Consumption of Non-Native Bigheaded Carps by Native Blue Catfish in an Impounded Bay of the Upper Mississippi River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blue Catfish Collection
2.2. Diet Collection
2.3. Diet Analysis
2.4. Genetic Analysis
2.5. Aging of Hard Structures
2.6. Data Analysis
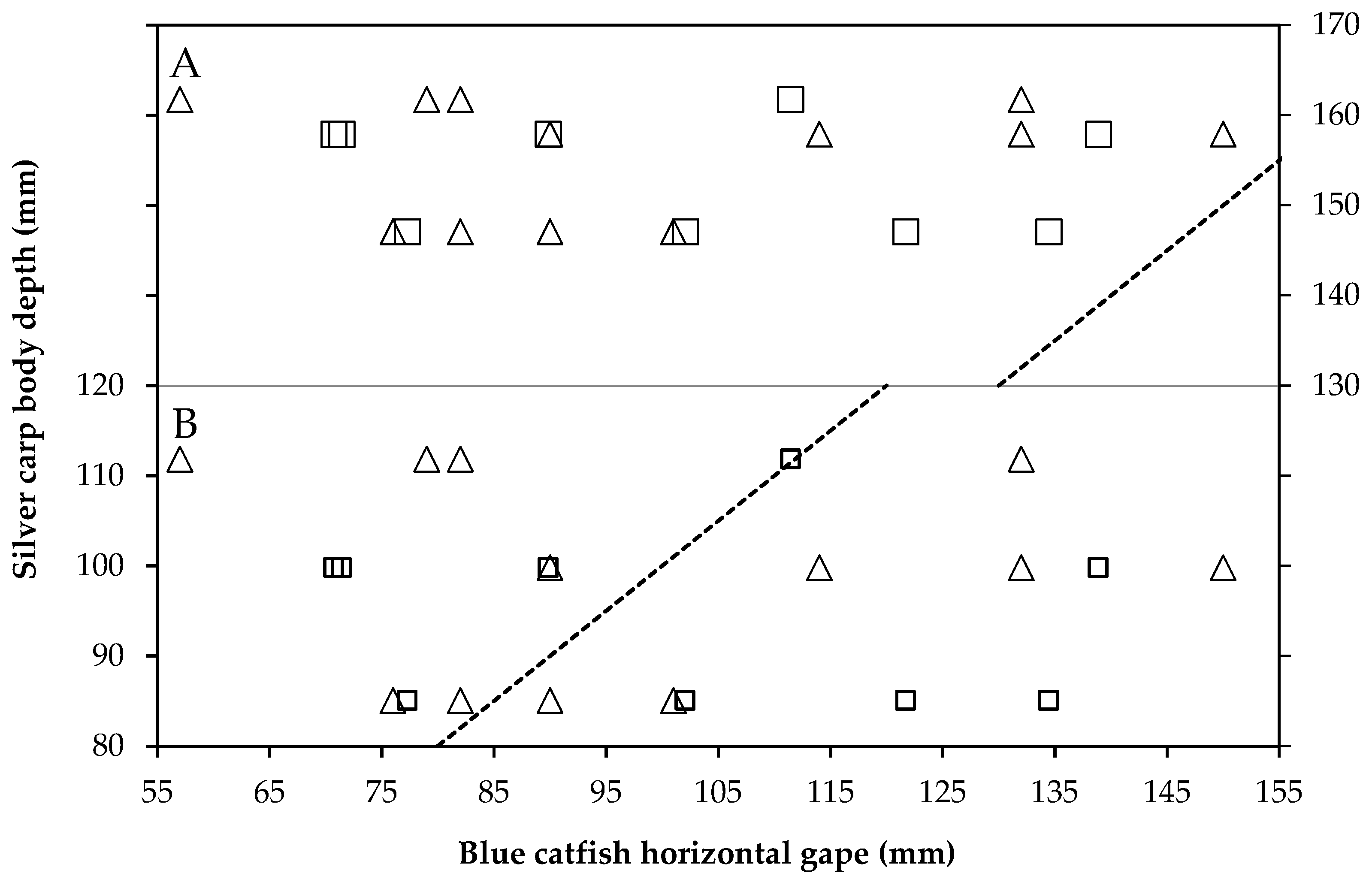
2.7. Size of Prey and Gape Limitation
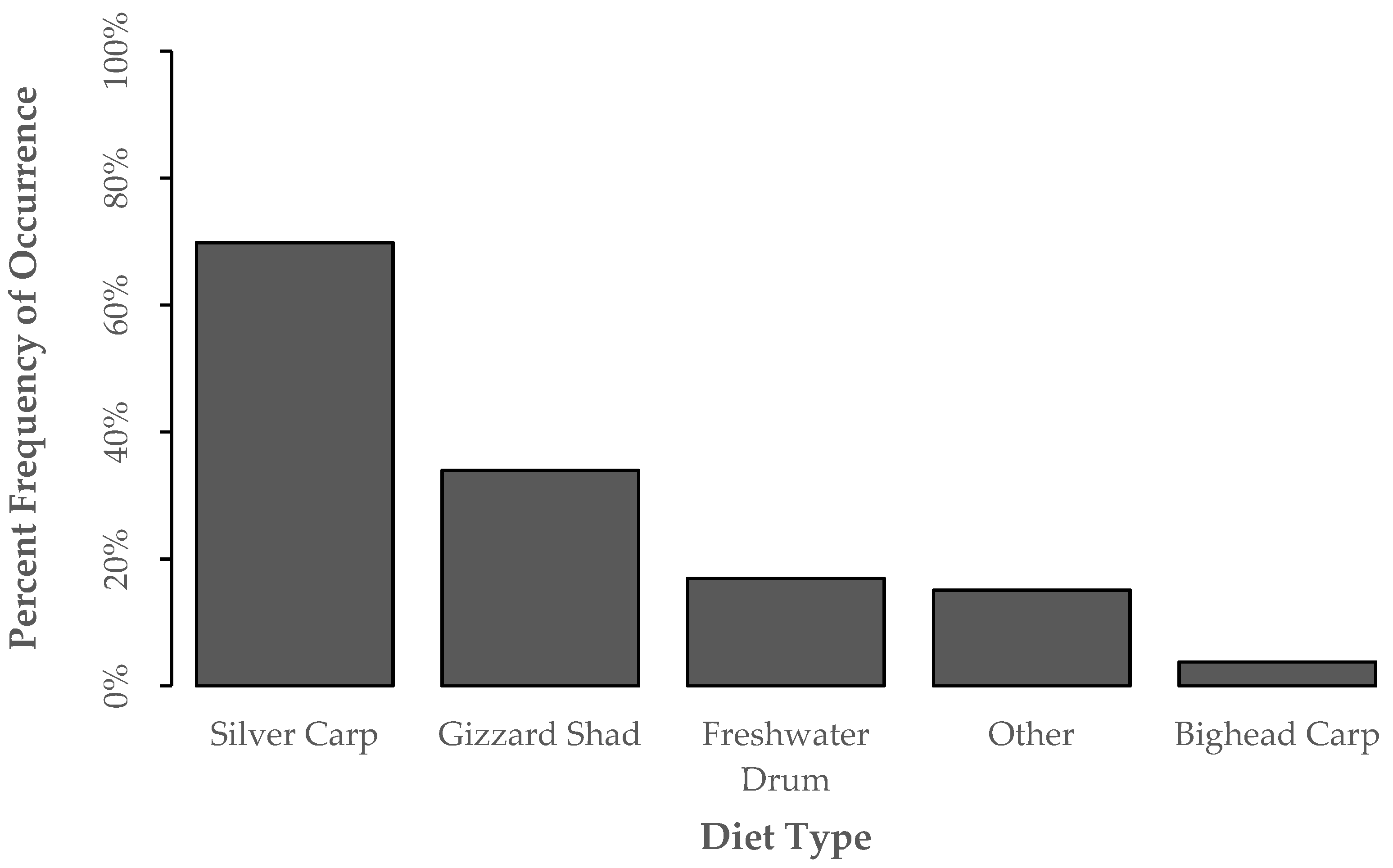
