitives and Purification of Phenazines
Measurement of phenazine productivity via UV/vis absorption: The ability of phenazine biosynthesis from P. Aeruginosa TUN03 was estimated through the determination of the yield of pyocyanin (PYO) pigment in the culture broth. This is the most applied method for estimation of phenazines in the culture medium [18]. The assay was performed according to the previous work reported by Murat Ozdal, 2019 [24]. A mixture of cell-free supernatant (2.5 mL) combined with chloroform solvent (1.5 mL) and HCl 0.2N (0.5 mL) was measured based on the absorbance at 520 nm (a recording of OD520 value). The yield of PYO was calculated via the following formula:
Phenazine content (PYO) = OD520 × 17.072
Furthermore, the content of the major phenazine compound was determined using high-performance liquid chromatography (HPLC; UHPLC-UV Ultimate 3000, Thermo US) technique.
Measurement of the major phenazine via HPLC analysis [31]: the residues and P. aeruginosa TUN03 biomass in culture broths were removed by centrifugation at 8000 rpm for 10 min, and the harvested culture supernatants were used to determine the concentration of hemi-pyocyanin (the major phenazine compound produced by P. aeruginosa TUN03. The culture supernatants (5 µL) were injected into the HPLC system then separated via a C18 column using the solvent systems of methanol/acidified 0.1% H3PO4 (70/30 v/v) with the flow rate of 0.2 mL/min. The compound was detected at 265 nm. The purified compound hemi-pyocyanin obtained from our previous work was used as the reference compound to establish the following equation for stimulation of hemi-pyocyanin content:
where x is the concentration of hemi-pyocyanin and y is the peak area of the reference compound, and all the tests were performed with triplicates. The reference compound hemi-pyocyanin was analyzed at the standard concentration range of 0.162–33.5 ppm.
Hemi-pyocyanin content: y = 0.3954x + 0.1584; R2 = 0.9998
The extraction of crude pigment: The method was referenced from the previous research [31]. Centrifugation of culture broth (obtained from the fermentation in the 14 L-bioreactor system) at 12,000 rpm for 10 min to collect the supernatant. Chloroform solvent was mixed with this supernatant at the ratio of 1/1 (v/v) and kept in the funnel for about 3 h with shaking every half hour. The chloroform layer containing pigment of phenazines was collected and concentrated in a rotary evaporator (IKA, Germany) at 55 °C under vacuum, then dried to obtain crude pigment in powder form (crude pigments) at 55 °C in an oven air drier.
The purification of the major compound: The crude pigment was purified using an opened silica column (Geduran® Si 60, size: 0.040–0.063 mm, 30 × 2 cm) with a gradient solvent system chloroform/methanol (100/0) and collecting this fraction. In our previous research, this fraction was identified as hemi-pyocyanin [31]. The hemi-pyocyanin purified in this work was determined its purity by HPLC using the reference compound for comparison. This pigment compound was also reconfirmed as hemi-pyocyanin by GC-MS analysis.
2.3. Assay of Nematodes Inhibition
The root-knot nematode inhibition assay was conducted according to the method presented in detail in our previous report [15]. The black pepper roots were collected from the sick trees (symptoms with yellow leaves) from Buon Ma Thuot, Dak Lak province, Vietnam, and the eggs and J2 nematodes were prepared according to Khan et al. [34]. The tested samples were dissolved in DMSO at various concentrations at 50, 25, 12.5, 6.25, 3.13, and 1.56 mg/mL and used for in vitro tests. The same treatment was also applied to two control samples (only containing DMSO or water). All the tests were carried out in triplicates. The antinematode activity is determined via the inhibition efficiency (%) and IC50 value (mg/mL). The IC50 value is defined as the concentration of a compound that may inhibit 50% nematode.
The assay of anti-J2 nematode in vitro: Mixing 200 μL sterile distilled water containing 30 individuals of J2 nematodes with 200 μL of sample solutions in a 96-well culture plate. This mixture was kept at 28 °C for 24 h before conducting a count of the immobilized nematodes under a stereoscopic microscope Olympus SZ5.
In vitro assay of egg-hatching inhibition: 100 μL of the sample solution at various concentrations was combined with sterile distilled water containing 200 nematode eggs; then, this mixture was incubated at 28 °C. The number of hatching eggs was counted per day after three days up to six days of incubation (based on J2 nematodes).
2.4. Statistical Analysis
The experiments of pigment production and tested bioactivity were designed randomly. Statistical analysis in this study used Statistical analysis software (SAS-9.4) purchased from SAS Institute Taiwan Ltd. (Taipei, Taiwan). The experimental data on phenazine production and bioactivity were obtained and analyzed via the simple variance (ANOVA) followed by Duncan’s multiple range tests (when the experiment contained ≥6 items that needed to be compared) and Fisher’s LSD tests (when the experiment contained ≤5 items that needed to be compared) at p = 0.01 were evaluated.
3. Results and Discussion
3.1. New Records of Fishery-Processing By-Product Squid Pens as Sole Carbon/Nitrogen Source for Phenazine Production via Fermentation
To choose the suitable discard material for fermentation, some C/N sources were screened. The experiment was designed for fermentation on three substrate sources, including marine by-products (1), commercial free protein (2), and combined marine by-products with free protein (3). The results are summarized in Table 1.
Among marine by-products (1), SPP showed a positive effect on phenazine production, with a high yield of 14.52 µg/mL, while production of phenazines was not credited for the de-SSP and de-CSP. Several commercial free proteins (2) were used as substrates for fermentation for comparison purposes. Among them, the media containing peptone or casein had a positive effect on phenazine synthesis; however, the recorded yield (3.54–6.91 µg/mL) was lower than that of using SPP (14.52 µg/mL) as the sole C/N source for fermentation. Moreover, by-products are also combined with free protein (peptone) to evaluate the improved ability of phenazine content compared to the oral use of these marine by-products. The supplements of peptone only helped to increase phenazine production on medium containing SSP and CSP. When combined with SPP, the yield of PYO reached 14.31 µg/mL, almost the same as when SPP was used (14.52 µg/mL). For cost-effective phenazine production, SPP was used as the sole C/N source for the next experiments.
Several SPP concentrations were tested to choose the most suitable concentration for phenazine production (Figure 1). Based on the experimental data, the medium containing SPP at 1% recorded the highest content of phenazines at 14.44 µg/mL. At higher concentrations (1.5–2.5%), the yield of phenazines decreased (11.94–10.93 µg/mL). Thus, the concentration of SPP 1% was chosen for further tests.
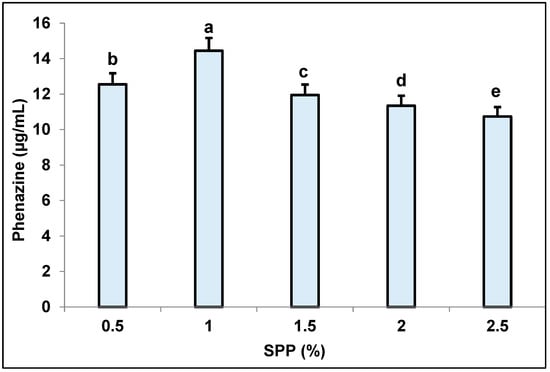
Figure 1.
