Abstract
Social stress can affect the ability of fish to respond to various stressors, such as pathogens or environmental variations. In this paper, the effects of social stress on gilt-head bream (Sparus aurata) were investigated. To study the effects of physiological stress, we evaluated biochemical and cellular parameters, such as cortisol, glucose, lactate, osmolarity, and phagocytosis, 24 h after the establishment of social hierarchy in a group of three fish. Social hierarchy was determined and characterized by behavioral observation (aggressive acts and feeding order) of the specimens (dominant: “α”; subordinate: “β” and “γ”). After the establishment of social hierarchy, we observed that, overall, levels of plasma cortisol and other biochemical plasmatic stress markers (glucose and lactate) were higher in subordinate individuals than in dominant individuals. In addition, the modulation of phagocytic activity of the peritoneal exudate cells (PECs) demonstrated that social stress appeared to affect immune response. Finally, principal component analysis clearly separated the subordinate fish groups from the dominant groups, based on stress markers and the phagocytic activity of peritoneal exudate cells. This study contributes to current knowledge on gilt-head sea bream, helping to understand the link between social stress, behavior, and physiology of this species, relevant in the aquaculture sector, where fish are subjected to several kinds of stress.
1. Introduction
Currently, there is a growing interest in fish welfare, both in fisheries and aquaculture [1]. In the aquaculture framework, the environment available to reared fish is very different from the environment in which their wild counterparts live [2]. Good food quality is readily available, as fish are protected from natural predators and disease and do not have to compete for mates. However, the physical environment is much simpler, as fish are disturbed by rearing activity and often restricted at high densities to limited and crowded spaces, with the consequent risk of disease spread and increased social interaction, including with aggressive fish. Because good fish welfare is correlated with good overall production, the welfare of farmed fish is important for the market, as well as being a matter of increasing public concern [3].
One of the important aspects to consider when evaluating aquaculture is the behavioral profile and interaction between animals [4]. Indeed, the social environment of a species can be a considerable source of stress, impairing the welfare status of fish, and social relationships can impact both mental and physical health [5]. Dominant–subordinate relationships can affect physiological status and animal responsiveness [6]. Social stress can be considered the result of physical contact between animals (high-density and agonistic interaction) and psychological components, such as hierarchical instability and submission [7]. Social interactions reflect agonistic competition for access to limited resources [8]. Responses to social stress depend on the life history of the species, sex, and age [9]. Social interactions between conspecifics are, for some fish species, dynamic processes, where subordinates frequently try to become dominants and dominants try to maintain their status by using direct attack or displaying cues to others [10]. Dominant and subordinate can be distinguished by characteristic behavioral differences in activity, feeding, and aggression, with subordinates being less active and aggressive and consuming less food. In addition to behavioral differences, dominant and subordinate fish can also differ from each other in their stress response [11]. In teleost fish, stress response is divided into different levels: primary, secondary, and tertiary. The primary response is coordinated by the neuroendocrine axes, which, in teleost fish, comprise the hypothalamic–pituitary–interrenal (HPI) axis and sympathochromaffin tissues and leads to increased levels of adrenocorticotropic hormone, cortisol, and catecholamine in the blood [12]. The increase in plasma cortisol levels under stress conditions typically causes an increase in plasma glucose and lactate levels, while the increase in plasma glucose is initially generated by catecholamine-mediated glycogenolysis and, in later stages, cortisol-mediated gluconeogenesis [13]. The increase in lactate concentration, as muscle lactate, is formed during anaerobiosis and is released into the plasma [13]. In the literature, it is reported that when fish cope with stress, an increase in osmolarity levels is observed in the plasma [14]. Indeed, secondary stress responses include changes in the concentration of circulating ions (Na+, K+, Mg2+, Ca2+, and Cl−). Prolonged stress exposition could also affect the tertiary response, including behavior, immune response, growth, and fitness [6]. Unnecessary stress can be considered deleterious, and, indeed, it can be seen from an adaptive point of view, which temporarily allows fish to cope with environmental changes, safeguarding single specimens and populations [15]. Cortisol, the major stress hormone in fish, mediates several physiological processes, such as glucose metabolism, ionic and osmotic regulation, and immune response. It plays a pivotal role in stress response through its action on both aerobic and anaerobic metabolism, osmoregulation, carbohydrate metabolism, immunity, and appetite [13,16]. Corticosteroid hormones play a central role in behavioral and neuroendocrine control in vertebrate species [17]. On the contrary, chronic stress, which is associated with elevated plasma cortisol levels, can result in a compromised physiological state. High levels of cortisol are considered a causal factor in many of the deleterious effects of stress in reared fish, such as reduced immune competence, reduced growth, flesh quality, or impaired reproduction [18,19]. For this reason, the mechanisms involved in stress coping strategies in fish are receiving significantly more attention. Most of the responses in organisms are species specific; therefore, it is necessary to investigate responses in different reared species.
Physiological differences are widely demonstrated to be correlated, in part, to different social positions in fish, and the consequent variation in response to changes in social status is well documented [11,20]. In aquaculture, inadequate rearing conditions may cause an increase in blood plasma cortisol levels, which can affect both the behavior and physiology of farmed species. During social interaction, the HPI axis is activated, resulting in variation in blood cortisol levels [7]. Social status (i.e., dominant or subordinate) is inextricably linked to hormonal regulation, such as testosterone and cortisol [21]. Different studies correlate social status and/or aggressive behavior with hormones, such as testosterone and cortisol, in gilt-head sea bream (Sparus aurata) [22,23,24]. In social animals, interactions between conspecifics are often initiated to obtain a higher position in the hierarchical structure. Physiological consequences of social interaction have been observed in the subordinate fish of gregarious species. The defeat of socially subordinate fish is perceived as a strong stressor [11,25], and it could also affect the secondary and tertiary stress responses. For instance, it has been demonstrated that physiological changes in subordinate fish might affect appetite and reduce aggression [26]. Moreover, contact between conspecific fish does not promote habituation [27], and prolongated stress exposition, as mentioned below, could ultimately trigger the tertiary stress response, with severe consequences on animals and aquaculture services. Indeed, aggressivity has been linked with several issues in aquaculture, such as decreased feed intake, growth dispersion, chronic stress and disease vulnerability [28]. Therefore, understanding the aggressive behavior of farmed fish, as well social interaction, is of great importance for the aquaculture practice, in order to both improve animal welfare and productivity [29].
Sea bream is a protandric hermaphrodite species, born as males and becoming female at about 3 years old, living either solitarily or in small aggregations. Mainly carnivorous, sea bream is one of the most important fish of Mediterranean marine aquaculture [30]. The important development and wide spreading of seabream aquaculture is due to its features; the robustness, plasticity, diet adaptability and illness resistance renders it able to adapt to a wide range of environmental conditions. These characteristics, in addition to significant technological advances in reproduction, using artificial conditions, permitted the expansion of this industry [31]. In that species, the overall dominant fish carry out more aggressive acts and bite at food particles more often than the subordinate, resulting in higher relative specific growth rate. Goldan, and Popper [32] concluded that direct competition for food is probably the major social mechanism regulating growth in small groups of juveniles of this species, when food is limited and defendable. Recently, Arechavala-Lopez et al. [33] showed that effects of social hierarchy could be modulated by aquaculture management in sea bream. For instance, self-feeding reinforces the social hierarchy, which might lead to a higher competitiveness for resources among fishes, increasing the social hierarchy and, therefore, stress, when compared to hand feeding. Therefore, understanding the link between aggressive and feeding behaviours with the social hierarchy and stress physiology of sea bream is of great interest for the aquaculture sector, in order to find the best farming practices for the species [23].