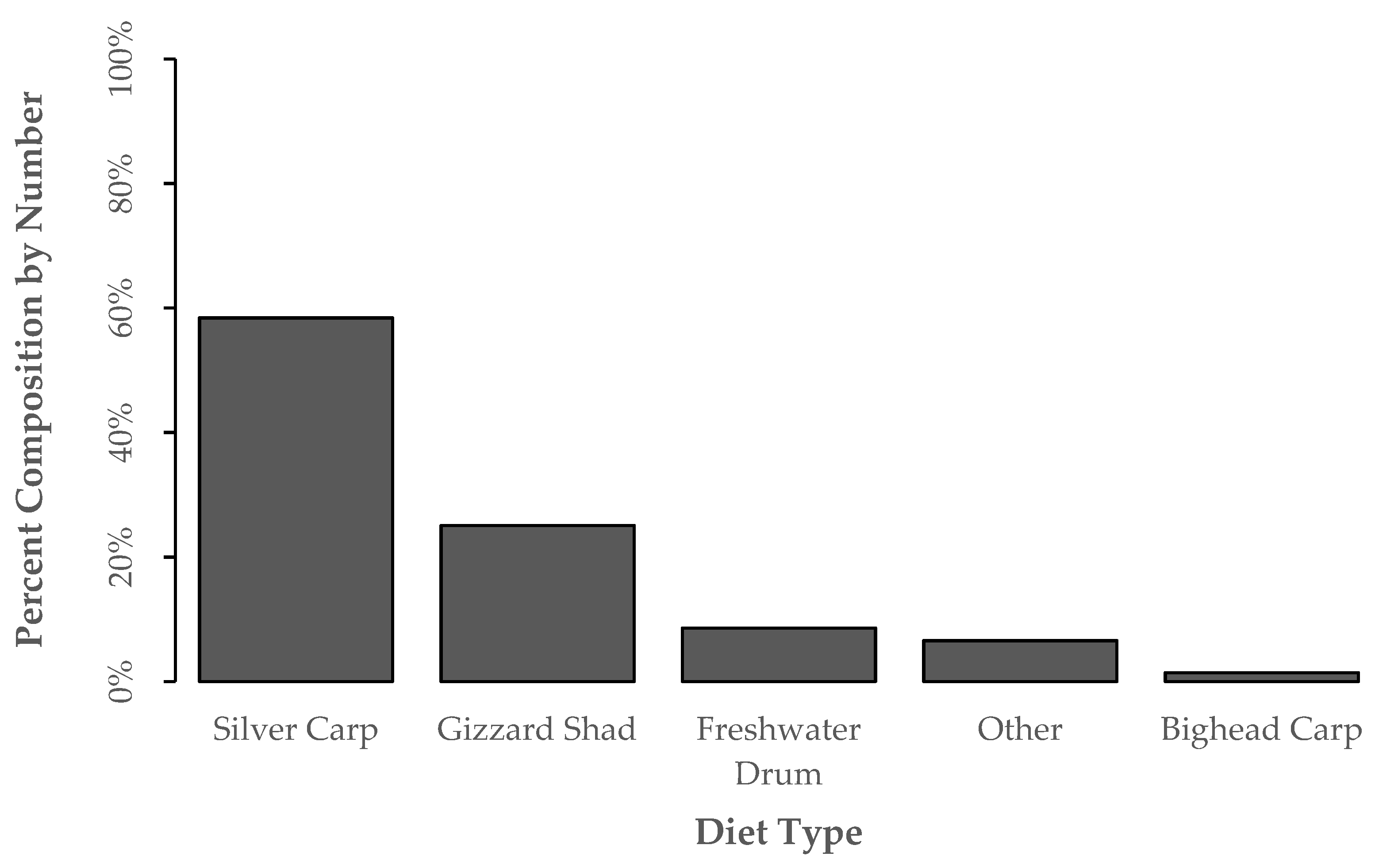
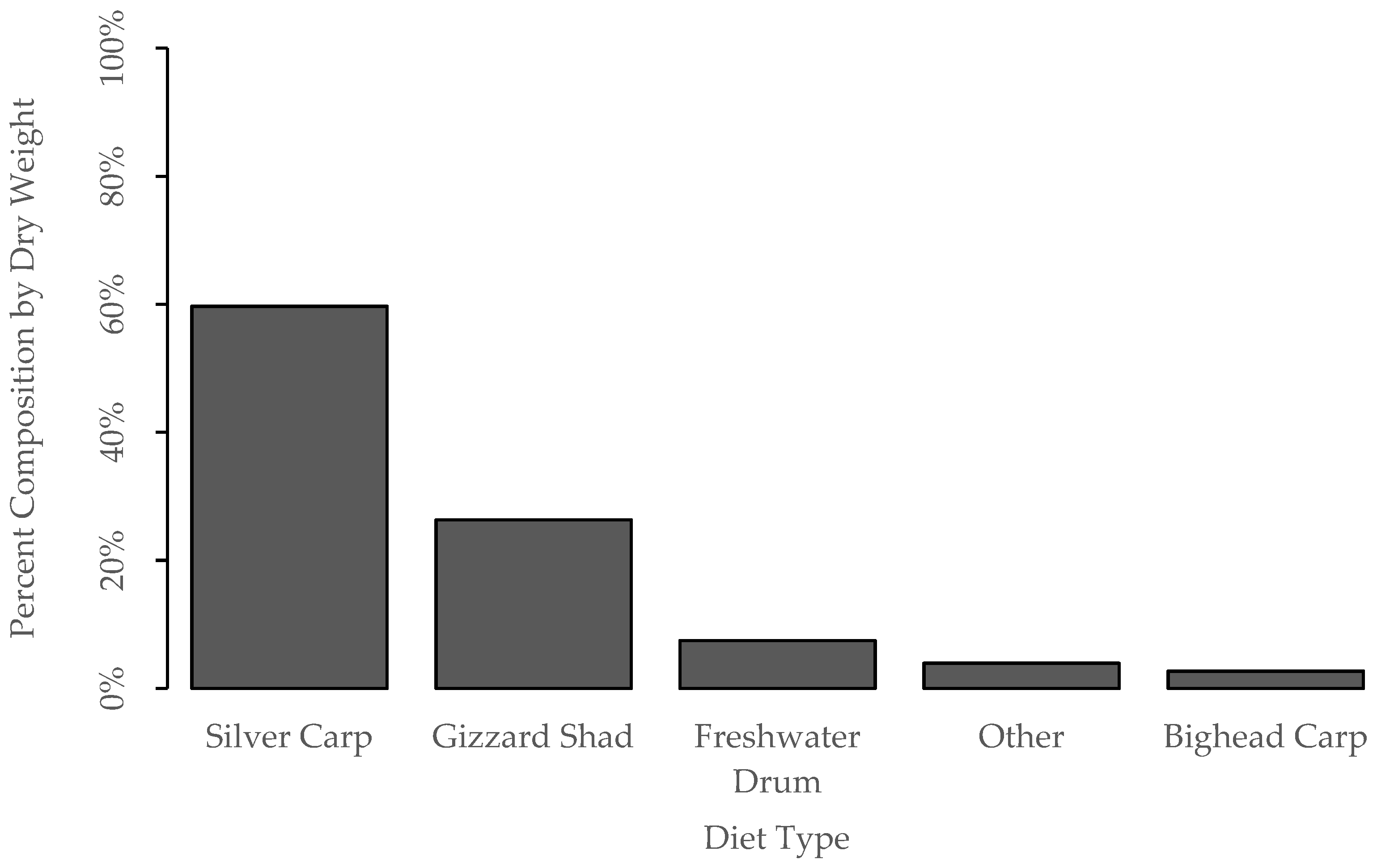
3. Results
3.1. Blue Catfish Collections and Diet Identifications
3.2. Diet Proportion, Age, and Gape Limitation Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magoulick, D.D.; Lewis, L.C. Predation on exotic zebra mussels by native fishes: Effects on predator and prey. Freshw. Biol. 2002, 47, 1908–1918. [Google Scholar] [CrossRef]
- Steinhart, G.B.; Stein, R.A.; Marschall, E.A. High growth rate of young-of- the-year smallmouth bass in Lake Erie: A result of the round goby invasion? J. Great Lakes Res. 2004, 30, 381–389. [Google Scholar] [CrossRef]
- Anderson, C.A.; Anderson, R.L.; Wang, J.; Gillespie, N.; Lampo, E.G.; McClelland, N.N.; Solomon, L.E.; Pendleton, R.; Lamer, J.T. Juvenile silver carp as forage for predatory fish in the LaGrange reach of the Illiois River. N. Am. J. Fish. Manag. 2022; Early online. [Google Scholar] [CrossRef]
- Kolar, C.S.; Chapman, D.C.; Courtenay, W.R., Jr.; Housel, C.M.; Williams, J.D.; Jennings, D.P. Asian carps of the genus Hypophthalmichthys (Pisces, Cyprinidae)—A biological synopsis and environmental risk assessment. Natl. Invasive Species Counc. Mater. 2005. Available online: https://digitalcommons.unl.edu/natlinvasive/5 (accessed on 4 November 2021).