Effect of supplementary squid pen powder (SPP) concentration on phenazine biosynthesis. The analysis result showed that the different letters are significantly different. LSD (Least Significant Difference): 0.0778; CV (Coefficient of Variation): 0.232777.
Some substrate sources used in phenazine PYO production were summarized in Table 2. Nutrient broth (NB) and improved NB medium were used for PYO biosynthesis; in addition, some other commercial media were reported, and the phenazine content reached about 3.2–25.5 µg/mL [21,22,23,24,25,32]. To reduce the cost of phenazine production, several low-cost organic materials were also added to the culture medium; however, the phenazine productivity achieved a low yield of only 0.34–4.0 µg/mL [25,26]. Considering cost effect and environmental issues, some by-products/wastes were utilized as C/N sources for fermentation and exhibited a positive effect on phenazine production [25,27,28,33,35,36]. According to the trend of production of the natural compounds using a cost-effective and environmentally friendly approach, in this study, SPP was found as a new and potential substrate for the cost-effective production of phenazine pigment via microbe fermentation.

Table 2.
Various substrate sources used for phenazine production.
3.2. The Effect of Salt Composition on Phenazine Productivity in Fermentation
In various studies, chloride salts were commonly used for phenazine production [22,37,38]. Therefore, we reconfirmed the effect of chloride salt (using NaCl) in this experiment at various concentrations, including 0.5, 1, 2, and 3% for phenazine production, and the combined salts (0.05% MgSO4 and 0.1% K2HPO4) used in the previous study [31] were also added to the culture broth for comparison. The results are presented in Figure 2. Generally, NaCl also impacted phenazine biosynthesis; herein, the highest phenazine content (13.25 µg/mL) was measured in the medium containing 1% NaCl; however, this value was lower than that of the culture medium supplemented with the combined salts (14.66 µg/mL). The sulfate and phosphate salts were also conducted to supplement into the culture media for fermentation to induce phenazines in some earlier works [39,40]. Burton et al. reported the formation of PYO at a high yield in the medium containing 2% MgSO4, 0.04% K2HPO4, and 0.001% FeSO4 [39]. The optimum production of PYO was achieved in a medium comprising 0.05% MgSO4 and 0.05% K2HPO4 in the study by Georgia and Poe, 1932 [40]. However, the effect of types of various sulfate and phosphate salts was not elucidated. Thus, in the next test, we evaluated the impact of several types of sulfate and phosphate salts for phenazine-producing activity.
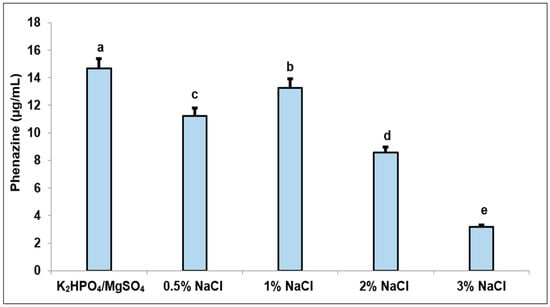
Figure 2.
The effect on phenazine production of K2HPO4 and MgSO4 system (control) compared to NaCl at concentrations including 0.5, 1, 2, 3%. Values with the different letters are significantly different. LSD value of 0.115; CV value of 0.412766.
Five types of sulfate salts were added into the medium at 0.05% to test their effect on phenazine production (Figure 3a). Of these, the medium containing MgSO4 showed the highest productivity of phenazines at 14.96 µg/mL, followed by (NH4)2SO4 with a relatively high phenazine content of 13.55 µg/mL, and the lowest value belonging to the medium supplemented with ZnSO4 (1.85–2.25 µg/mL). Various types of phosphate salts at 0.1% were also tested for their effect on phenazine production (Figure 3b). The maximum phenazine content (16.15 µg/mL) was obtained when adding 0.1% of Ca3(PO4)2 into the medium while other phosphate salts gave a lower yield of phenazines (10.74–13.45 µg/mL). Overall, the final composition of culture medium in the small flask for the highest phenazine yield production included 1% SPP combined with newly recorded salt compositions of 0.05% MgSO4 and 0.1% Ca3(PO4)2.
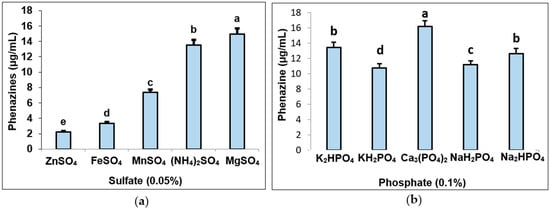
Figure 3.
The effect of sulfate (a) and phosphate (b) salts for phenazine production by Pseudomonas aeruginosa TUN03. Thirty milliliters of culture broth including 1% SPP and respective salt type for each test was fermented at 28 °C for 3 d and shaking at 150 rpm. The data showed that the different letters are significantly different. The LSD values; CV for the test of 0.05% sulfate (0.0731; 0.468512, respectively) and for the test of phosphate 0.1% (0.0982; 0.246744, respectively) are presented.
3.3. Determination of Suitable Culture Parameters for Pigment Biosynthesis
To reach more effective phenazine production, some fermentation parameters, such as initial pH of culture medium (pH in the range of 6.0, 6.5, 6.0, 7.5, 8.0, 8.5, 9.0), the temperature of cultivation (20, 25, 30, 35, 40 °C), and the fermentation time (0, 1, 2, 3, and 4 d), were evaluated. The experimental data presented in Figure 4 indicate that P. aeruginosa TUN03 produced phenazine pigments with the highest productivity at the medium initial pH 7.0, fermentation temperature of 30 °C in 2 d. The results of the most appropriate pH and temperature are similar to those in previous reports [21,33]. The yield of maximum phenazines was obtained by P. Aeruginosa OSh1 at 28 °C, pH 7 [21]. The optimal conditions in the medium using maize by-product were defined at 29.6 °C and pH 6.92 [33].
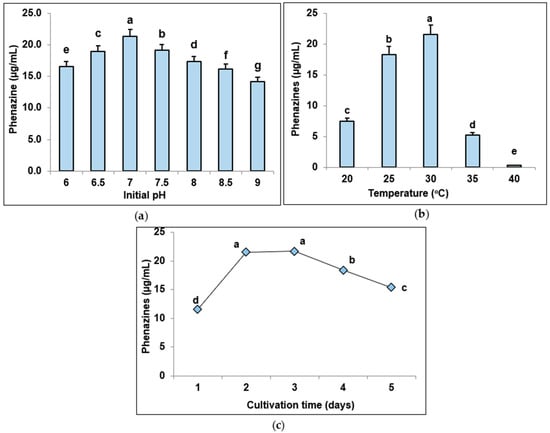
Figure 4.