In Cammarata et al. [34], we have previously demonstrated, using the paired fish model, that the established hierarchy between two specimens of gilt-head bream, determines a change of the principal biochemical (cortisol, glucose and osmolarity) and cellular (phagocytic activity) parameters in subordinate individuals, in a short period of time after pairing (at 24 h). The biochemical and cellular stress markers considered (e.g., cortisol, glucose, lactate) were higher in subordinate individuals than in dominant [34]. This has also been reported for cortisol in groups of two, five or ten sea breams by Montero et al. [35]. As reported by Vazzana et al., stressors affect the immuno-cells activity, such as the respiratory burst and cytotoxic activity of leucocytes in the head kidney and peritoneal cavity of fish [36]. Moreover, in vivo and in vitro experiments showed that increased cortisol levels act on phagocytic activity via the cytosolic receptor DlGR1 in sea bass [37]. Peritoneal exudates cells (PECs) play important roles in both non-specific and specific immune mechanisms [38,39], and the peritoneal exudate has been used as a source of leucocytes for studies of innate immunity, including phagocytosis and cytotoxicity [40,41]. In this study, introducing one extra specimen in the experimental design, with respect to the previous work, we increased the complexity of the interactions among the individuals of the gilt-head bream. The social ranks were determined using various parameters, such as aggression and feeding priority, with a novelty of a clear determination of sequential hierarchy, with dominant α, subordinate β and γ. After the establishment of the hierarchy, we examined the plasma levels of stress parameters (cortisol, glucose, lactate, osmolarity) and the phagocytic activity of the PECs to compare physiological status between dominant α and subordinates β and γ. Well established physiological and immunity bioindicators (plasma concentrations of cortisol, glucose, lactate, osmolality and major ions, and phagocytosis) were monitored in this study [42].
Overall, throughout the behavioural observation of sea bream, and analysis of relevant plasmatic biochemical indicators, this study aims to contribute to the improvement of knowledges on an important marine farmed species—the gilt-head seabream. This study would help to understand the link between social stress, behaviour and physiology of this species in a confined environment, such as in aquaculture environments, where fish are subjected to several kinds of stress.
2. Materials and Methods
2.1. Animals
Twenty-seven male specimens (125 ± 25 g body weight) of the seawater proterandrous teleost gilt-head seabream were obtained from a commercial fish farm (Ittica Trappeto, Sicily, Italy). Fish were selected to have the same weight, age and sexual maturity in order to reduce variability and obtain comparable results. The experimental design involved the observation of specimens of gilt-head bream in glass tanks (200 L) in which the fish were placed simultaneously in groups of three. The experiment was replicated nine times, each time three different specimens, resulting in the monitoring of 27 fish in total. The tanks were obscured on three sides to eliminate reflection or external disturbance on fish. Experiments were carried out after an acclimatising period of one week [34]. The animals were subdivided and placed in tanks in groups of three. The sampling and analysis were done for each group at 24 h after hierarchical establishment. The hierarchy had been clearly distinguished after one hour for each experiment. New fish were utilised for the replicate experiments in order to avoid influences in new hierarchical scheme. No fish died or showed signs of disease during the experiments.
2.2. Experimental Conditions and Behaviour Observation
The aquaria seawater was monitored daily and maintained at an average temperature of 18 ± 1 °C, at a salinity of 38 ± 1%, oxygen 6 ± 0.5 mg L−1 by Dissolved Oxygen Meter (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and total ammonia-nitrogen concentration of <0.2 mg L−1 by commercial kit (Tetra, Spectrum Brands, Blacksburg, VA, USA) under a photoperiod of 12 h dark and 12 h light. The fish were fed with commercial pellet diet (Skretting USA, Tooele, Utah) once a day ad libitum. To be able to identify the three individuals in an objective manner the fish were marked as follows: a fish was clipped at the dorsal fin, another was clipped on the caudal fin, and one was not clipped [43]. Fin clipping is a technique extensively used to recognize individuals within a group; it is commonly used in breeding work and animal tracking without affecting behaviour [44,45,46]. The behavioural changes were recorded to assign a hierarchical position to each individual and observed to detect changes in the social status until the social positions were established. Every group was observed for 24 h and recorded by a digital camera for the data acquisition (Panasonic sdr-h85 hybrid; Panasonic corporation, Kadoma, Japan) during light period. The choice of 24-h monitoring period following introduction of fish into experiment tank was chosen based on previous study on sea bream showing that the hierarchy establishment occurs after 24 h of cohabitation with a duration of at least 6 months [34]. The method used for behavioural monitoring was continuous sampling. For each specimen, 15 videos of 15-min each were randomly sampled during daylight, resulting in the monitoring of 225 min for each fish. During the monitoring period, all behavioural acts were recorded using the Behavioral Observation Research Interactive Software v. 7.12.2 (BORIS) [47,48,49]. The individual behaviour was examined by the continuous check of the different behaviour categories, and individuals from each group were distinguished as dominant α, subordinate β and subordinate γ. High social status has been correlated with increased aggressiveness and preferential access to food [48]. To define this social distinction, the number of aggressive acts (A+) were observed and defined as a bite or a rapid approach without biting that resulted in the displacement of the subordinate [50] classifiable as: charge, nip, chase, butting, mouth fighting and circling [51,52]. Feeding order (FO) of each group was determined according to the methods of McCarthy et al. [48] counting the number of accessions to feeding area for all the specimens. Data are expressed as percentage of total interactions among fish.
2.3. Blood Sampling and Peritoneal Cell Preparation
After 24 h of cohabitation, the fish were anaesthetised with 0.05% w/v MS222 (3-aminobenzoicacid ethyl ester, Merck & Co., Darmstadt, Germany) in seawater for three minutes. After the anaesthetic exposure, the blood samples were collected via caudal venepuncture into heparin-coated syringes (2500 IU mL−1 heparin sodium salt, Merck & Co., Darmstadt, Germany) and centrifuged (10,000× g for 2 min). Plasma was extracted and stored at −80 °C for later analysis of cortisol, glucose, lactate and osmolarity levels. The peritoneal exudates cells (PECs) were obtained as follows: the fish were anesthetized and after disinfection of the body ventral surface with 70% ethyl alcohol, the peritoneal cavity was injected with 15 mL of isotonic (370 mOsm kg−1) medium (Leibovitz L15 medium containing 2% foetal calf serum, 100 units penicillin mL−1, 100 units streptomycin mL−1 and 10 units heparin mL−1, Merck & Co., Darmstadt, Germany). During the procedure fish gills were wetted by continued water flow by a pipe inserted in mouth during the ten minutes. Ventral surface was disinfected again with 70% ethyl alcohol. After massaging the ventral surface for 10 min, the medium containing the PECs was collected by withdrawing with a syringe needle from the peritoneal cavity. The PECs were isolated by centrifugation at 400× g for 10 min at 4 °C. The dead cells were determined by light microscopy after addition of 0.01% trypan blue to the medium.