- Sass, G.G.; Cook, T.R.; Irons, K.S.; McClelland, M.A.; Michaels, N.M.; O’Hara, T.M.; Stroub, M.R. A mark-recapture population estimate for invasive silver carp (Hypophthalmichthys molitrix) in the La Grange Reach, Illinois River. Biol. Invasions 2010, 12, 433–436. [Google Scholar] [CrossRef]
- Sampson, S.J.; Chick, J.H.; Pegg, M.A. Diet overlap among two Asian carp and three native fishes in backwater lakes on the Illinois and Mississippi Rivers. Biol. Invasions 2009, 11, 483–496. [Google Scholar] [CrossRef]
- Irons, K.S.; Sass, G.G.; McClelland, M.A.; Stafford, J.D. Reduced condition factor of two native fish species coincident with invasion of non-native Asian carps in the Illinois River, U.S.A. Is this evidence for competition and reduced fitness? J. Fish. Biol. 2007, 71, 258–273. [Google Scholar] [CrossRef]
- Solomon, L.E.; Pendleton, R.M.; Chick, J.H.; Casper, A.F. Long-term changes in fish community structure in relation to the establishment of Asian carps in a large floodplain river. Biol. Invasions 2016, 18, 2883–2895. [Google Scholar] [CrossRef]
- Sanft, E.; Parkos, J.J.; Collins, S.F.; Porreca, A.P.; Wahl, D.H. Vulnerability of juvenile bighead and silver carps to predation by largemouth bass. Trans. Am. Fish. Soc. 2018, 147, 1207–1214. [Google Scholar] [CrossRef]
- Wolf, M.C.; Phelps, Q.E. Prey selectivity of common predators on silver carp (Hypophthalmichthys molitrix): Controlled laboratory experiments support field observations. Environ. Biol. Fish. 2017, 100, 1139–1143. [Google Scholar] [CrossRef]
- Graham, K. A review of the biology and management of blue catfish. In Catfish 2000: Proceedings of the International Ictalurid Symposium; American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 1999; Volume 24, pp. 37–49. [Google Scholar]
- Schmitt, J.D.; Peoples, B.K.; Castello, J.; Orth, D.J. Feeding ecology of generalist consumers: A case study of invasive blue catfish Ictalurus furcatus in Chesapeake Bay, Virginia, USA. Environ. Biol. Fish. 2019, 102, 443–465. [Google Scholar] [CrossRef]
- Schmitt, J.D.; Peoples, B.K.; Bunch, A.J.; Castello, L.; Orth, D.J. Modeling the predation dynamics of invasive blue catfish (Ictalurus furcatus) in Chesapeake Bay. Fish. Bull. 2019, 117, 277–290. [Google Scholar] [CrossRef]
- Moran, Z.; Orth, D.J.; Schmitt, J.D.; Hallerman, E.M.; Aguilar, R. Effectiveness of DNA barcoding for identifying piscine prey items in stomach contents of piscivorous catfishes. Environ. Biol. Fish. 2016, 99, 161–167. [Google Scholar] [CrossRef]
- Jolly, J.C.; Irwin, E.R. Food habits of catfishes in tailwater and reservoir habitats in a section of the Coosa River, Alabama. In Proceedings of the Annual Conference Southeastern Association of Fish and Wildlife Agencies; American Fisheries Society: Bethesda, MD, USA, 2003; Volume 57, pp. 124–140. [Google Scholar]
- Jolly, J.C.; Irwin, E.R. Catfish population characteristics in tailwater and reservoir habitats of the Coosa River, Alabama. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2011; Volume 77, pp. 155–166. [Google Scholar]
- Schlosser, R.W.; Fabrizio, M.C.; Latour, R.J.; Garman, G.C.; Greenlee, B. Ecological role of blue catfish in Chesapeake Bay communities and implications for management. In Conservation, Ecology, and Management of Catfish: 2nd International Catfish Symposium 2010; American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2011; Volume 77, pp. 369–382. [Google Scholar]
- Bonvechio, T.F.; Jennings, C.A.; Harrison, D.R. Diet and population metrics of the introduced blue catfish on the Altamaha River, Georgia. In Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies; American Fisheries Society: Bethesda, MD, USA, 2011; Volume 65, pp. 112–118. [Google Scholar]