The effect of initial pH of culture broth (a), the fermentation temperature (b), and fermentation time (c) on phenazine biosynthesis by Pseudomonas aeruginosa TUN03. The different letters show a statistically significant difference. The LSD values; CV for the test of initial pH (0.0542; 0.123263, respectively), for the test of fermentation temperature (0.1108; 0.382522, respectively), and for the test of fermentation temperature (0.4742; 0.978673, respectively) are presented.
Overall, the biosynthesis of phenazines by P. aeruginosa TUN03 reached the highest yield (21.8 µg/mL) in the novel designed culture broth containing 1% SPP, 0.05% MgSO4 h0.1% Ca3(PO4)2 at initial pH 7.0, cultivation temperature at 30 °C in 2 d of cultivation, and the phenazines yield increased more than 1.5-fold (from 14.52 to 21.8 µg/mL) after optimization of medium ingredients and culture conditions. Further, to enhance the production of pigment, fermentation was conducted on a larger scale in a 14 L bioreactor system.
3.4. Scale-Up Production of Phenazines via Using a 14 L Bioreactor System and Purification of Phenazines
Bioreactors are strong tools in fermentation technology that help in mass bioproduction of active compounds, significantly reducing the fermentation time, and may also enhance the yield of products compared to the traditional fermentation in flasks [9,10,12,14,15]. To approach the aim of phenazine biosynthesis at a mass scale, the 14 L bioreactor system was used for fermentation. The novel designed medium (5 L) obtained in the previous experiments (in Section 3.1, Section 3.2 and Section 3.3 of this study) was fermented by P. aeruginosa TUN03 in the bioreactor systems in 14 h, and the phenazine productivity was recorded per 2 h (Figure 5). The phenazine production started early and gradually increased to the max yield (22.73 µg/mL) at 12 h of fermentation. Compared to fermentation in flasks, the utilization of bioreactor systems for fermentation resulted in a slightly higher phenazine yield (22.73 µg/mL) produced by P. aeruginosa TUN03 than that of fermentation in flasks (21.8 µg/mL). However, the time of fermentation in a bioreactor in this study was much shorter (12 h) than that of fermentation in flasks (2–3 days).
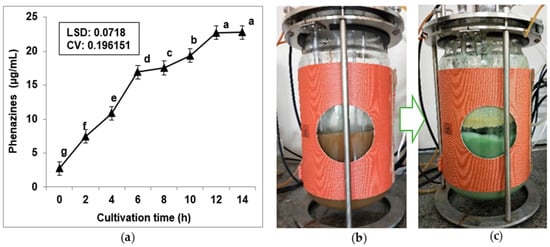
Figure 5.
Scale-up production of phenazines by Pseudomonas aeruginosa TUN03 using a 14 L Bioreactor system. The recorded data of phenazine yield produced during the process of fermentation (a). The reaction tube of the 14 L Bioreactor system at 0 h (b) and 12 h (c) of fermentation. The 4.5 L of medium contained 1% SPP, 0.05% MgSO4, and 0.1% Ca3(PO4)2 mixed with 500 mL of bacteria seeds. The condition parameters for fermentation were set up with a shaking speed of 250 rpm, culture temperature at 30 °C, a dissolved oxygen content of 1.2 vvm, and a fermentation period of 14 h. The phenazine yield was determined per 2 h.
Because they hold vast arrays of valuable applications, phenazines have been studied for their production in numerous reports [16,17,19,20,21,22,23,24]. However, almost all the previous studies reported the bioproduction of phenazines on a small scale, in flasks, and used commercial media as a C/N source for fermentation. Few studies used bioreactors for the mass production of phenazines [41]. Recently, a phenazine was successfully produced in mass using a 5 L fermenter with a true working volume of 2 L (commercial medium) for fermentation, with the highest productivity of 3.6 mg/mL in 54 h of fermentation [41]. In this study, phenazines were produced on a large scale in 14 L bioreactor systems with a true working volume of 5 L, the fishery waste was utilized as the sole C/N source for fermentation, and phenazines were produced with a yield of 22.73 µg/mL in a short time of cultivation (12 h).
3.5. Determination, Extraction, and Purification of the Main Phenazine Compound from the Culture Broth
To determine the major phenazine compounds contained in the fermented culture broth, the supernatant was extracted with chloroform to enrich the phenazine content, and this crude pigment was further analyzed for its phenazine compound content using GCMS and HPLC techniques. Based on the output data of GCMS (summarized in Table 3), the crude extract containing four major constituents, including hemi-pyocyanin (1), phenazine (2), Phthalic acid, monodecyl ester (3), and Phthalic acid, hex-3-yl octyl ester (4) were detected and were identified their area percentage of 11,28, 2.67, 2.11, and 71.82 %, respectively. The GCMS spectra, including Figures S1–S4 were presented in the Supplementary Materials section. Of these, two phenazine compounds (compounds 1 and 2) were detected, and hemi-pyocyanin (1) was found as the major phenazine compound with its area percentage of 11.28% in the crude sample, while phenazine (2) was recorded at a lower area percentage value of 2.67%. The result of identification of the major phenazines in this work was similar to various previous reports [19,22], which also revealed pyocyanin or hemipyocyanin as the major compounds synthesized by genus P. Aeruginosa. In this study, phthalic acid, the hex-3-yl octyl ester (4) was newly found to be produced by P. Aeruginosa as a coproduct with phenazine. Notably, this metabolite was produced in a significant amount (71.82% of area percentage).

Table 3.
The major compounds in the crude pigments produced by P. aeruginosa TUN03.
Although there are still controversies surrounding the polluting impact of Phthalic acid esters (PAEs) from synthesis sources, in fact, the effect of natural PAEs biosynthesized from various natural sources (algae, bacteria, fungi) on ecology has been not elucidated. PAEs were also reported showing some bioactivities such as antimicrobial, insecticidal, and enhancing the competitiveness of plants, algae, and microorganisms to better accommodate stress. Thus, PAEs should not be treated solely as a “human-made pollutant”; the useful bioactivities of these natural compounds also should be researched further [42], whereby the new finding in our study may be an interesting topic for further investigation on cost-effective bioproduction of this compound (Phthalic acid, hex-3-yl octyl ester) from organic wastes by P. aeruginosa fermentation, and the potential applications of this major compound should be further investigated.
To confirm that these five molecules were produced by fermentation and did not exist in the medium beforehand, the chloroform extract of the unfermented medium (control medium) was also analyzed via GC-MS under the same condition as the crude pigments. In the GC profile of the crude pigment (Figure S5A), these identified molecules, hemi-pyocyanin (1), phenazine (2), Phthalic acid, monodecyl ester (3), and Phthalic acid, hex-3-yl octyl ester (4) appeared at the retention time of 12.81, 13.44, 16.93, 17.66, and 19.42 min, respectively. However, these compounds were not detected in the GC profile of the control medium (Figure S5B). This evidence indicated that the above molecules were biosynthesized by fermentation.