2.4. Plasmatic Biochemical Parameters
The concentrations of total cortisol were measured in the plasma sample using a commercially available kit (Intermedical Diagnostics srl, Villaricca, Italy) according to the manufacturer’s instructions and confirmed by radioimmunoassay (RIA) [53]. The glucose and lactate plasma levels were determined using the Accutrend Plus Kit (Roche Diagnostics, Rotkreuz, Switzerland) according to the manufacturer’s instructions. The osmolarity of the plasma samples was measured using a freezing-point depression osmometer type 4b (Hermann Roebling MESSTECHNIK, Berlin, Germany).
2.5. Phagocytosis Assay
The method established in Cammarata et al. [34] using Saccharomyces cerevisiae (Merck & Co., Darmstadt, Germany) as a target for evaluating the percentage of phagocytosis, was performed with slight modifications. Briefly, yeast was prepared in distilled water as a 0.25% (w/v) solution (approximately 1 × 107 yeast mL−1), autoclaved for 15 min, washed 2 times at 2000 g at 4 °C for 5 min and incubated for 1 h at 20 °C with eosin Y (4-Bromo-fluorescein) to a final concentration of 0.05%. The yeast was washed four times in distilled water and resuspended to a final concentration of 0.0125% w/v in phosphate buffered saline (PBS: 103.6 mM NaCl, 1.46 mM KH2PO4, 0.8 mM Na2HPO4, 2.6 mM KCl, 0.9 mM CaCl2 and 0.49 mM MgCl2, pH 7.4) and stored at −20 °C for a maximum of 2 weeks. The yeast suspension was added (v/v) to 100 µL of leucocyte suspension (2.5 × 106) and placed in a 1 mL plastic tube. The mixture was incubated for 30 min at 20 °C with gentle stirring. To observe the phagocytosis, 50 µL of a quenching solution (QS) (2 mg mL−1 trypan blue and 2 mg mL−1 crystal violet in 0.02 M citrate buffer, pH 4.4 containing 33 mg mL−1 NaCl) was added to the reaction mixture. The slides were examined under a microscope equipped with a Normarski interferential contrast device and fluorescence apparatus (450–490 nm filter) (Diaplan, Leica, Wetzlar, Germany). A total of 200 cells, 35 cells on each slide, were counted. The results were expressed as a percentage of cells containing yeasts.
2.6. Statistical Analyses
Statistical analyses were performed using the R software version 4.0.4 [54] and carried out at the 95% level of significance. Generalized linear mixed model (GLMM) was carried out using the library lme4 [55]. The principal component analysis (PCA) was performed using packages ade4 [56] and FactoMineR library [57]. The data are expressed as mean ± standard deviation unless otherwise mentioned. All the recorded plasmatic biochemical markers were analysed as function of social rank (fixed factor) and experimental tank (i.e., replicate; random factor) one by one using GLMM. When social status appeared significant, Tukey HSD post-hoc test was carried out to determine differences between groups (i.e., dominant α, subordinate β and subordinate γ). GLMM was fitted using gaussian distribution family and log link for all variables. Log cortisol data were analysed instead of raw cortisol data in GLM to better fit model assumptions. Visual inspection of the residuals revealed no violation of the statistical assumptions by the model. In addition, to examine the interrelation between the two sets of variables (stress plasmatic biochemical markers and phagocytic activity of the PEC) and social status, principal component analysis (PCA) was performed for multiple groups of principal component analysis including five variables (cortisol, glucose, lactate levels, osmolarity and phagocytosis). The relevant dimensions of the PCA were selected using the acceleration factor method [58]. Individual PCA scores of fish were downloaded for each relevant component of the PCA, and then analysed as function of fish social position using Kruskal–Wallis test followed by Tukey HSD when significant.
3. Results
3.1. Determination of Social Hierarchy
The social hierarchy was established after about an hour of cohabitation and interaction and remained unchanged throughout the 24-h period of observation. During the experimentation, we did not identify any fish behavioural differences or particular hierarchical positions attributable to the different clipped fins (Table 1). This is consistent with previous works, demonstrating that fin clipping does not induce negative effects for fish (e.g., [43,46,59]).

Table 1.
Hierarchical classification of the fish according to the mark position (dorsal fin, caudal fin, or no mark).
Social status and hierarchical position have been correlated with increased aggressiveness and preferential access to the food (Table 2). As shown in Table 2, the percentage of aggressive acts for each hour (A+) and preferential access to the food (FO) allowed us to distinguish the fish, identifying them as dominant α or β and γ subordinates in each group, attributing a social status in the hierarchy to each fish, with the order of: dominant α < subordinate β < subordinate γ (p < 0.001). The hierarchy was maintained for the total observation time.

Table 2.
Mean percentage (%; ±SD) of aggressive acts (A+) and preferential access at the food (FO) during experimental period in sea bream (Sparus aurata). Nine fish were monitored for each social status (dominant (α), subordinate (β and γ).
3.2. Stress Biochemical Profile Related to Social Position
After 24 h of cohabitation-interaction among the three sea breams, the cortisol levels in plasma appeared different, depending on the social rank acquired by fish (p < 0.001 for subordinate β and subordinate γ, respectively, vs. dominants; Figure 1B); the highest levels were measured in subordinate individuals, following the order: dominant α < subordinate β < subordinate γ (p < 0.001 for all). In a similar way, the lactate level in plasma is affected by the social position (p < 0.001 for subordinate β and subordinate γ, respectively, vs. dominants; Figure 1D); the highest levels were measured in subordinate individuals, following the order: dominant α < subordinate β < subordinate γ (p < 0.001 for all). The plasmatic glucose level is also affected by the social position gained, 24 h after introduction in the novel tank (p < 0.001 for subordinate β and subordinate γ, respectively, vs. dominants; Figure 1C). This time, the greater glucose plasmatic level is still observed in subordinate fish γ, but with a different order: subordinate β < dominant α < subordinate γ (p < 0.001 for all; Figure 1C). The pattern of osmolarity, depending on social position, has been found similar to that of plasmatic glucose level (p < 0.001 for subordinate β and subordinate γ, respectively, vs. dominants; Figure 1C), with the following order: subordinate β < dominant α < subordinate γ (p < 0.001 for all; Figure 1E).
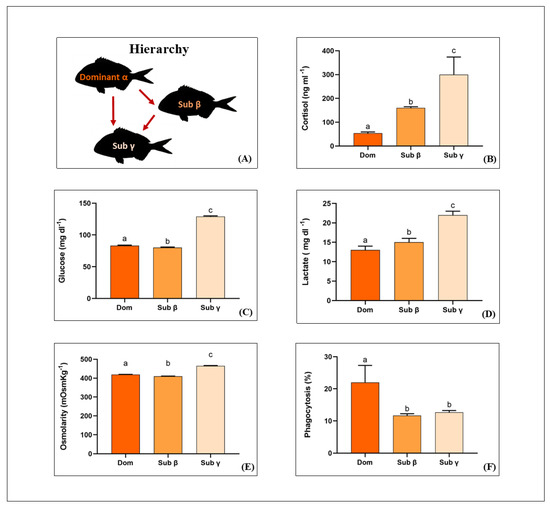
Figure 1.