- Edds, D.R.; Matthews, W.J.; Gelwick, F.P. Resource use by large catfishes in a reservoir: Is there evidence for interactive segregation and innate differences? J. Fish Biol. 2002, 60, 739–750. [Google Scholar] [CrossRef]
- Eggleton, M.A.; Schramm, H.L., Jr. Feeding ecology and energetic relationships with habitat of blue catfish, Ictalurus furcatus, and flathead catfish, Pylodictis olivaris, in the lower Mississippi River, U.S.A. Environ. Biol. Fish. 2004, 70, 107–121. [Google Scholar] [CrossRef]
- Lamer, J.T.; Ruebush, B.C.; Arbieva, Z.H.; McClelland, M.A.; Epifanio, J.M.; Sass, G.G. Diagnostic SNPs reveal widespread introgressive hybridization between introduced bighead and silver carp in the Mississippi River Basin. Mol. Ecol. 2015, 24, 3931–3943. [Google Scholar] [CrossRef]
- Butler, S.E.; Porreca, A.P.; Collins, S.F.; Freedman, J.A.; Parkos, J.J., III; Diana, M.J.; Wahl, D.H. Does fish herding enhance catch rates and detection of invasive bigheaded carp? Biol. Invasions 2019, 21, 775–785. [Google Scholar] [CrossRef]
- Waters, D.S.; Kwak, T.J.; Arnott, J.B.; Pine, W.E., III. Evaluation of stomach tubes and gastric lavage for sampling diets from blue catfish and flathead catfish. N. Am. J. Fish. Manag. 2004, 24, 258–261. [Google Scholar] [CrossRef] [Green Version]
- Bowen, S.H. Quantitative description of diet. In Fisheries Techniques, 2nd ed.; Murphy, B.R., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 513–532. [Google Scholar]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D.N. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Schmitt, T.R.; Bielawski, J.P.; Gold, J.R. Molecular phylogenetics and evolution of the cytochrome b gene in the cyprinid genus Lythurus (Actinopterygii: Cypriniformes). Copeia 1998, 1998, 14–22. [Google Scholar] [CrossRef]
- De Barba, M.; Miquel, C.; Boyer, F.; Mercier, C.; Rioux, D.; Coissac, E.; Taberlet, P. DNA metabarcoding multiplexing and validation of data accuracy for diet assessment: Application to omnivorous diet. Mol. Ecol. Resour. 2014, 14, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Sarri, C.; Stamatis, C.; Sarafidou, T.; Galara, I.; Godosopoulos, V.; Kolovos, M.; Liakou, C.; Tastsoglou, S.; Mamuris, Z. A new set of 16S rRNA universal primers for identification of animal species. Food Control 2014, 43, 35–41. [Google Scholar] [CrossRef]
- Leray, M.; Yang, J.Y.; Meyer, C.P.; Mills, S.C.; Agudelo, N.; Ranwez, V.; Boehm, J.T.; Machida, R.J. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: Application for characterizing coral reef fish gut contents. Front. Zool. 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuevo, M.; Sheehan, R.J.; Heidinger, R.C. Accuracy and precision of age determination techniques for Mississippi River bighead carp Hypophthalmichthys nobilis (Richardson 1845) using pectoral spines and scales. Arch. Hydrobiol. 2004, 160, 45–56. [Google Scholar] [CrossRef]
- Williamson, C.J.; Garvey, J.E. Growth, fecundity, and diets of newly established silver carp in the middle Mississippi River. Trans. Am. Fish. Soc. 2005, 134, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Schrank, S.J.; Guy, C.S. Age, growth, and gonadal characteristics of adult bighead carp, Hypophthalmichthys nobilis, in the lower Missouri River. Environ. Biol. Fish. 2002, 64, 443–450. [Google Scholar] [CrossRef]
- Nikolskii, G.V. Special Ichthyology; Soretskaya Nauka: Moscow, Russia, 1954; 538p. [Google Scholar]
- USGS (U.S. Geological Survey); Upper Midwest Environmental Sciences Center; UMRR (Upper Mississippi River Restoration); LTRM (Long Term Resource Monitoring). Graphical Fisheries Database Browser. USGS Upper Midwest Environmental Sciences Center, LaCrosse, WI. Available online: www.umesc.usgs.gov/data_library/fisheries/graphical/fish_front.html (accessed on 31 January 2022).