Recently, hemi-pyocyanin was also determined as the major compound produced by P. aeruginosa TUN03 in our previous work [31]. For more careful confirmation, this major phenazine compound was purified, then identified based on GC-MS, and its purity was confirmed via HPLC. Based on the comparison of the 10 largest mass fragments of the purified compound to those of the reference compound, this purified pigment was identified as hemi-pyocyanin. The GCMS profile of the purified hemi-pyocyanin is shown in Figure 6.
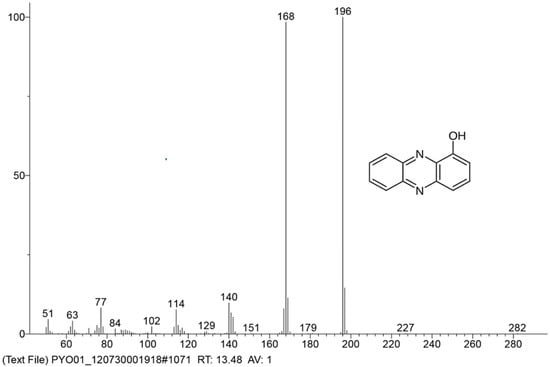
Figure 6.
The GCMS profile of purified hemi-pyocyanin produced by Pseudomonas aeruginosa TUN03.
The crude pigments, hemi-pyocyanin isolated in this study, and the purified hemi-pyocyanin obtained from the previous work were analyzed for comparison (Figure 7). As shown in Figure 7a, hemi-pyocyanin (purified in this work) appeared clearly as the single peak with retention time (RT) at 3.323 min, which is approximately similar to its reference compound appearing with RT at 3.317 min (Figure 7b). This profile confirmed that the hemi-pyocyanin isolated in this study possesses a high grade of purity and could be applied for biological investigation. A major peak was also found at the RT of 3.330 min on the HPLC profile of crude pigments (Figure 7c). This result also supports the confirmation that hemi-pyocyanin is a major phenazine compound produced by P. aeruginosa TUN03. Since hemi-pyocyanin is the main compound and clearly appeared on the HPLC profile of crude pigments, we thus used the HPLC technique for determining the phenazine yield of the culture broth fermented in bioreactor system at 12 h based on the method presented in Section 2.2.3 (Quantities and Purification of Phenazines). The result showed that hemi-pyocyanin was produced at a concentration of 20.11 µg/mL. This result is approximately the same as the phenazine content estimated by the UV/vis method (22.73 µg/mL).
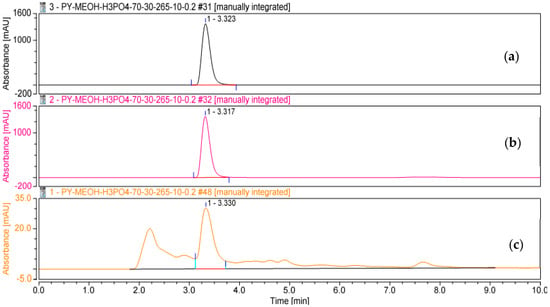
Figure 7.
The HPLC fingerprinting of hemi-pyocyanin (purified in this work (a), the reference compound (b), and the crude pigments (c)).
3.6. The Inhibitory Activity of Phenazines on Black Pepper Nematodes
Black pepper is one of the key crops in Vietnam; however, it is seriously destroyed by root diseases [43]. Of these, Meloidogyne incognita is a major harmful species [44]. In the strategy for management of nematodes, beneficial microbes, or natural compounds with effective antinematode effects have been wildly reported [45,46,47,48,49,50]. These green methods have been increased due to their cost-effectiveness, safety, and being environmentally friendly [45]. Of the natural compounds, the studies on the utilization of nematode inhibitors from microbial fermentation have received much interest since they may be produced on an industrial scale for available use [15]. The prospective nematicidal candidates are considered to inhibit both juveniles (J2) nematodes and egg hatching. Thus, we evaluated the effect of samples against both J2 nematodes and the egg hatching using the assays presented in the Section 2.2. The tested samples included crude pigments and hemi-pyocyanin purified in this work, and prodigiosin, an antinematode compound, was used as a positive control for comparison. DMSO was used for dissolving the samples; thus, an equal volume of DMSO was used instead of samples in the negative control tests for the calculation of the results (Figure 8).
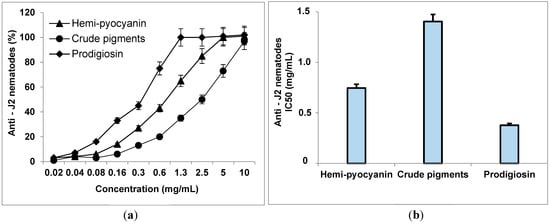
Figure 8.
The anti-J2 activity (%) of purified hemi-pyocyanin, crude pigments, and prodigiosin (a), and their effects were expressed as IC50 values (b). The experiments of the anti-J2 nematode were conducted in three repetitions. The samples were tested at various concentrations for 2 d. The mobilized and immobilized J2 nematodes were counted using stereoscopic microscope Olympus SZ5 for estimation of the inhibitory activity.
The anti-J2 nematodes effect: In the negative control tests (the J2 nematode treated with DMSO), the survival rate of J2 is approximate 94%; thus, DMSO had a negligible effect on J2 nematodes, making it appropriate for dissolving the samples as well as a negative control in the experiments. As showed in Figure 8a, hemi-pyocyanine, crude pigments, and prodigiosin demonstrated strong anti-J2 nematode activity with max inhibition values of 99, 90, and 100%, respectively, at the tested concentration of 10 mg/mL. However, at low concentration (1.25 mg/mL), crude pigments showed low inhibition activity of 55%, while the two compounds, hemi-pyocyanin and prodigiosin, demonstrated stronger activity, with their inhibition value up to 77% and 100%, respectively. For more clarification of the activity, the IC50 values were also calculated, as presented in Figure 8b. The IC50 value was defined as the concentration of an inhibitor compound that may inhibit 50% of J2 nematodes; as such, the smaller the value the inhibitor obtained, the stronger activity it showed. The crude pigments showed the highest IC50 values; thus, it demonstrated the minimized anti-J2 nematodes among the tested samples. Since hemi-pyocyanin and prodigiosin inhibited J2 nematodes with IC50 values of 0.377 and 0.746 µg/mL, respectively, hemi-pyocyanin was ranked as weaker than prodigiosin in terms of activity. However, in the independent dose tests, hemi-pyocyanin may inhibit J2 nematodes with max inhibition of 99%. Thus, this compound was also considered as a promising natural antinematode compound with moderate effect. For morphological characteristics of the J2 nematode, the normal J2 in the control test (treated with DMSO) is still able to move flexibly (Figure 9a), while in the tests treated hemi-pyocyanin (Figure 9b), crude pigments (Figure 9c), and prodigiosin (Figure 9d), the J2 nematodes possessed similar characteristics as the body straightened and immobilized.