Mean (±SD) of plasmatic parameters of dominant (α) (orange histogram) (n = 9), subordinate (β) (pale orange histogram) (n = 9), and subordinate (γ) (pinkish histogram) (n = 9) sea bream (Sparus aurata). The panel (A) presents the social relationship between dominant (α) and subordinates (β and γ). Arrow indicates social domination from an individual toward the other. (B) Cortisol concentration (ng mL−1), (C) glucose concentration (mg dL−1), (D) lactate concentration (mg dL−1), (E) osmolarity level (mOsmKg−1) and (F) Phagocytosis (%). Different lowercase letters over the bars indicate significant statistical difference between groups, same lowercase letters over the bars indicate absence of statistical differences (GLMM followed by Tukey HSD post-hoc test, p < 0.05).
3.3. Phagocytic Activity Related to Social Position
The percentage value of the PECs’ phagocytic activity is also affected by the social position of sea bream (p < 0.001 for subordinate β and subordinate γ, respectively, vs. dominants; Figure 1F). Post-hoc test indicates that, after 24 h of cohabitation-interaction between specimens, the phagocytic activity of the dominant is higher compared to both the subordinate β and γ (p < 0.001 for both), while it remains significantly unchanged between the subordinates β and γ fish (p = 0.85; Figure 1F).
3.4. Principal Component Analysis of Plasmatic Biochemical and Cellular Parameters
The PCA analysis was performed on five monitored physiological variables (Table 3). According to the acceleration factor method, the relevant components of the PCA were the first two, together explaining 93.4% of the data variability. The first component of the PCA, which explained 75.3% of the observed data variability, is mainly driven by the variables related to fish stress response, including cortisol, glucose and lactate plasmatic levels, and osmolarity (Table 3). The phagocytosis also significantly drives component 1, but less than the other variables (Table 3). Individuals displaying high values on this component are those that showed higher levels of glucose, cortisol and lactate (Table 3), suggesting more stressed individuals, while the second component of the PCA, which explained 18.1% of the observed data variability, is driven only by the variable phagocytosis (Table 3). Individuals displaying high values on this component are those that showed higher level of glucose, cortisol and lactate (Table 3).

Table 3.
Contribution of the five variables to the two first components of the principal component analysis (PCA) and data variance explained by each component. Bold values indicate a variable contribution higher than |0.5| to the component.
Looking at the individual positioning on the first component of the PCA (Figure 2A), fish from the subordinate group Υ displayed greater values than fish from the subordinate group β (p < 0.001), displaying greater values than dominant fish (p = 0.04). The second component of the PCA is also affected by the social position of the sea bream (p < 0.001). In more detail, the subordinate fish β displayed lower values on the second component of the PCA than both dominant fish and subordinate Υ (p < 0.001 and p = 0.007, respectively). Dominant and subordinate Υ are displaying similar values in component 2 of the PCA (p = 0.48). Overall, the results of the PCA support the previous results, suggesting that subordinate sea breams displayed higher level of stress than dominant ones in triads.
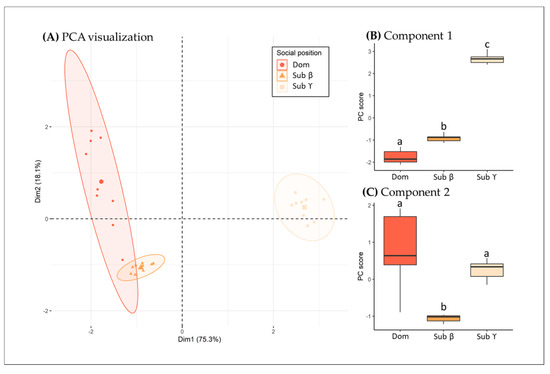
Figure 2.
Principal component analysis. (A) Scatter plot of the principal component analysis including the haematological parameters and immune activities. Cortisol concentration (ng mL−1), glucose concentration (mg dL−1), lactate concentration (mg dL−1), osmolarity level (mOsmKg−1) and phagocytosis (%) according to social status of sea bream (Sparus aurata). Orange colour represents dominant fish (α) (n = 9), pale orange represents subordinate fish (β) (n = 9) and pink represents subordinate fish (γ). Confidence ellipses drawn around the levels of the categorical variable social status with a confidence level of 0.95. (B) Individual PC score for the first component as a function of diet treatment; (C) individual PC score for the first component as a function of diet treatment. For (B,C) different lowercase letters over the bars indicate significant statistical difference between groups, same lowercase letters over the bars indicate absence of statistical differences (Tukey HSD, p < 0.05).
4. Discussion
In this study, we provide valuable insights into the dominance and social interactions of sea bream, aiming to establish the link between social interactions and behavioural patterns (feeding order and aggressivity), and stress physiological and immunity blood indicators. Here, with respect to previous work (Cammarata et al. [34]), we modified the experimental design, introducing one more fish to the observation group than the paired model previously used to evaluate the social dynamics, in a more complex pattern between fish that were confined, but maintaining a restricted number of specimens [21].
Following the establishment of hierarchy (24 h after introduction in tank), greater levels of cortisol and glucose, lactate and osmolarity were observed in fish, regardless their social position, which is indicative of stress. In previous work, the basal plasmatic cortisol and glucose concentrations were 18 ± 3 ng mL−1 and 50 ± 4 mg dL−1, respectively, in sea bream, while in paired fish, the concentrations of cortisol were 114 ± 5 ng mL−1 for the dominant and 204 ± 4 ng mL−1 for the subordinate, and the concentrations of glucose were 130 ± 8 mg dL−1 for dominant and 117 ± 7 mg dL−1 in subordinate, clearly supporting the induction of stress state flowing the establishment of social hierarchy, as already demonstrated in paired sea bream [34]. In the current study, stress state appeared to be greater in subordinate fish (β and γ) than in dominant ones, because higher levels of cortisol, glucose, lactate and osmolarity were measured in these fish. In addition, cortisol, glucose, lactate and osmolarity values were significantly higher in subordinate fish γ compared to fish β. This is also consistent with previous work carried out in sea bream, where subordinate fish were characterized by the elevation of plasma cortisol levels, in groups of two, five or ten fish [35]. Overall, this is consistent with results in other fish species, such as rainbow trout or sea bass [11,27,60], and in fishes in general, as recently reviewed by Bessa et al. [21], which showed that dominants generally exhibit lower basal cortisol levels than subordinates in a small group. This effect is, however, modulated by many factors, such as group size, habitat temperature, fish size, sexual maturity. All these factors, in larger groups, contribute to the complexity with which social hierarchies can elicit stress.