- Ickles, B.S.; Schlifer, B.; Hansen, D.; Bartels, A.; Sauer, J. Graphical Fish Database Browser for Synthesized Long Term Resource Monitoring Fisheries Data; Upper Midwest Environmental Sciences Center Project Status Report; Report 2003-01; USGS: Charleston, SC, USA, 2003.
- Anderson, K.R.; Chapman, D.C.; Wynne, T.T.; Masagounder, K.; Paukert, C.P. Suitability of Lake Erie for bigheaded carps based on bioenergetics models and remote sensing. J. Great Lakes Res. 2015, 41, 358–366. [Google Scholar] [CrossRef]
- Eggleton, M.A.; Schramm, H.L., Jr. Caloric densities of selected fish prey organisms in the lower Mississippi River. J. Freshw. Ecol. 2002, 17, 409–414. [Google Scholar] [CrossRef]
- Wanner, G.A.; Klumb, R.A. Length-weight relationships for three Asian carp species in the Missouri River. J. Freshw. Ecol. 2009, 24, 489–495. [Google Scholar] [CrossRef]
- Miller, R.R. Systematics and biology of the gizzard shad (Dorosoma cepedianum) and related fishes. Fish. Bull. Fish Wildl. Serv. 1960, 173, 371–392. [Google Scholar]
- Koel, T.M. Spatial variation in fish species richness of the Upper Mississippi River system. Trans. Am. Fish. Soc. 2004, 133, 984–1003. [Google Scholar] [CrossRef]
- Schramm, H.L., Jr.; Eggleton, M.A. Applicability of the flood-pulse concept in a temperate floodplain river ecosystem: Thermal and temporal components. River Res. Appl. 2006, 22, 543–553. [Google Scholar] [CrossRef]

| Visual ID | Genetics ID | N | N Aged |
|---|---|---|---|
| Silver carp | NA | 27 | 17 |
| Gizzard shad | NA | 12 | 0 |
| Freshwater drum | NA | 6 | 0 |
| Crawfish | NA | 3 | 0 |
| Bighead carp | NA | 1 | 1 |
| Coot | NA | 1 | 0 |
| Silver carp | Silver carp | 12 | 4 |
| Silver carp | Gizzard shad | 22 | 3 |
| Silver carp | Freshwater drum | 3 | 0 |
| Silver carp | Bigmouth buffalo | 2 | 0 |
| Silver carp | Bighead carp | 1 | 0 |
| Silver carp | Bluegill | 1 | 0 |
| Unknown | Bluegill | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locher, T.; Wang, J.; Holda, T.; Lamer, J. Consumption of Non-Native Bigheaded Carps by Native Blue Catfish in an Impounded Bay of the Upper Mississippi River. Fishes 2022, 7, 80. https://doi.org/10.3390/fishes7020080
Locher T, Wang J, Holda T, Lamer J. Consumption of Non-Native Bigheaded Carps by Native Blue Catfish in an Impounded Bay of the Upper Mississippi River. Fishes. 2022; 7(2):80. https://doi.org/10.3390/fishes7020080
Chicago/Turabian StyleLocher, Tad, Jun Wang, Toby Holda, and James Lamer. 2022. "Consumption of Non-Native Bigheaded Carps by Native Blue Catfish in an Impounded Bay of the Upper Mississippi River" Fishes 7, no. 2: 80. https://doi.org/10.3390/fishes7020080
APA StyleLocher, T., Wang, J., Holda, T., & Lamer, J. (2022). Consumption of Non-Native Bigheaded Carps by Native Blue Catfish in an Impounded Bay of the Upper Mississippi River. Fishes, 7(2), 80. https://doi.org/10.3390/fishes7020080