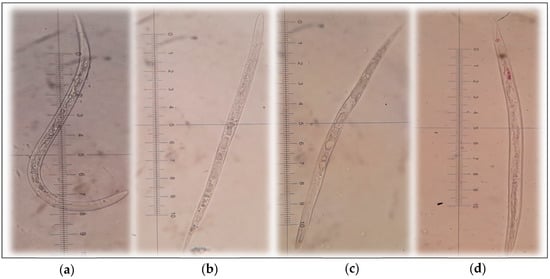
Figure 9.
The images of mobilized J2 nematodes in control samples (a) immobilized J2 nematodes after being treated by hemi-pyocyanin (b), the crude pigments (c), and prodigiosin (d). The images of J2 were taken under stereoscopic microscope Olympus SZ5.
The inhibitory activity against nematode egg-hatching: In the control tests (the nematode eggs treated with DMSO), DMSO presented a negligible effect on eggs; the egg-hatching amount remained at a high level (about 93.2%) until the sixth day. Based on the daily observation, the nematode eggs in control tests started hatching from day 2 of incubation, while the eggs treated with hemi-pyocyanin, crude pigments, and prodigiosin appeared to hatch at day 3 of incubation. This means the tested samples have an effect to delay the egg hatching of nematodes. The egg hatching was counted daily and expressed as inhibition values (%, Figure 10a–c) and IC50 values (mg/mL, Figure 10d). As shown in Figure 10, all the tested samples of hemi-pyocyanin, crude pigments, and prodigiosin demonstrated significant activity from day 3 of incubation, with max inhibition (%) values of 97%, 88%, 98%, respectively, and IC50 values 0.441, 1.9, and 0.301 mg/mL, respectively. These values were greatly maintained until day 6 of incubation, with max inhibition and IC50 values in the range of 53.1–70% and 1.8–8.8 mg/mL, respectively. In the comparison, hemi-pyocyanin and prodigiosin demonstrated potent activity at an equal level, while the crude pigments displayed lower activity due to their lower max inhibition value and higher IC50 value than those of hemi-pyocyanin and prodigiosin at all the tested concentrations during the incubation. The experimental data of this work indicated that hemi-pyocyanin demonstrated moderate anti-J2 nematodes and potential inhibition against nematode egg hatching; as such, it may be suggested as a novel and potential candidate for management of black pepper nematodes. The morphological change was also observed and described in Figure 11. The eggs in the control tests developed normally to generate J2 nematodes, while the membranes of most of the eggs treated with hemi-pyocyanin, crude pigments, and prodigiosin were broken, leading to the leakage of intracellular components.
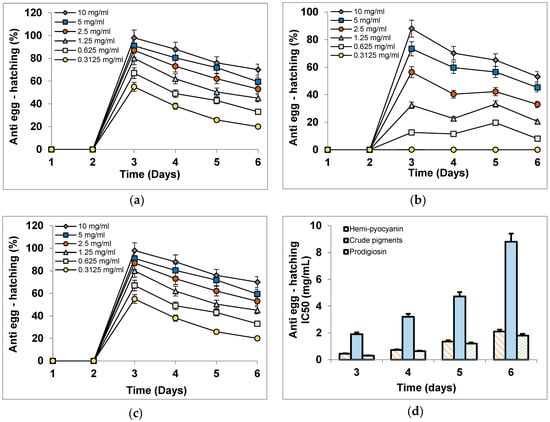
Figure 10.
The egg-hatching inhibitory effect (%) of hemi-pyocyanin (a), crude pigments (b), and prodigiosin (c), and their effect was expressed as IC50 values (d). The graph bioactivity was constructed by Microsoft Excel 2010. The nematode eggs were treated with six concentrations of samples (0.313, 0.625, 1.25, 2.5, 5, and 10 mg/mL) and followed up by counting the amount of egg hatching using stereoscopic microscope Olympus SZ5 for estimation of the activity during the 6 days of treatment. All the experiments were conducted in three repetitions.
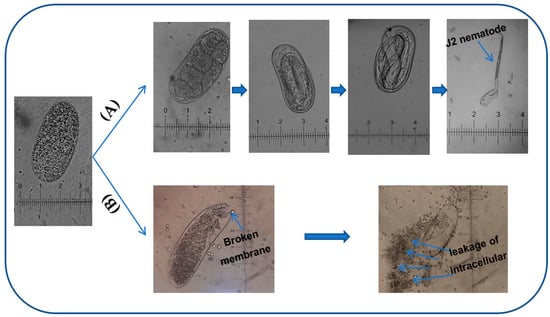
Figure 11.
The nematode egg develops normally, creating J2 nematode in control samples (A) and the egg was broken, causing the leakage of intracellular ingredients after the treatment by hemi-pyocyanin and crude pigments (B).
Some molecules produced by microorganisms, such as Verrucarin A, Roridin A [51], Kojic acid [52], Indole acetic acid [47], and 2-Furoic [53], Fungichromin B [54], and prodigiosin [29] were also previously reported showing root-knot nematode inhibitory effects with IC50 values in the range of 1.5–238 µg/mL. In this work, HPC showed lower anti-J2 nematode activity (the IC50 value of 746 µg/mL) than that of some reported molecules. However, HPC showed a comparable egg-hatching inhibitory activity (the IC50 value of 301 µg/mL) than that of Kojic acid (the IC50 value of 238 µg/mL) [52] and prodigiosin (the IC50 value of 320 µg/mL) [29]. HPC may be suggested as a potent nematode inhibitor due to its effective egg-hatching inhibition and moderate anti-J2 nematode activity.
Phenazines have been reported with applications in many fields, such as medicine, industry, and technical uses [16,17]; in particular, phenazines possess many valuable bioactivities with potential application in agriculture. These chemical groups are recognized and proven as potential agrochemicals with excellent properties of inhibition against various pathogens, fungal and bacterial strains, and plant-growth-promoting effects [55,56,57,58,59,60,61,62,63]. However, the antinematode effect of phenazines was rarely reported in several previous works [55,64]. In the earlier study reported by Brent Cezairliyan et al. [55] a phenazine compound 1-hydroxyphenazine was found to have efficient inhibition against the nematode Caenorhabditis elegans, while other phenazines demonstrated moderate or weak activity toward this genus nematode. Recently, phenazines including 2-hydroxy-phenazine, phenazine I-carboxylic acid, and 2-hydroxy-phenazine I-carboxylic acid were reported to have a potent effect on the root-knot nematodes; however, the nematicidal effect was due to which phenazine compound was not clarified because the tested sample was in the crude form [64]. In this study, we first reported the inhibition effect of crude phenazines against egg-hatching and J2 nematodes inhabiting the black pepper plant. Notably, the moderate anti-J2 and potential anti-egg hatching of purified compound hemi-pyocyanin were the new findings in this work; therefore, the results of this work may contribute to enriching the data on the biological activity of phenazines, and also presents the novel use of phenazines in the management of black pepper root-knot nematodes.