Pottinger and Carrick [61], with their study on rainbow trout, and Fatsini et al. [8], with their study on Senegalese sole (Solea senegalensis), used the classical paired model and reported that fish position within a tank, locomotor activity, agonistic behaviour, feeding, and plasma cortisol levels, are useful criteria for the determination of social dominance. Here, the dominant gilt-head seabream individuals showed a highly aggressive attitude and monopolized the access to food, with respect to the subordinates. These latter fish showed behavioural inhibition, such as suppressed aggression and low competition for feed intake with the dominant [11,62]. Feed utilization and lower growth performances have also been demonstrated in subordinate sea bream [32,35]. Low feed intake could not, however, only be the result of monopolization from the dominant. Indeed, it has been widely demonstrated, with studies on different species, that the dominant and subordinate relationships could have consequences for the animals’ physiological status. Behaviour may affect glucocorticoid levels, and, in many cases, when changes in behaviour and glucocorticoid hormones co-occur, causes and effects cannot be easily disentangled [11,63] However, many examples in vertebrates are often characterised by chronically high plasma cortisol levels, coupled with suppressed aggressiveness and reduced food intake [61,64,65]. As reported by Gregory and Wood, chronic exposure at the cortisol level influences behaviour activities; for example, the research of food [66]. Confirming the data in Cammarata et al. [34] obtained by studies using a paired model, we observed that the establishment of the hierarchy in sea bream involves not only behavioural patterns, such as aggressiveness and the order of food access, but also different stress physiological profiles. It was also demonstrated, in that species, as well as in rainbow trout, that more aggressive individuals also display lower cortisol response when coping with other stress events, such as confinement or restraining tests [23]. In this study, we also find that the glucose levels measured were increased in subordinate fish. Glucose release in blood is generally associated with the secondary stress response being modulated by the action of cortisol, which influences glucidic metabolism in fish [6]. Mommsen et al. [13] reported that plasmatic, hepatic and muscular glucose levels in teleosts might not be univocally correlated with the stress condition (i.e., cortisol level). Further, in this paper, we have observed an increase in lactate levels in the blood of subordinate individuals compared to dominant ones. An increase in blood lactate level is generally reported as a secondary response to stress [67,68]. The secondary responses are, in part, triggered by the cortisol increase (primary response). Cortisol plays a role in stimulating some processes (e.g., gluconeogenesis) and inhibiting others (e.g., digestion). According to Peters et al. [69], increased plasma glucose could be originated catabolizing the hepatic glycogen. Overall, it indicates the mobilisation of the required energy to face and fight future interactions with other fish to maintain the top hierarchical position. Changes in plasmatic lactate level, as a product of the anaerobic metabolism, suggest that energy could be subtracted from muscle and/or the reproductive system [70]. Since the increased glucocorticoid levels exhibited during stress might also serve to mitigate defence mechanisms [71], we examined the correlation between the social rank in the hierarchy establishment, as a stress source, and its effects on innate immunity.
It is well known that continuous agonistic interactions between conspecifics constitute social stress in fishes, which translates as chronic cortisol elevation [27,35,60]. Cammarata et al. [34] demonstrated the cortisol effect on gilthead sea bream PECs, treating cells with three different cortisol concentrations and showing a dose-dependent decrease in cells’ phagocytic activity. Moreover, in vivo and in vitro experiments showed that increased cortisol levels affect phagocytosis via the cortisol cytosolic receptor DlGR1 (Dicentrarchus labrax glucocorticoid receptor1) in European sea bass [36]. This receptor was localised in the head, kidney, spleen, gills, intestine, heart and liver tissues [37,72], highlighting the crucial role of cortisol in the regulation of homeostasis. It is known that stress-induced hormonal responses, lead to osmotic imbalances in fish [14]. Thus, stress causes the elevation of plasma cortisol and electrolyte loss in freshwater fish [73,74]. In agreement with previous studies, in this work, we have also observed a significant increase in the levels of osmolarity in subordinate individuals compared to dominant α. Moreover, we have evaluated the effects of social stress on the phagocytic activity of peritoneal cavity cells. In Cammarata et al. [34], we proved that cohabitation and hierarchy have a physiological effect, after a 24 h pairing period, affecting the PECs, with respect to phagocytic activity. Further, we showed, after this cohabitation-interaction period, PECs’ phagocytic activity was significantly higher in the dominant fish, with respect to subordinates β and γ, highlighting a rapid (24 h) and strong effect of social interaction on the peritoneal exudate leucocytes responses. Indeed, the social stress mainly affects the PECs’ response in subordinate individuals, as revealed by phagocytosis and respiratory burst activity [34]. In addition to PEC, Montero et al. [35] also demonstrated in sea bream that lysozyme activity can be reduced in subordinates. Reduced lysozyme activity in subordinate fish has also been observed in other fish species, such as sea bass or Nile tilapia [11,75]. Overall, this indicates that subordinate sea bream display reduced immunity status, indicative of chronic stress, which could be deleterious in case of disease outbreak [35]. In addition to immunity markers (e.g., PECs, lysozyme, enzyme activities), further experiments could study specific immunological response to bacteria/virus, depending on fish social rank.
In this paper, the testosterone levels, which have a role in the regulation of cellular immune functions [76], have not been evaluated. However, sea bream is a proterandrous hermaphrodite species. At the stage examined in this study (i.e., 125 ± 25 g), fish are all immature males. Further experiments could also study possible changes in testosterone levels, following the establishment of social hierarchy in sea bream, as well as how hierarchy establishment works later in sea bream life, when males become mature or become female, as well as the underlying physiology.
The results of the PCA analysis, overall, support the analysis of the different variables one by one. Indeed, the first component of the PCA, significantly driven by all variables (cortisol, glucose, lactate, osmolarity and phagocytosis) and explaining a high percentage of data variability (i.e., 75.3%), is significantly affected by social rank. Insights obtained from the second component of the PCA are few, since only phagocytosis is significantly driving the component overall, explaining the low percentage of data variability (18.1%). These results moderate, in some way, the result from the GLM analysis of the percentage of phagocytosis, depending on the social rank of sea bream, where subordinate β and Υ displayed similar levels of phagocytosis, both lower than the level measured in the dominant fish. Other indicators of immunity, such as oxidative stress markers, cellular cytotoxic activity, could be measured in the future to bring further insights on immunity differences, according to social rank in sea beam. Overall, the differences observed between the groups, measured both in GLMM and PCA analyses, could be attributed to different allostatic load and adaptation time in the responses, indicating that these could be used as allostatic load biomarkers of social stress responses and impact on fish health. Our results show that stress physiological status can be determined from social interactions and from the territorial disputes activating the stress response, through cortisol release in gilt-head bream, as occurs in response to other stressors and in vertebrates [77].
Interestingly, as for aggressivity, the boldness behaviour has also been observed to affect the growth of sea bream, reared at different density over long periods of time [78]. These results may be correlated with the aggressive behaviour of dominant sea bream (i.e., eat first and more), coupled with different physiological features, depending on the boldness/aggressiveness level [23,79]. In the rainbow trout, Gesto et al. [80] also found that fish with different abilities compete for food, showing different behavioural responses to cope with hypoxia and ammonia exposure. In addition, stress physiological responses toward other stressors (e.g., confinement, netting) could be different, depending on fish social rank. Thus, the behavioural indicators of social position (e.g., boldness, aggressive behaviour, feeding behaviour) in sea bream could be a relevant proxy of the physiological status and stress response [23,79,81].
Furthermore, since it has been demonstrated that hierarchy is a cause of chronic stress, our data support the need to find solutions to mitigate its effects on the condition of the fish, preserving the aquaculture final product from its consequent negative effect. Solutions could be to improve the rearing condition of fish, implementing the rearing methods, as well as structural complexity of rearing tanks, which in fish farming are under-implemented [82]. For instance, self-feeding reinforces the social hierarchy, which might lead to a higher competitiveness for resources among fishes, increasing the social hierarchy and, therefore, stress, when compared to hand feeding. Thus, hand feeding could reduce the deleterious effect of social hierarchy, but this is not really feasible in intensive farming conditions. Increasing the complexity of the physical environment can also lead to benefits for the species, such as a reduction in aggressivity and related stress [83]. Indeed, an articulated space, introducing the possibility to avoid direct interaction, to defend territory, to escape during social conflict, investigate and interact with enrichment, could reduce stress. Studies report the relevant importance of environment on gilt-head sea bream behaviour and aggressivity; for example, the presence of blue or red-brown substrate on the tank bottom resulted in suppression of aggressive behaviour, compared to green substrate and no-substrate tanks [84]. This hypothesis is supported by Arechavala-Lopez et al., which demonstrated the influence of environmental enrichment on the enhancement of cognition, exploratory behaviour and brain physiological functions of sea bream [85].
