Cytoprotective and Antigenotoxic Properties of Organic vs. Conventional Tomato Puree: Evidence in Zebrafish Model
Abstract
:1. Introduction
2. Materials and Methods
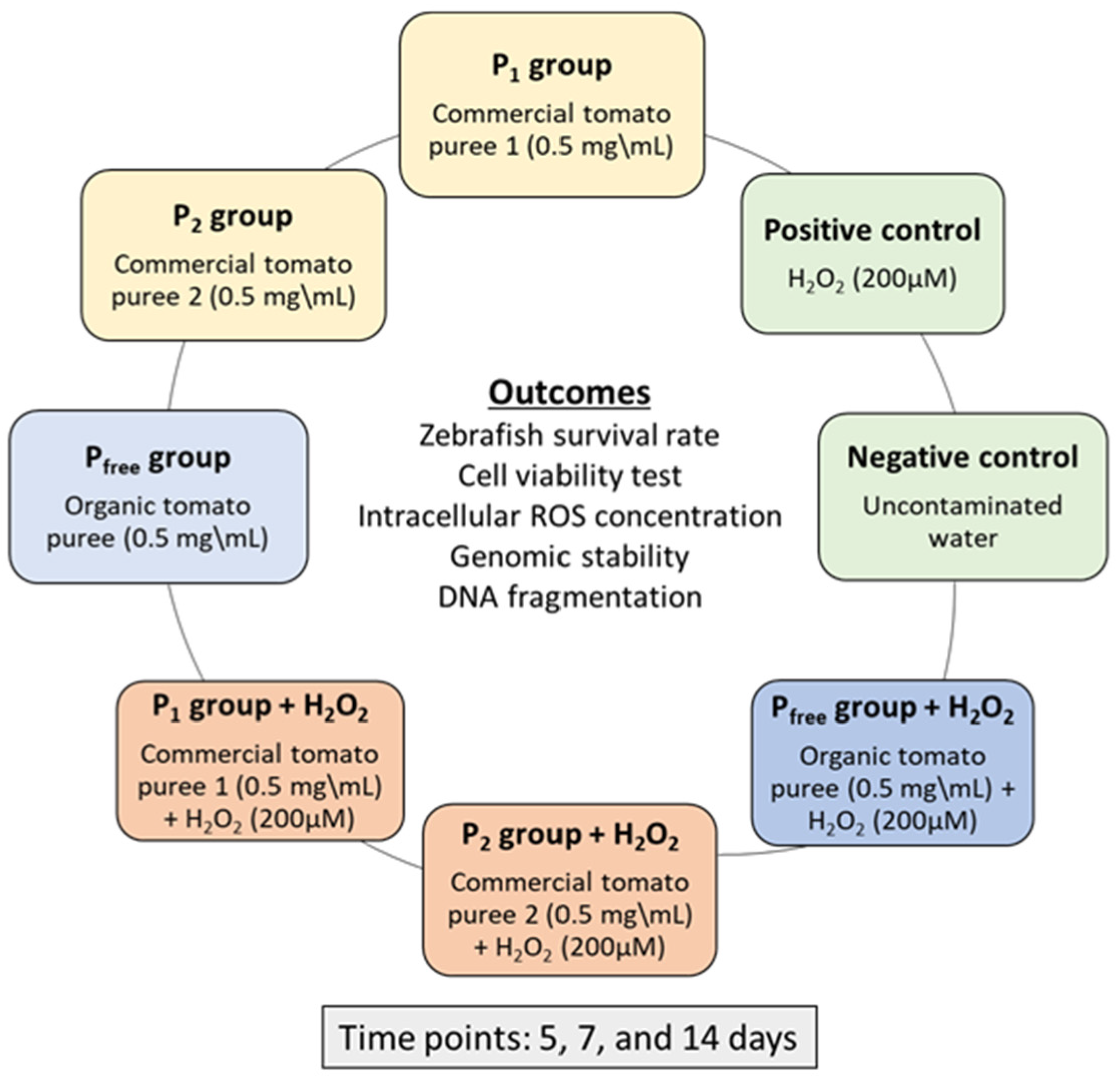
2.1. Study Design
2.2. Cell Viability Test
2.3. ROS Assay
2.4. DNA Extraction from Muscle Tissue
2.5. RAPD–PCR Protocol
2.6. TUNEL Assay
2.7. Statistical Analysis
3. Results
3.1. Zebrafish Survival
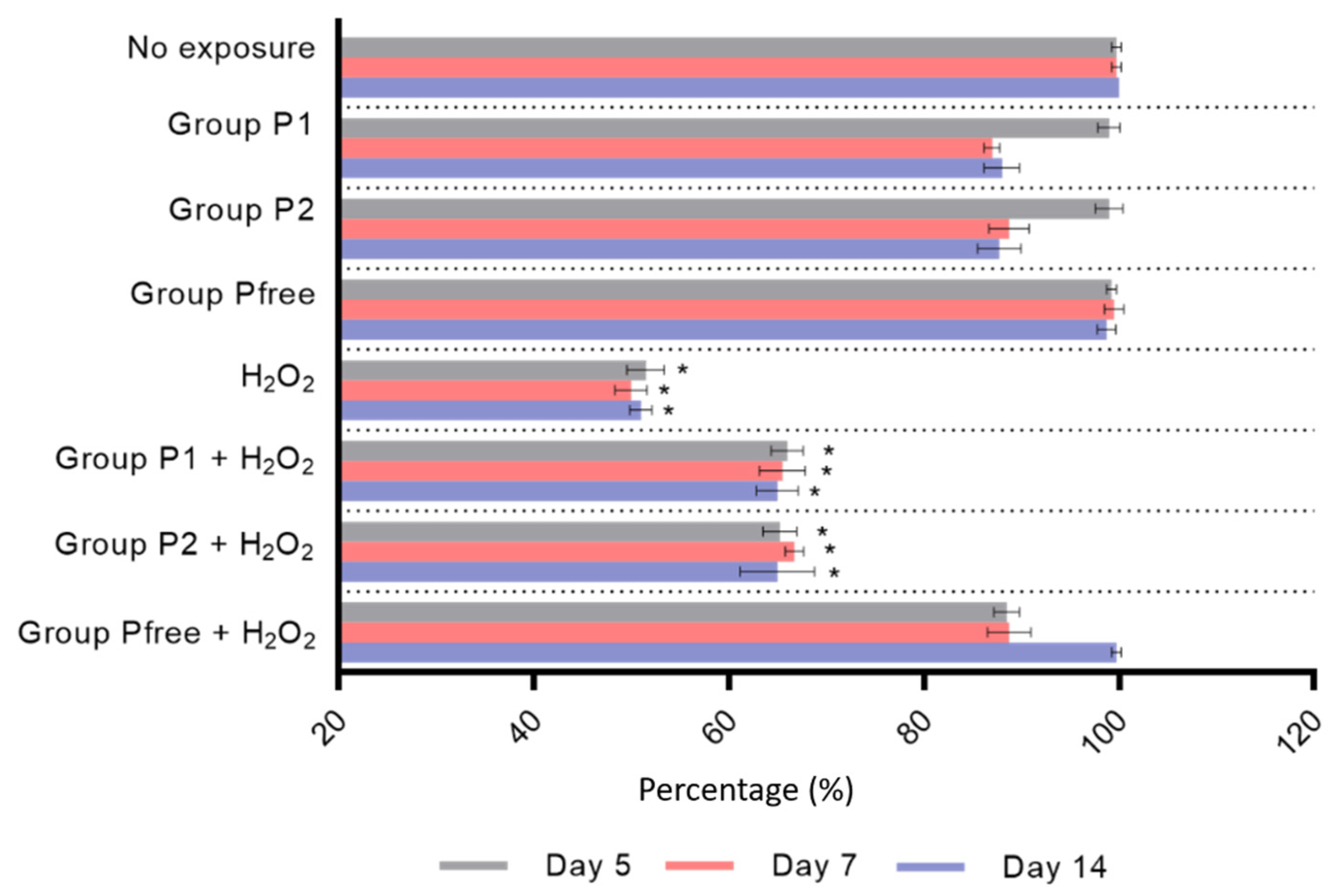
3.2. Cell Viability Test
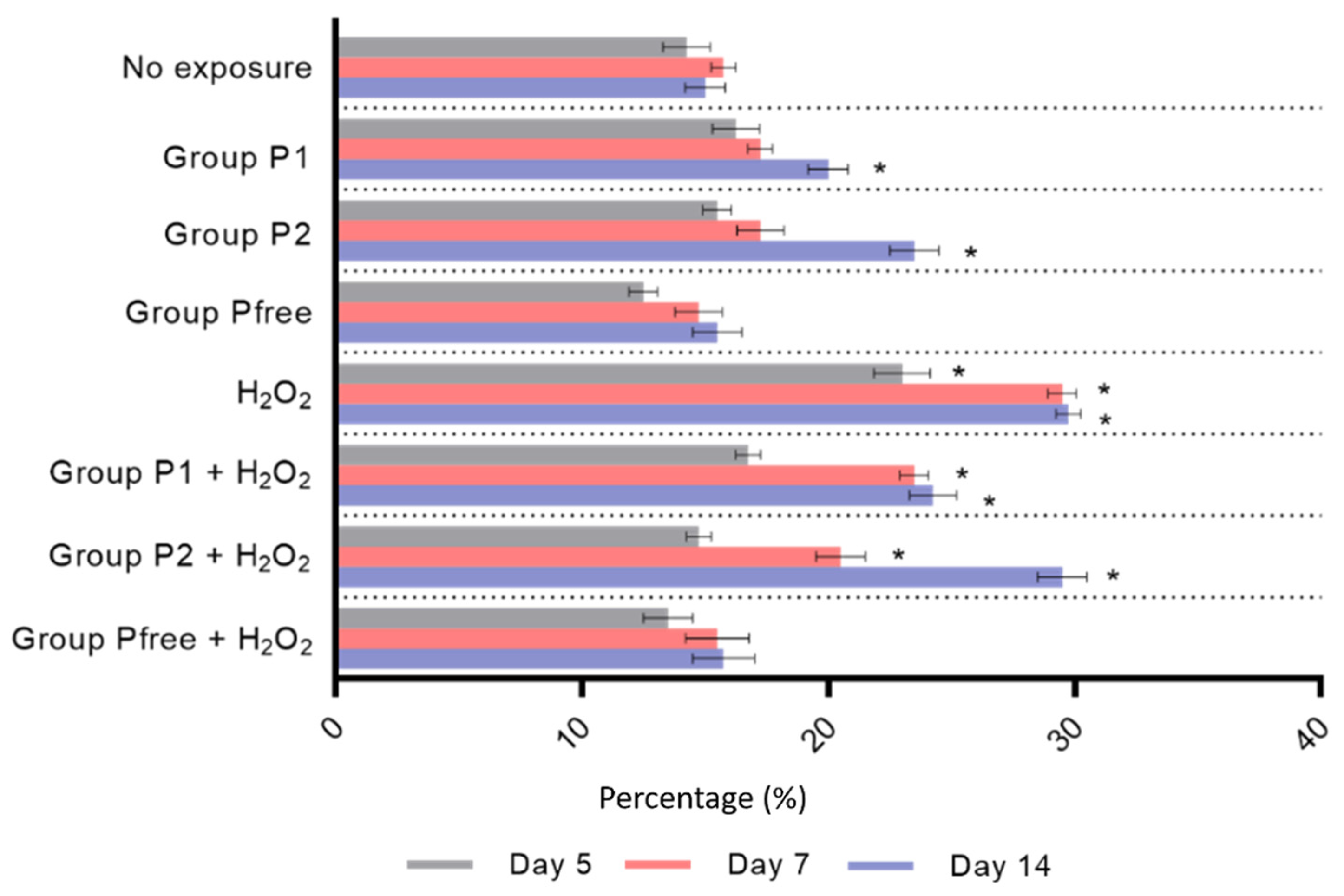
3.3. ROS Assay
3.4. RAPD–PCR Assay and GTS%
3.5. TUNEL Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carillo, P.; Cacace, D.; De Pascale, S.; Rapacciuolo, M.; Fuggi, A. Organic vs. traditional potato powder. Food Chem. 2012, 133, 1264–1273. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Occurrence of environmental pollutants in foodstuffs: A review of organic vs. conventional food. Food Chem. Toxicol. 2019, 125, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kapeleka, J.A.; Sauli, E.; Ndakidemi, P.A. Pesticide exposure and genotoxic effects as measured by DNA damage and human monitoring biomarkers. Int. J. Environ. Health Res. 2021, 31, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Leisegang, K.; Roychoudhury, S.; Slama, P.; Finelli, R. The mechanisms and management of age-related oxidative stress in male hypogonadism associated with non-communicable chronic disease. Antioxidants 2021, 10, 1834. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Analytical determination of antioxidants in tomato: Typical components of the Mediterranean diet. J. Sep. Sci. 2007, 30, 452–461. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Rouphael, Y.; Corrado, G.; Colla, G.; De Pascale, S.; Dell’aversana, E.; D’amelia, L.I.; Fusco, G.M.; Carillo, P. Biostimulation as a means for optimizing fruit phytochemical content and functional quality of tomato landraces of the San Marzano area. Foods 2021, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ren, B.; Guo, R.; Zhang, W.; Ma, S.; Yao, Y.; Yuan, T.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem. Toxicol. 2017, 109, 505–516. [Google Scholar] [CrossRef]
- Li, W.; Jiang, B.; Cao, X.; Xie, Y.; Huang, T. Protective effect of lycopene on fluoride-induced ameloblasts apoptosis and dental fluorosis through oxidative stress-mediated Caspase pathways. Chem. Biol. Interact. 2017, 261, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S.; Kadiroğlu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef]
- Knockaert, G.; Pulissery, S.K.; Colle, I.; Van Buggenhout, S.; Hendrickx, M.; Loey, A. Van Lycopene degradation, isomerization and in vitro bioaccessibility in high pressure homogenized tomato puree containing oil: Effect of additional thermal and high pressure processing. Food Chem. 2012, 135, 1290–1297. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Changes in phenolic profile and antioxidant activity during production of diced tomatoes. Food Chem. 2011, 126, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Hoet, P.; Vilain, F.; Lison, D. Occupational exposure to pesticides and Parkinson’s disease: A systematic review and meta-analysis of cohort studies. Environ. Int. 2012, 46, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Starling, A.P.; Umbach, D.M.; Kamel, F.; Long, S.; Sandler, D.P.; Hoppin, J.A. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup. Environ. Med. 2014, 71, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Dyck, R.; Karunanayake, C.; Pahwa, P.; Hagel, L.; Lawson, J.; Rennie, D.; Dosman, J. Prevalence, risk factors and co-morbidities of diabetes among adults in rural Saskatchewan: The influence of farm residence and agriculture-related exposures. BMC Public Health 2013, 13, 7. [Google Scholar] [CrossRef] [Green Version]
- Andersen, H.R.; Debes, F.; Wohlfahrt-Veje, C.; Murata, K.; Grandjean, P. Occupational pesticide exposure in early pregnancy associated with sex-specific neurobehavioral deficits in the children at school age. Neurotoxicol. Teratol. 2015, 47, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Toepel, K.; Irish, R.; Fenske, R.A.; Barr, D.B.; Bravo, R. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ. Health Perspect. 2006, 114, 260–263. [Google Scholar] [CrossRef]