4. Conclusions
Among various fishery by-products, squid pens are newly found to be the most suitable material and are used as the sole C/N source for phenazine production via P. aeruginosa TUN03 fermentation. The salt compositions of MgSO4 and Ca3(PO4)2 were also a novel finding for the enhancement of phenazine productivity. Phenazine production was further investigated for scale-up production on a large scale of a 14 L Bioreactor system, and a high yield (22.73 µg/mL) was achieved in a short fermentation time (12 h). A major phenazine compound in the fermented product was purified and identified as hemi-pyocyanin. This pure compound and crude pigment demonstrate potential nematicidal activity. Notably, the potential anti-J2 and egg hatching of purified compound hemi-pyocyanin were reported for the first time in this work. The results of this study provided the novel use of SPP for cost-effective bioproduction of hemi-pyocyanin for a novel potential use as an inhibitor of nematodes. However, for the development of hemi-pyocyanin as an applicable antinematode agent, further studies, including the effect of hemi-pyocyanin on J2 nematodes, egg hatching, soil microbiota, black pepper seedlings, and black pepper trees in the greenhouse, as well as in field conditions, should be conducted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes7030113/s1, Figure S1. The GCMS analysis of Hemi-pyocyanin produced by Pseudomonas aeruginosa TUN03; Figure S2. The GCMS analysis of Phenazine produced by Pseudomonas aeruginosa TUN03; Figure S3. The GCMS analysis of The GCMS analysis of Phthalic acid, monodecyl ester produced by Pseudomonas aeruginosa TUN03; Figure S4. The GCMS analysis of The GCMS analysis of Phthalic acid, hex-3-yl octyl ester produced by Pseudomonas aeruginosa TUN03; Figure S5. The GC profiles of crude phenazines (A) and the extract of unfermented medium (B).
Author Contributions
Conceptualization, V.B.N., A.D.N. and S.-L.W.; methodology, T.H.N. (Thi Hanh Nguyen); formal analysis, V.B.N., T.H.T.T., V.A.N., C.T.D., T.N.T., N.D.H., V.C.D. and S.-L.W.; investigation, T.H.N. (Thi Hanh Nguyen), T.H.N. (Thi Huyen Nguyen), M.D.D. and V.B.N.; writing—original draft preparation, T.H.N. (Thi Hanh Nguyen); writing—review and editing, V.B.N., A.D.N. and S.-L.W.; project administration, V.B.N. and S.-L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from Tay Nguyen University (T2021-52CBTĐ) and sup-ported in part by the Ministry of Science and Technology, Taiwan (MOST 110-2320-B-032-001; MOST-111-2923-B-032-001).
Data Availability Statement
Data available on request due to restrictions e.g., privacy or ethical.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Lima, M.A.B.; Franco, L.O.; Campos-Takaki, G.M. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery wastes as a yet undiscovered treasure from the sea: Biomolecules sources, extraction methods and valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradit, S.; Noppradit, P.; Goh, B.P.; Sornplang, K.; Ong, M.C.; Towatana, P. Occurrence of microplastics and trace metals in fishand shrimp from Songkhla Lake, Thailand during the COVID-19 pandemic. Appl. Ecol. Environ. Res. 2021, 19, 1085–1106. [Google Scholar] [CrossRef]
- Hue, H.T.T.; Pradit, S.; Lim, A.; Goncalo, C.; Nitiratsuwan, T. Shrimp and fish catch landing trends in Songkhla Lagoon, Thailandduring 2003–2016. Appl. Ecol. Environ. Res. 2018, 16, 3061–3078. [Google Scholar] [CrossRef]
- Venugopal, V. Valorization of seafood processing discards: Bioconversion and biorefinery approaches. Front. Sustain. Food Syst. 2021, 5, 611835. [Google Scholar] [CrossRef]
- Sanuddin, M.; Yulianis, Y.; Annisaq, N. Synthesis chitosan from squid pens waste. ALKIMIA J. Ilmu Kim. Dan Terap. 2020, 4, 6–11. [Google Scholar]
- Huang, Y.L.; Tsai, Y.H. Extraction of chitosan from squid pen waste by high hydrostatic pressure: Effects on physicochemical properties and antioxidant activities of chitosan. Int. J. Biol. Macromol. 2020, 160, 677–687. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L.; Nguyen, A.D.; Phan, T.Q.; Techato, K.; Pradit, S. Bioproduction of prodigiosin from fishery processingwaste shrimp heads and evaluation of its potential bioactivities. Fishes 2021, 6, 30. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.L.; Nguyen, D.N.; Nguyen, A.D.; Nguyen, T.H.; Doan, M.D.; Ngo, V.A.; Doan, C.T.; Kuo, Y.H.; Nguyen, V.B. Bioprocessing of marine chitinous wastes for the production of bioactive prodigiosin. Molecules 2021, 26, 3138. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, D.N.; Nguyen, A.D.; Ngo, V.A.; Ton, T.Q.; Doan, C.T.; Pham, T.P.; Tran, T.P.H.; Wang, S.L. Utilization of crab waste for cost-effective bioproduction of prodigiosin. Mar. Drugs 2020, 18, 523. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, D.N.; Wang, S.L. Microbial reclamation of chitin and protein-containing marine by-products for the production of prodigiosin and the evaluation of its bioactivities. Polymers 2020, 12, 1328. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Chen, S.P.; Nguyen, T.H.; Nguyen, M.T.; Tran, T.T.T.; Doan, C.T.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Novel efficient bioprocessing of marine chitins into active anticancer prodigiosin. Mar. Drugs 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, L.T.; Techato, K.; Nguyen, V.B.; Wang, S.L.; Nguyen, A.D.; Phan, T.Q.; Doan, M.D.; Phoungthong, K. Utilization of cassava wastewater for low-cost production of prodigiosin via Serratia marcescens TNU01 fermentation and its novel potent α-glucosidase inhibitory effect. Molecules 2021, 26, 6270. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Wang, S.L.; Doan, M.D.; Nguyen, T.H.; Tran, T.H.T.; Tran, T.N.; Doan, C.T.; Ngo, V.A.; Ho, N.D.; Do, V.C.; et al. Utilization of by-product of groundnut oil processing for production of prodigiosin by microbial fermentation and its novel potent anti-nematodes effect. Agronomy 2022, 12, 41. [Google Scholar] [CrossRef]