5. Conclusions
In conclusion, in this study, the links between behaviour, stress physiological profile (cortisol, glucose and lactate) and immunity, in relation to social hierarchy, were investigated in gilt-head bream triads for the first time. We confirmed previous work in pairs, subordinate sea bream displayed greater stress level (plasmatic level of cortisol, glucose, and lactate), as well lower immunity (lower percentage of phagocytosis) than dominant fish. In this optic, further research is needed to study the hierarchic relationship in different rearing conditions, such as larger and sex-mixed groups and in an enriched environment. Further, this would help to improve the health and welfare of farmed sea bream by finding best management practices, depending on the need of the species.
Author Contributions
Conceptualization, M.C. and M.V.; methodology, M.D. (Maria Dioguardi) and D.A. and I.V. and M.D. (Mariano Dara); validation, M.C.; investigation, M.D. (Maria Dioguardi), D.A., and I.V., S.A. and M.C.; data curation D.A. and M.D. (Mariano Dara) and P.C.; writing—original draft preparation, M.D. (Maria Dioguardi) and M.V.; writing—review and editing, M.C. and M.D. (Mariano Dara) and P.C. and S.A.; visualization, M.D. (Mariano Dara), P.C. and M.C; supervision, M.C.; project administration, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from MIUR, and MC RITMARE, project SP2-WP4-AZ3-UO3 (CNR and CONISMA), FFR Cammarata (PJ_RIC_FFABR_2017_004312 MC, Ministero dell’Istruzione, dell’Università e della Ricerca).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ministero della Sanità, Ufficio Veterinario degli adempimenti C.E.E. della Sicilia Institutional Review Board, Decreto ministeriale n.62/94-A del 1/6/1994. The experiments were performed in full compliance with the national guidelines (D.Lgs 116/92 art.4 comma 3 e art 6 subsequent amendments) and the international European Commission Recommendation guidelines for the accommodation and care of animals used for experimental and other scientific purposes (2007/526/EC and 2010/63/UE).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cerqueira, M.; Millot, S.; Felix, A.; Silva, T.; Oliveira, G.A.; Oliveira, C.C.V.; Rey, S.; MacKenzie, S.; Oliveira, R. Cognitive appraisal in fish: Stressor predictability modulates the physiological and neurobehavioural stress response in sea bass. Proc. R. Soc. B Biol. Sci. 2020, 287. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.R. One Species with Two Biologies: Atlantic Salmon (Salmo salar) in the Wild and in Aquaculture; IATP: Washington, DC, USA, 1998. [Google Scholar]
- Braithwaite, V.A.; Huntingford, F.A. Fish and welfare: Do fish have the capacity for pain perception and suffering? Anim. Welf. 2004, 13, S87–S92. [Google Scholar]
- Huntingford, F.A. Implications of domestication and rearing conditions for the behaviour of cultivated fishes. J. Fish Biol. 2004, 65, 122–142. [Google Scholar] [CrossRef]
- DeVries, A.C.; Craft, T.K.S.; Glasper, E.R.; Neigh, G.N.; Alexander, J.K. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology 2007, 32, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Cepa, I.; Ruiz-Jarabo, I. Physiology: An important tool to assess the welfare of aquatic animals. Biology 2021, 10, 61. [Google Scholar] [CrossRef]
- Maguire, S.M.; DeAngelis, R.; Dijkstra, P.D.; Jordan, A.; Hofmann, H.A. Social network dynamics predict hormone levels and behavior in a highly social cichlid fish. Horm. Behav. 2021, 132, 104994. [Google Scholar] [CrossRef]
- Fatsini, E.; Rey, S.; Ibarra-Zatarain, Z.; Mackenzie, S.; Duncan, N.J. Dominance behaviour in a non-aggressive flatfish, Senegalese sole (Solea senegalensis) and brain mRNA abundance of selected transcripts. PLoS ONE 2017, 12, e0184283. [Google Scholar] [CrossRef] [PubMed]
- Magnhagen, C.; Borcherding, J. Risk-taking behaviour in foraging perch: Does predation pressure influence age-specific boldness? Anim. Behav. 2008, 75, 509–517. [Google Scholar] [CrossRef]
- Korzan, W.J.; Höglund, E.; Watt, M.J.; Forster, G.L.; Øverli, Ø.; Lukkes, J.L.; Summers, C.H. Memory of opponents is more potent than visual sign stimuli after social hierarchy has been established. Behav. Brain Res. 2007, 183, 31–42. [Google Scholar] [CrossRef][Green Version]
- Carbonara, P.; Dioguardi, M.; Cammarata, M.; Zupa, W.; Vazzana, M.; Spedicato, M.T.; Lembo, G. Basic knowledge of social hierarchies and physiological profile of reared sea bass Dicentrarchus labrax (L.). PLoS ONE 2019, 14, e0208688. [Google Scholar] [CrossRef] [PubMed]
- Kittilsen, S.; Ellis, T.; Schjolden, J.; Braastad, B.O.; Øverli, Ø. Determining stress-responsiveness in family groups of Atlantic salmon (Salmo salar) using non-invasive measures. Aquaculture 2009, 298, 146–152. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Tracey, S.R.; Hartmann, K.; Leef, M.; McAllister, J. Capture-induced physiological stress and postrelease mortality for southern bluefin tuna (Thunnus maccoyii) from a recreational fishery. Can. J. Fish. Aquat. Sci. 2016, 73, 1547–1556. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Ellis, T.; Yildiz, H.Y.; López-Olmeda, J.; Spedicato, M.T.; Tort, L.; Øverli, Ø.; Martins, C.I.M. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Øverli, Ø.; Pottinger, T.G.; Carrick, T.R.; Øverli, E.; Winberg, S. Brain monoaminergic activity in rainbow trout selected for high and low stress responsiveness. Brain Behav. Evol. 2001, 57, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Bird, D.J. Modulation of the fish immune system by hormones. Vet. Immunol. Immunopathol. 2000, 77, 163–176. [Google Scholar] [CrossRef]
- Schreck, C.B.; Contreras-Sanchez, W.; Fitzpatrick, M.S. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 2001, 197, 3–24. [Google Scholar] [CrossRef]
- Gilmour, K.M.; DiBattista, J.D.; Thomas, J.B. Physiological causes and consequences of social status in salmonid fish. Integr. Comp. Biol. 2005, 45, 263–273. [Google Scholar] [CrossRef]
- Bessa, E.; Sadoul, B.; Mckenzie, D.J.; Geffroy, B. Group size, temperature and body size modulate the effects of social hierarchy on basal cortisol levels in fishes. Horm. Behav. 2021, 136, 105077. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.F.; Conceição, L.E.C.; Millot, S.; Rey, S.; Bégout, M.L.; Damsgård, B.; Kristiansen, T.; Höglund, E.; Øverli, Ø.; Martins, C.I.M. Coping styles in farmed fish: Consequences for aquaculture. Rev. Aquac. 2017, 9, 23–41. [Google Scholar] [CrossRef]