- Oates, L.; Cohen, M.; Braun, L.; Schembri, A.; Taskova, R. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ. Res. 2014, 132, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Bradman, A.; Quirós-Alcalá, L.; Castorina, R.; Schall, R.A.; Camacho, J.; Holland, N.T.; Barr, D.B.; Eskenazi, B. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ. Health Perspect. 2015, 123, 1086–1093. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elhaleem, Z.A. Pesticide residues in tomato and tomato products marketed in Majmaah province, KSA, and their impact on human health. Environ. Sci. Pollut. Res. Int. 2020, 27, 8526–8534. [Google Scholar] [CrossRef]
- Williamson, S.; Ball, A.; Pretty, J. Trends in pesticide use and drivers for safer pest management in four African countries. Crop Prot. 2008, 27, 1327–1334. [Google Scholar] [CrossRef]
- EL-Saeid, M.H.; AL-Dosari, S.A. Monitoring of pesticide residues in Riyadh dates by SFE, MSE, SFC, and GC techniques. Arab. J. Chem. 2010, 3, 179–186. [Google Scholar] [CrossRef] [Green Version]
- European Commission Commission Directive 2002/63/EC Establishing Community Methods of Sampling for the Official Control of Pesticide Residues in and on Products of Plant and Animal Origin and Repealing–Directive 79/700/EEC. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC034557/ (accessed on 30 November 2021).
- Food and Agriculture Organization of the United Nations and World Health Organization Codex Alimentarius–International Food Standards. Available online: https://www.fao.org/fao-who-codexalimentarius/en/?provide=committeeDetail&idList=29 (accessed on 30 November 2021).