- Nikolaus, G.; Wulf, B.; Rolf, B. Recent developments in the isolation, biological function, biosynthesis, and synthesis of phenazine natural products. Bioorg. Med. Chem. 2017, 25, 6149–6166. [Google Scholar]
- Alka, R.; Wamik, A. An overview on biosynthesis and applications of extracellular pyocyanin pigment and its role in Pseudomonas aeruginosa pathogenesis. Ann. Phytomed. 2019, 8, 28–42. [Google Scholar]
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behaviour of bacteria in the environment and biotechnological process. Appl. Microbiol. Biotechnolol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.T.; Ye, F.C.; Pang, C.P.; Yong, T.Q.; Tang, W.D.; Xiao, J.; Shang, C.H.; Lu, Z.J. Isolation and identifcation of bioactive substance 1-hydroxyphenazine from Pseudomonas aeruginosa and its antimicrobial activity. Lett. Appl. Microbiol. 2020, 71, 303–310. [Google Scholar] [CrossRef]
- Gruber, J.D.; Wei, C.; Stuart, P.; Kevin, B.; Peter, M.; Patrick, A.F.; Zhang, Y.M. The role of 2,4-dihydroxyquinoline (DHQ) in Pseudomonas aeruginosa pathogenicity. PeerJ 2016, 4, e1495. [Google Scholar] [CrossRef] [Green Version]
- Barakat, K.M.; Mattar, M.Z.; Sabae, S.Z.; Darwesh, O.M.; Hassan, S.H. Production and characterization of bioactive pyocyanin pigment by marine Pseudomonas aeruginosa Osh1. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 933–943. [Google Scholar]
- Devnath, P.; Uddin, M.K.; Ahmed, F.; Hossain, M.T.; Manchur, M.A. Extraction, purification and characterization of pyocyanin produced by Pseudomonas aeruginosa and evaluation for its antimicrobial activity. Int. Res. J. Biol. Sci. 2017, 6, 1–7. [Google Scholar]
- Ozdal, M.; Gurkok, S.; Ozdal, O.G.; Kurbanoglu, E.B. Enhancement of pyocyanin production by Pseudomonas aeruginosa via the addition of n-hexane as an oxygen vector. Biocatal. Agric. Biotechnol. 2019, 22, 101365. [Google Scholar] [CrossRef]
- Ozdal, M. A new strategy for the efficient production of pyocyanin, a versatile pigment, in Pseudomonas aeruginosa OG1 via toluene addition. 3 Biotech. 2019, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- El-Fouly, M.Z.; Sharaf, A.M.; Shahin, A.A.M.; El-Bialy, H.A.; Omara, A.M.A. Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J. Radiat. Res. Appl. Sci. 2015, 8, 36–48. [Google Scholar] [CrossRef] [Green Version]
- DeBritto, S.; Gajbar, T.D.; Satapute, P.; Lalitha, S.; Ramachandra, Y.L.; Sudisha, J.; Shin-ichi, I. Isolation and characterization of nutrient dependent pyocyanin from Pseudomonas aeruginosa and its dye and agrochemical properties. Sci. Rep. 2020, 10, 1542. [Google Scholar] [CrossRef] [Green Version]
- Hüseyin, K.; Cennet, C.K. Pyocyanine production, twitching motility and hydrophobicity of different wastes on Pseudomonas aeruginosa. Pol. J. Environ. Stud. 2021, 30, 1–5. [Google Scholar]
- Bianca, T.M.O.; Patrik, S.Z.B.; Thiago, G.C.; Ian, P.G.A.; Ulrich, V. Craft beer waste as substrate for pyocyanin synthesis. IOSR-JPBS 2019, 14, 21–25. [Google Scholar]
- Nguyen, D.N.; Wang, S.L.; Nguyen, A.D.; Doan, M.D.; Tran, D.M.; Nguyen, T.H.; Ngo, V.A.; Doan, C.T.; Tran, T.N.; Do, V.C.; et al. Potential application of rhizobacteria isolated from the central highland of Vietnam as an effective biocontrol agent of robusta coffee nematodes and as a bio-fertilizer. Agronomy 2021, 11, 1887. [Google Scholar] [CrossRef]
- Wang, S.L.; Yeh, P.Y. Production of a surfactant and solvent-stable alkaliphilic protease by bioconversion of shrimp shell wastes fermented by Bacillus subtilis TKU007. Process. Biochem. 2006, 41, 1545–1552. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.-L.; Nguyen, A.D.; Doan, M.D.; Tran, T.N.; Doan, C.T.; Nguyen, V.B. Novel α-Amylase inhibitor hemi-pyocyanin produced by microbial conversion of chitinous discards. Mar. Drugs 2022, 20, 283. [Google Scholar] [CrossRef]
- Elbargisy, R.M. Optimization of nutritional and environmental conditions for pyocyanin production by urine isolates of Pseudomonas aeruginosa. Saudi J. Biol. Sci. 2021, 28, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.B.V.; Jesús, A.P.G.; Mayra, L.F.M.; Fabricio, E.A.; Luis, A.O.F.; Yolanda, R.V. Optimized production of a redox metabolite (pyocyanin) by Pseudomonas aeruginosa NEJ01R using a maize by-product. Microorganisms 2020, 8, 1559. [Google Scholar]
- Khan, Z.; Kim, S.G.; Jeon, Y.H.; Khan, H.U.; Son, S.H.; Kim, Y.H. A plant growth promoting rhizobacterium, Paenibacillus polymyxastrain GBR-1, suppresses root-knot nematode. Bioresour. Technol. 2008, 99, 3016–3302. [Google Scholar] [CrossRef] [PubMed]
- Onbasli, D.; Aslim, A. Determination of antimicrobial activity and production of some metabolites by Pseudomonas aeruginosa B1 and B2 in sugar beet molasses. Afr. J. Biotechnol. 2008, 7, 4614–4619. [Google Scholar]
- Onbasli, D.; Aslim, B.; Yuvalicelik, G. Investigation of metabolite productions and degradation of hazardous organic pollutants by Pseudomonas spp. J. Appl. Biol 2011, 5, 9–14. [Google Scholar]
- Li, S.; Mou, Q.; Feng, N.; Leung, P.H.M. A selective medium for pyocyanin-dependent fast electrochemical detection of Pseudomonas aeruginosa in environmental microbial samples. Int. J. Electrochem. Sci. 2018, 13, 3789–3798. [Google Scholar] [CrossRef]
- Hicham, D.; Rachid, D.; Saïd, N. Antimicrobial, antioxidant and hemolytic effects of Pyocyanin produced by Pseudomonas aeruginosa isolated from saline soil of Mina river, Algeria. Int. J. Biosci. 2016, 9, 134–143. [Google Scholar]
- Burton, M.O.; Eagles, B.A.; Campbell, J.R. The amino acid requirements for pyocyanin production. Can. J. Res. 1947, 25, 121–128. [Google Scholar] [CrossRef]
- Georgia, F.R.; Poe, C.F. Study of bacterial fluorescence in various media. The production of fluorescence in media made from peptones. J. Bacteriol. Res. 1932, 23, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Yupeng, W.; Hongchen, L.; Mo, X.; Wei, H. Biosynthesis and metabolic engineering of 1-hydroxyphenazine in Pseudomonas chlororaphis H18. Microb. Cell Factories 2021, 20, 235. [Google Scholar]
- Ling, H.; Xunzhi, Z.; Shixing, Z.; Zhenrui, C.; Kai, S.; Chi, Z.; Hua, S. Phthalic acid esters: Natural sources and biological. Toxins 2021, 13, 495. [Google Scholar]