- Castanheira, M.F.; Herrera, M.; Costas, B.; Conceição, L.E.C.; Martins, C.I.M. Linking cortisol responsiveness and aggressive behaviour in gilthead seabream Sparus aurata: Indication of divergent coping styles. Appl. Anim. Behav. Sci. 2013, 143, 75–81. [Google Scholar] [CrossRef]
- Herrera, M.; Castanheira, M.F.; Conceição, L.E.C.; Martins, C.I. Linking risk taking and the behavioral and metabolic responses to confinement stress in gilthead seabream Sparus aurata. Appl. Anim. Behav. Sci. 2014, 155, 101–108. [Google Scholar] [CrossRef]
- Edeline, E.; Haugen, T.O.; Weltzien, F.A.; Claessen, D.; Winfield, I.J.; Stenseth, N.C.; Asbjørn Vøllestad, L. Body downsizing caused by non-consumptive social stress severely depresses population growth rate. Proc. R. Soc. B Biol. Sci. 2010, 277, 843–851. [Google Scholar] [CrossRef]
- Backström, T.; Winberg, S. Serotonin coordinates responses to social stress—What we can learn from fish. Front. Neurosci. 2017, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-de-Castilho, M.; Pottinger, T.G.; Volpato, G.L. Chronic social stress in rainbow trout: Does it promote physiological habituation? Gen. Comp. Endocrinol. 2008, 155, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Huntingford, F.; Adams, C. Behavioural syndromes in farmed fish: Implications for production and welfare. Behaviour 2005, 142, 1207–1221. [Google Scholar] [CrossRef]
- Conte, F.S. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004, 86, 205–223. [Google Scholar] [CrossRef]
- Frimodt, C. Multilingual Illustrated Guide to the World’s Commercial Coldwater Fish; Fishing News Books Ltd.: Oxford, UK, 1995; ISBN 0852382138. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Yearbook of the United Nations 1998; FAO: Rome, Italy, 1998; pp. 1377–1379. [Google Scholar] [CrossRef]
- Goldan, O.; Popper, D.; Karplus, I. Food competition in small groups of juvenile gilthead sea bream (Sparus aurata). Isr. J. Aquac. -Bamidgeh 2003, 55, 94–106. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Nazzaro-Alvarez, J.; Jardí-Pons, A.; Reig, L.; Carella, F.; Carrassón, M.; Roque, A. Linking stocking densities and feeding strategies with social and individual stress responses on gilthead seabream (Sparus aurata). Physiol. Behav. 2020, 213, 112723. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, M.; Vazzana, M.; Accardi, D.; Parrinello, N. Seabream (Sparus aurata) long-term dominant-subordinate interplay affects phagocytosis by peritoneal cavity cells. Brain Behav. Immun. 2012, 26, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Lalumera, G.; Izquierdo, M.S.; Caballero, M.J.; Saroglia, M.; Tort, L. Establishment of dominance relationships in gilthead sea bream Sparus aurata juveniles during feeding: Effects on feeding behaviour, feed utilization and fish health. J. Fish Biol. 2009, 74, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, M.; Cammarata, M.; Cooper, E.L.; Parrinello, N. Confinement stress in sea bass (Dicentrarchus labrax) depresses peritoneal leukocyte cytotoxicity. Aquaculture 2002, 210, 231–243. [Google Scholar] [CrossRef]
- Vizzini, A.; Vazzana, M.; Cammarata, M.; Parrinello, N. Peritoneal cavity phagocytes from the teleost sea bass express a glucocorticoid receptor (cloned and sequenced) involved in genomic modulation of the in vitro chemiluminescence response to zymosan. Gen. Comp. Endocrinol. 2007, 150, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Olivier, G.; Moore, A.R.; Fildes, J. Toxicity of Aeromonas salmonicida cells to atlantic salmon Salmo salar peritoneal macrophages. Dev. Comp. Immunol. 1992, 16, 49–61. [Google Scholar] [CrossRef]
- Suzuki, K. Morphological and phagocytic characteristics of peritoneal exudate cells in tilapia, Oreochromis niloticus (Trewavas), and carp, Cyprinus carpio L. J. Fish Biol. 1986, 29, 349–364. [Google Scholar]
- Cammarata, M.; Vazzana, M.; Cervello, M.; Arizza, V.; Parrinello, N. Spontaneous cytotoxic activity of eosinophilic granule cells separated from the normal peritoneal cavity of Dicentrarchus labrax. Fish Shellfish Immunol. 2000, 10, 143–154. [Google Scholar] [CrossRef]
- Vazzana, M.; Parrinello, D.; Cammarata, M. Chemiluminescence response of β-glucan stimulated leukocytes isolated from different tissues and peritoneal cavity of Dicentrarchus labrax. Fish Shellfish Immunol. 2003, 14, 423–434. [Google Scholar] [CrossRef]
- Gesto, M. Chapter 9—Characterization of the neuroendocrine stress status as part of the multiparametric assessment of welfare in fish. In Cellular and Molecular Approaches in Fish Biology; Academic Press: London, UK, 2022; pp. 285–308. ISBN 978-0-12-822273-7. [Google Scholar]
- Culbert, B.M.; Gilmour, K.M. Rapid recovery of the cortisol response following social subordination in rainbow trout. Physiol. Behav. 2016, 164, 306–313. [Google Scholar] [CrossRef]
- Gunnes, K.; Refstie, T. Cold-branding and fin-clipping for marking of salmonids. Aquaculture 1980, 19, 295–299. [Google Scholar] [CrossRef]
- Hammer, S.A.; Lee Blankenship, H. Cost comparison of marks, tags, and mark-with-tag combinations used in salmonid research. North Am. J. Aquac. 2001, 63, 171–178. [Google Scholar] [CrossRef]
- Thompson, J.M.; Hirethota, P.S.; Eggold, B.T. A comparison of elastomer marks and fin clips as marking techniques for Walleye. North Am. J. Fish. Manag. 2005, 25, 308–315. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- McCarthy, I.D.; Gair, D.J.; Houlihan, D.F. Feeding rank and dominance in Tilapia rendalli under defensible and indefensible patterns of food distribution. J. Fish Biol. 1999, 55, 854–867. [Google Scholar] [CrossRef]
- Sleet, D.A. Paul Martin and Patrick Bateson: Measuring behavior: An introductory guide. Cambridge University Press, Cambridge, England, 1993, Second Edition, 222 pages, ISBN 0521 446147 (paperback). Behav. Sci. 1995, 40, 77–80. [Google Scholar] [CrossRef]
- Øverli, Ø.; Kotzian, S.; Winberg, S. Effects of cortisol on aggression and locomotor activity in rainbow trout. Horm. Behav. 2002, 42, 53–61. [Google Scholar] [CrossRef]
- Baerends, G.P.; Baerends-van Roon, J.M. An introduction to the Study of the Ethology of the Cichlid Fishes; Brill Publishers: Leiden, The Netherlands, 1950; 242p. [Google Scholar]
- Oliveira, R.F.; Almada, V.C.; Canario, A.V.M. Social modulation of sex steroid concentrations in the urine of male cichlid fish Oreochromis mossambicus. Horm. Behav. 1996, 30, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Espelid, S.; Løkken, G.B.; Steiro, K.; Bøgwald, J. Effects of cortisol and stress on the immune system in Atlantic Salmon (Salmo salar L.). Fish Shellfish Immunol. 1996, 6, 95–110. [Google Scholar] [CrossRef]