- Rodrigues, A.A.Z.; De Queiroz, M.E.L.R.; De Oliveira, A.F.; Neves, A.A.; Heleno, F.F.; Zambolim, L.; Freitas, J.F.; Morais, E.H.C. Pesticide residue removal in classic domestic processing of tomato and its effects on product quality. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, G.; Zomer, P.; Atreya, K.; Mol, H.G.J.; Yang, X.; Geissen, V. Pesticide residues in Nepalese vegetables and potential health risks. Environ. Res. 2019, 172, 511–521. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Carrasco Cabrera, L.; Medina Pastor, P. The 2019 European Union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar]
- Duan, J.; Duan, J.; Zhang, Z.; Tong, T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int. J. Biochem. Cell Biol. 2005, 37, 1407–1420. [Google Scholar] [CrossRef]
- Dulull, N.K.; Dias, D.A.; Thrimawithana, T.R.; Kwa, F.A.A. L-Sulforaphane confers protection against oxidative stress in an in vitro model of age-related macular degeneration. Curr. Mol. Pharmacol. 2018, 11, 237–253. [Google Scholar] [CrossRef]
- Castino, R.; Fiorentino, I.; Cagnin, M.; Giovia, A.; Isidoro, C. Chelation of lysosomal iron protects dopaminergic SH-SY5Y neuroblastoma cells from hydrogen peroxide toxicity by precluding autophagy and Akt dephosphorylation. Toxicol. Sci. 2011, 123, 523–541. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.H.; Wu, J.H.; Ge, H.M.; Tan, R.X. Protective effect of hopeahainol A, a novel acetylcholinesterase inhibitor, on hydrogen peroxide-induced injury in PC12 cells. Environ. Toxicol. Pharmacol. 2009, 28, 30–36. [Google Scholar] [CrossRef]
- Schmidt, L.J.; Gaikowski, M.P.; Gingerich, W.H. Evironmental assessment for the use of hydrogen peroxide in aquaculture for treating external fungal and bacterial diseases of cultured fish and fish eggs. USGS 2006, 54603, 180. [Google Scholar]
- Castranova, D.; Lawton, A.; Lawrence, C.; Baumann, D.; Best, J.; Coscolla, J.; Doherty, A.; Ramos, J.; Hakkesteeg, J.; Wang, C.; et al. The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 2011, 8, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, 18432654. [Google Scholar] [CrossRef]
- Mottola, F.; Finelli, R.; Iovine, C.; Carannante, M.; Santonastaso, M.; Rocco, L. Anti-genotoxicity evaluation of ellagic acid and curcumin—An in vitro study on zebrafish blood cells. Appl. Sci. 2021, 11, 8142. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Colacurci, N.; Iovine, C.; Pacifico, S.; Cammarota, M.; Cesaroni, F.; Rocco, L. In vitro genotoxic effects of titanium dioxide nanoparticles (n-TiO2) in human sperm cells. Mol. Reprod. Dev. 2019, 86, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Mottola, F.; Santonastaso, M.; Iovine, C.; Feola, V.; Pacifico, S.; Rocco, L. Adsorption of Cd to TiO2-NPs forms low genotoxic aggregates in Zebrafish cells. Cells 2021, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.J.; Li, H.; Wu, D.T.; Zhuang, Q.G.; Li, H.B.; Geng, F.; Gan, R.Y. Recent development in zebrafish model for bioactivity and safety evaluation of natural products. Crit. Rev. Food Sci. Nutr. 2021, 1–29. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Breinholt, V.; Lauridsen, S.T.; Daneshvar, B.; Jakobsen, J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000, 154, 201–210. [Google Scholar] [CrossRef]
- Palozza, P.; Colangelo, M.; Simone, R.; Catalano, A.; Boninsegna, A.; Lanza, P.; Monego, G.; Ranelletti, F.O. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, A.E.; Hong, Y.J.; Koh, E.; Barrett, D.M.; Bryant, D.E.; Denison, R.F.; Kaffka, S. Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J. Agric. Food Chem. 2007, 55, 6154–6159. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remón, A.; Lamuela-Raventós, R.M. Evaluation of a method to characterize the phenolic profile of organic and conventional tomatoes. J. Agric. Food Chem. 2012, 60, 3373–3380. [Google Scholar] [CrossRef] [PubMed]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, R.M.; Gustafson, L.; Hewitt, S.; Kilian, B.; Crabb, J.; Hendrickson, C.; Jiwan, D.; Andrews, P.; Dhingra, A. Concomitant phytonutrient and transcriptome analysis of mature fruit and leaf tissues of tomato (Solanum lycopersicum L. Cv. Oregon Spring) grown using organic and conventional fertilizer. PLoS ONE 2020, 15, e0227429. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Elgueta, S.; Valenzuela, M.; Fuentes, M.; Meza, P.; Manzur, J.; Liu, S.; Zhao, G.; Correa, A. Pesticide residues and halth risk assessment in tomatoes and lettuces from farms of metropolitan region Chile. Molecules 2020, 25, 355. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Qian, Y.; Chen, Q.; Tao, C.; Li, C.; Li, Y. Evaluation of pesticide residues in fruits and vegetables from Xiamen, China. Food Control 2011, 22, 1114–1120. [Google Scholar] [CrossRef]
- Bakirci, G.T.; Yaman Acay, D.B.; Bakirci, F.; Ötleş, S. Pesticide residues in fruits and vegetables from the Aegean region, Turkey. Food Chem. 2014, 160, 379–392. [Google Scholar] [CrossRef]
- Lozowicka, B.; Abzeitova, E.; Sagitov, A.; Kaczynski, P.; Toleubayev, K.; Li, A. Studies of pesticide residues in tomatoes and cucumbers from Kazakhstan and the associated health risks. Environ. Monit. Assess. 2015, 187, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasem Mahmoud, E.; Mohamed Ghoneim, A. Effect of polluted water on soil and plant contamination by heavy metals in El-Mahla El-Kobra, Egypt. Solid Earth 2016, 7, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Rashtian, J.; Chavkin, D.E.; Merhi, Z. Water and soil pollution as determinant of water and food quality/contamination and its impact on female fertility. Reprod. Biol. Endocrinol. 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.; Huang, X.; Huang, J.; Li, Y.; Zhang, C.; Yin, Y.; Chen, Z.; Jin, Y.; Cai, J.; Cui, F. Long- and short-term health effects of pesticide exposure: A cohort study from China. PLoS ONE 2015, 10, e0128766. [Google Scholar] [CrossRef]
- Naughton, S.X.; Terry, A.V. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018, 408, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Mineau, P. A review and analysis of study endpoints relevant to the assessment of “long term” pesticide toxicity in avian and mammalian wildlife. Ecotoxicology 2005, 14, 775–799. [Google Scholar] [CrossRef]



| Exposure | Day 5 * | Day 7 * | Day 14 * |
|---|---|---|---|
| Group P1 | / | +600 | +600 |
| Group P2 | / | +600 | +600 |
| Group Pfree | / | / | / |
| Group P1 + H2O2 | +600, 620 −200 | +850 −200, 310 | +350, 650, 850 |
| Group P2 + H2O2 | +600, 620 −200 | +850 −200, 310 | +350, 650, 850 |
| Group Pfree + H2O2 | / | +650 | +850 |
| H2O2 | +250, 350, 600 −310 | +250, 350, 600 −310 | +350, 650 −250, 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottola, F.; Finelli, R.; Santonastaso, M.; Carillo, P.; Rocco, L. Cytoprotective and Antigenotoxic Properties of Organic vs. Conventional Tomato Puree: Evidence in Zebrafish Model. Fishes 2022, 7, 103. https://doi.org/10.3390/fishes7030103
Mottola F, Finelli R, Santonastaso M, Carillo P, Rocco L. Cytoprotective and Antigenotoxic Properties of Organic vs. Conventional Tomato Puree: Evidence in Zebrafish Model. Fishes. 2022; 7(3):103. https://doi.org/10.3390/fishes7030103
Chicago/Turabian StyleMottola, Filomena, Renata Finelli, Marianna Santonastaso, Petronia Carillo, and Lucia Rocco. 2022. "Cytoprotective and Antigenotoxic Properties of Organic vs. Conventional Tomato Puree: Evidence in Zebrafish Model" Fishes 7, no. 3: 103. https://doi.org/10.3390/fishes7030103
APA StyleMottola, F., Finelli, R., Santonastaso, M., Carillo, P., & Rocco, L. (2022). Cytoprotective and Antigenotoxic Properties of Organic vs. Conventional Tomato Puree: Evidence in Zebrafish Model. Fishes, 7(3), 103. https://doi.org/10.3390/fishes7030103