- Wiratno, M.S.; Ankardiansyah, P.P.; Ahmed, I.A.Y. Biological control of root-knot nematode (Meloidogyne spp.) in pepper plants utilizing endophytic bacteria Pseudomonas sp. and Micrococcus sp. J. Pepper Ind. 2018, 9, 11–22. [Google Scholar]
- Thuy, T.T.T.; Chi, N.T.M.; Yen, N.T.; Anh, L.T.N.; Te, L.L.; De Waele, D. Fungi associated with black pepper plants in Quang Tri province (Vietnam), and interaction between Meloidogyne incognita and Fusarium solani. Int. J. Sci. Technol. Res. 2013, 46, 470–482. [Google Scholar]
- Singh, H.B.; Keswani, C.; Reddy, M.S.; Sansinenea, E.; Carlos, G.E. Biological control of nematodes by plant growth promoting rhizobacteria: Secondary metabolites involved and potential applications. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Harikesh, B.S., Chetan, K., Reddy, M.S., Estibaliz, S., Carlos, G.E., Eds.; Springer: Singapore, 2019; Chapter 13; pp. 253–264. [Google Scholar]
- Javed, N.; Gowen, S.R.; Inam, M.; Abdullah, K.; Shahina, F. Systemic and persistent effect of neem (Azadirachta indica) formulations against root-knot nematodes, Meloidogyne javanica and their storage life. Crop. Prot. 2007, 26, 911–916. [Google Scholar] [CrossRef]
- Bogner, C.W.; Kamdem, R.S.; Sichtermann, G.; Matthäus, C.; Hölscher, D.; Popp, J.; Proksch, P.; Grundler, F.M.; Schouten, A. Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microb. Biotechnol. 2017, 10, 175–188. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, X.; Chen, L.; Liu, W.; Chen, J.; Wang, S.; Zang, J.; Duan, Y.; Liu, X. Toxicity of a secondary metabolite produced by Simplicillium chinense Snef5 against the root-knot nematode Meloidogyne incognita. Acta. Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 550–555. [Google Scholar] [CrossRef]
- Puja, O.; Satinder, K.P. Effect of phenolic compounds on nematodes—A review. J. Nat. Appl. Sci. 2010, 2, 344–350. [Google Scholar]
- Sabrina, C.; Carolyn, A.B.; Ulrike, M. Functions of flavonoids in plant–nematode interactions. Plants 2018, 7, 85. [Google Scholar]
- Nguyen, T.L.T.; Jang, J.Y.; Kim, T.Y.; Yu, N.H.; Park, A.R.; Lee, S.; Bae, C.H.; Yeo, J.H.; Hur, J.S.; Park, H.W.; et al. Nematicidal activity of verrucarin A and roridin A isolated from Myrothecium verrucaria against Meloidogyne incognita. Pestic. Biochem. Phys. 2018, 148, 133–143. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jang, J.Y.; Jeon, S.J.; Lee, H.W.; Bae, C.H.; Yeo, J.H.; Lee, H.B.; Kim, I.S.; Park, H.W.; Kim, J.C. Nematicidal activity of kojic acid produced by Aspergillus oryzae against Meloidogyne incognita. J. Microbiol. Biotechnol. 2016, 26, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Linsong, W.; Yukun, Q.; Zhaoqian, F.; Kun, G.; Jiang, Z.; Ronge, X.; Song, L.; Pengcheng, L. Novel lead compound discovery from Aspergillus fumigatus 1T-2 against Meloidogyne incognita based on a chemical ecology study. J. Agric. Food Chem. 2022, 70, 4644–4657. [Google Scholar]
- Zeng, Q.; Huang, H.; Zhu, J.; Fang, Z.; Sun, Q.; Bao, S. A new nematicidal compound produced by Streptomyces albogriseolus HA10002. Antonie Leeuwenhoek 2013, 103, 1107–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brent, C.; Nawaporn, V.; Daniel, G.L.; Grace, J.Y.; Alan, S.; Frederick, M.A. Identification of Pseudomonas aeruginosa phenazines that kill Caenorhabditis elegans. PLoS Pathog. 2014, 9, e1003101. [Google Scholar]
- Zahraa, J.J.; Anaam, F.H.; Muthana, A.A.; Nuha, F.A.; Eman, S.A. Bioactivity of pyocyanin of Pseudomonas aeruginosa clinical isolates against a variety of human pathogenic bacteria and fungi species. Int. J. Antimicrob. Agents 2017, 7, 1–7. [Google Scholar]
- Sunish, K.R.; Ayyadurai, N.; Pandiaraja, P.; Reddy, A.V.; Venkateswarlu, Y.; Prakash, O.; Sakthivel, N. Characterization of anti-fungal metabolite produced by a new strain Pseudomonas aeruginosa PUPa3 that exhibits broad spectrum antifungal activity and biofertilizing traits. J. Appl. Microbiol. 2004, 98, 145–154. [Google Scholar]
- Simionato, A.S.; Navarro, M.O.; Jesus, M.; Barazetti, A.R.; Silva, C.S.; Simões, G.C.; Balbi-Peña, M.I.; Mello, J.C.; Panagio, L.A.; Almeida, R.S.; et al. The Effect of Phenazine-1-carboxylic acid on mycelial growth of Botrytis cinerea produced by Pseudomonas aeruginosa LV strain. Front. Microbiol. 2017, 8, 1102. [Google Scholar] [CrossRef]
- Makarand, R.R.; Prashant, D.S.; Bhushan, L.C.; Sudhir, B.C. Detection, isolation and identification of phenazine-1-carboxylic acid produced by biocontrol strains of Pseudomonas aeruginosa. J. Sci. Ind. Res. 2007, 66, 627–631. [Google Scholar]
- Jasim, B.; Anisha, C.; Rohini, S.; Kurian, J.M.; Jyothis, M.; Radhakrishnan, E.K. Phenazine carboxylic acid production and rhizome protective effect of endophytic Pseudomonas aeruginosa isolated from Zingiber officinale. World J. Microbiol. Biotechnol. 2014, 30, 1649–1654. [Google Scholar] [CrossRef]
- Lee, K.W.; Omar, D.; Cheng, G.L.E.; Nasehi, A.; Wong, M.Y. Characterization of phenazine and phenazine-1-carboxylic acid isolated from Pseudomonas aeruginosa UPMP3 and their antifungal activities against Ganoderma boninense. Pertanika J. Trop. Agric. Sci. 2018, 41, 1795–1809. [Google Scholar]
- Aunchalee, N.; Sukanya, A.; Chanokporn, P.; Paweena, P.; Saksit, C.; Chalerm, R. Synthesis, isolation of phenazine derivatives and their antimicrobial activities. Walailak J. Sci. Tech. 2009, 6, 79–91. [Google Scholar]
- Zhang, L.; Tian, X.; Shan, K.; Liu, G.; Zhang, C.; Sun, C. Antagonistic activity and mode of action of Phenazine-1-carboxylic acid, produced by marine bacterium Pseudomonas aeruginosa PA31x, against Vibrio anguillarum in vitro and in a Zebrafish in vivo model. Front. Microbiol. 2017, 8, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumaresan, K.; Subramanian, M.; Vaithiyanathan, S.; Sevagaperumal, N.; Gopal, C.; Fernando, W.G.D. Broad spectrum action of phenazine against active and dormant structures of fungal pathogens and root knot nematode. Arch. Phytopathol. Pflanzenschutz 2005, 38, 69–76. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).