- R Software R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Raîche, G.; Walls, T.A.; Magis, D.; Riopel, M.; Blais, J.G. Non-graphical solutions for Cattell’s scree test. Methodology 2013, 9, 23–29. [Google Scholar] [CrossRef]
- Hand, D.M.; Brignon, W.R.; Olson, D.E.; Rivera, J. Comparing Two Methods Used to Mark Juvenile Chinook Salmon: Automated and Manual Marking. North Am. J. Aquac. 2010, 72, 10–17. [Google Scholar] [CrossRef]
- Sloman, K.A.; Metcalfe, N.B.; Taylor, A.C.; Gilmour, K.M. Plasma cortisol concentrations before and after social stress in rainbow trout and brown trout. Physiol. Biochem. Zool. 2001, 74, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Pottinger, T.G.; Carrick, T.R. Stress responsiveness affects dominant-subordinate relationships in rainbow trout. Horm. Behav. 2001, 40, 419–427. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Sakai, R.R.; McEwen, B.; Weiss, S.M.; Blanchard, R.J. Subordination stress: Behavioral, brain, and neuroendocrine correlates. Behav. Brain Res. 1993, 58, 113–121. [Google Scholar] [CrossRef]
- Øverli, Ø.; Harris, C.A.; Winberg, S. Short-erm effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 1999, 54, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.C.; Dunsbrack, R.L.; Orr, C.D. The interaction of size and experience in dominance relationships of juvenile steelhead trout (Salmo gairdneri). Behaviour 1985, 92, 241–253. [Google Scholar]
- Winberg, S.; Lepage, O. Elevation of brain 5-HT activity, POMC expression, and plasma cortisol in socially subordinate rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 274, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Gregory, T.R.; Wood, C.M. The effects of chronic plasma cortisol elevation on the feeding behaviour, growth, competitive ability, and swimming performance of juvenile rainbow trout. Physiol. Biochem. Zool. 1999, 72, 286–295. [Google Scholar] [CrossRef]
- Caruso, G.; Genovese, L.; Maricchiolo, G.; Modica, A. Haematological, biochemical and immunological parameters as stress indicators in Dicentrarchus labrax and Sparus aurata farmed in off-shore cages. Aquac. Int. 2005, 13, 67–73. [Google Scholar] [CrossRef]
- Montero, D.; Marrero, M.; Izquierdo, M.S.; Robaina, L.; Vergara, J.M.; Tort, L. Effect of vitamin E and C dietary supplementation on some immune parameters of gilthead seabream (Sparus aurata) juveniles subjected to crowding stress. Aquaculture 1999, 171, 269–278. [Google Scholar] [CrossRef]
- Peters, G.; Delventhal, H.; Klinger, H. Physiological and morphological effects of social stress on the eel, Anguilla anguilla L. In Fish Diseases; Springer: Berlin/Heidelberg, Germany, 1980; pp. 225–227. [Google Scholar]
- Dinkel, K.; Ogle, W.O.; Sapolsky, R.M. Glucocorticoids and central nervous system inflammation. J. NeuroVirol. 2002, 8, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Øverli, Ø.; Korzan, W.J.; Larson, E.T.; Winberg, S.; Lepage, O.; Pottinger, T.G.; Renner, K.J.; Summers, C.H. Behavioral and neuroendocrine correlates of displaced aggression in trout. Horm. Behav. 2004, 45, 324–329. [Google Scholar] [CrossRef]
- Vazzana, M.; Vizzini, A.; Salerno, G.; Di Bella, M.L.; Celi, M.; Parrinello, N. Expression of a glucocorticoid receptor (DlGR1) in several tissues of the teleost fish Dicentrarchus labrax. Tissue Cell 2008, 40, 89–94. [Google Scholar] [CrossRef]
- Barton, B.A.; Zitzow, R.E. Physiological responses of juvenile walleyes to handling stress with recovery in saline water. Progress. Fish-Cult. 1995, 57, 267–276. [Google Scholar] [CrossRef]
- Cech, J.J.; Bartholow, S.D.; Young, P.S.; Hopkins, T.E. Striped Bass Exercise and Handling Stress in Freshwater: Physiological Responses to Recovery Environment. Trans. Am. Fish. Soc. 1996, 125, 308–320. [Google Scholar] [CrossRef]
- Caruso, D.; Lazard, J. Subordination stress in Nile tilapia and its effect on plasma lysozyme activity. J. Fish Biol. 1999, 55, 451–454. [Google Scholar] [CrossRef]
- Gesquiere, L.R.; Learn, N.H.; Simao, M.C.M.; Onyango, P.O.; Alberts, S.C.; Altmann, J. Life at the top: Rank and stress in wild male baboons. Science 2011, 333, 357–360. [Google Scholar] [CrossRef]
- Sloman, K.A. Social and Reproductive Behaviors| Dominance Behaviors; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 1, ISBN 9780080923239. [Google Scholar]
- Carbonara, P.; Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Pretto, T.; Spedicato, M.T.; Lembo, G. Behavioral and physiological responses to stocking density in sea bream (Sparus aurata): Do coping styles matter? Physiol. Behav. 2019, 212, 112698. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Stress coping styles: Is the basal level of stress physiological indicators linked to behaviour of sea bream? Appl. Anim. Behav. Sci. 2020, 231, 105085. [Google Scholar] [CrossRef]
- Gesto, M.; Zupa, W.; Alfonso, S.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Using acoustic telemetry to assess behavioral responses to acute hypoxia and ammonia exposure in farmed rainbow trout of different competitive ability. Appl. Anim. Behav. Sci. 2020, 230, 105084. [Google Scholar] [CrossRef]
- Øverli, Ø.; Korzan, W.J.; Ho, E.; Winberg, S.; Bollig, H.; Watt, M.; Forster, G.L.; Barton, B.A.; Øverli, E.; Renner, K.J.; et al. Stress Coping Style Predicts Aggression and Social Dominance in Rainbow Trout; Elsevier: Amsterdam, The Netherlands, 2004; Volume 45, pp. 235–241. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Diaz-Gil, C.; Saraiva, J.L.; Moranta, D.; Castanheira, M.F.; Nuñez-Velázquez, S.; Ledesma-Corvi, S.; Mora-Ruiz, M.R.; Grau, A. Effects of structural environmental enrichment on welfare of juvenile seabream (Sparus aurata). Aquac. Rep. 2019, 15, 100224. [Google Scholar] [CrossRef]
- Batzina, A.; Karakatsouli, N. The presence of substrate as a means of environmental enrichment in intensively reared gilthead seabream Sparus aurata: Growth and behavioral effects. Aquaculture 2012, 370–371, 54–60. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Caballero-Froilán, J.C.; Jiménez-García, M.; Capó, X.; Tejada, S.; Saraiva, J.L.; Sureda, A.; Moranta, D. Enriched environments enhance cognition, exploratory behaviour and brain physiological functions of Sparus aurata. Sci. Rep. 2020, 10, 11252. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).