Molecular Characterization and Nutrition Regulation of the Neutral Amino Acid Transporter ASCT2 in Triploid Crucian Carp
Abstract
1. Introduction
2. Materials and methods
2.1. Fish
2.2. RNA Isolation and cDNA Synthesis
2.3. Cloning of cDNA of ASCT2
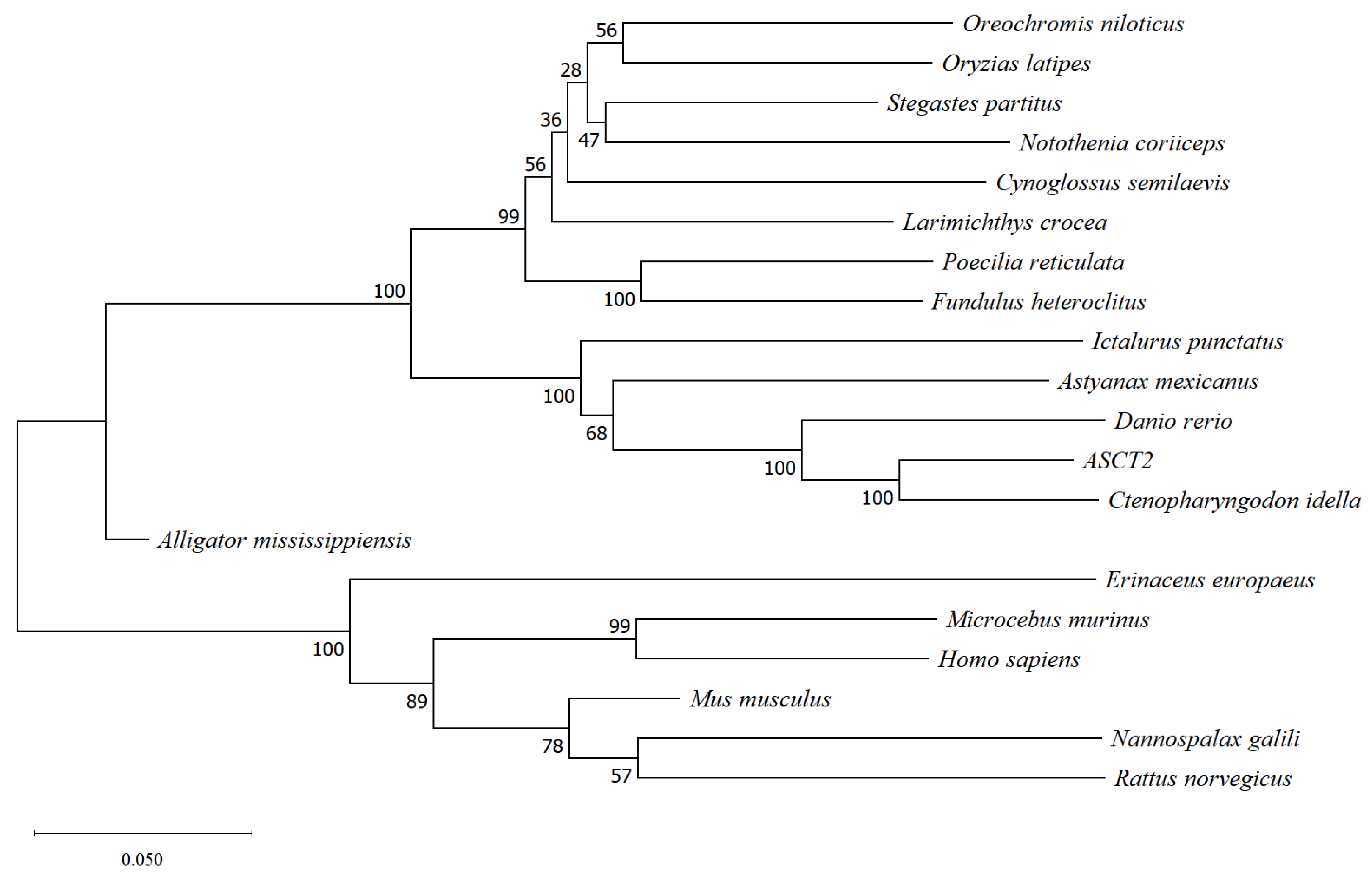
2.4. Phylogenetic Relationships
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. ASCT2 Expression in Various Tissues and Diurnal Variation
2.7. The Effect of Glutamate and Glutamine on ASCT2
2.8. Regulation of Dietary Protein Levels and Protein Sources on ASCT2
2.9. Statistical Analysis
3. Results
3.1. Isolation and Sequence Analysis of ASCT2 cDNA from Triploid Crucian Carp
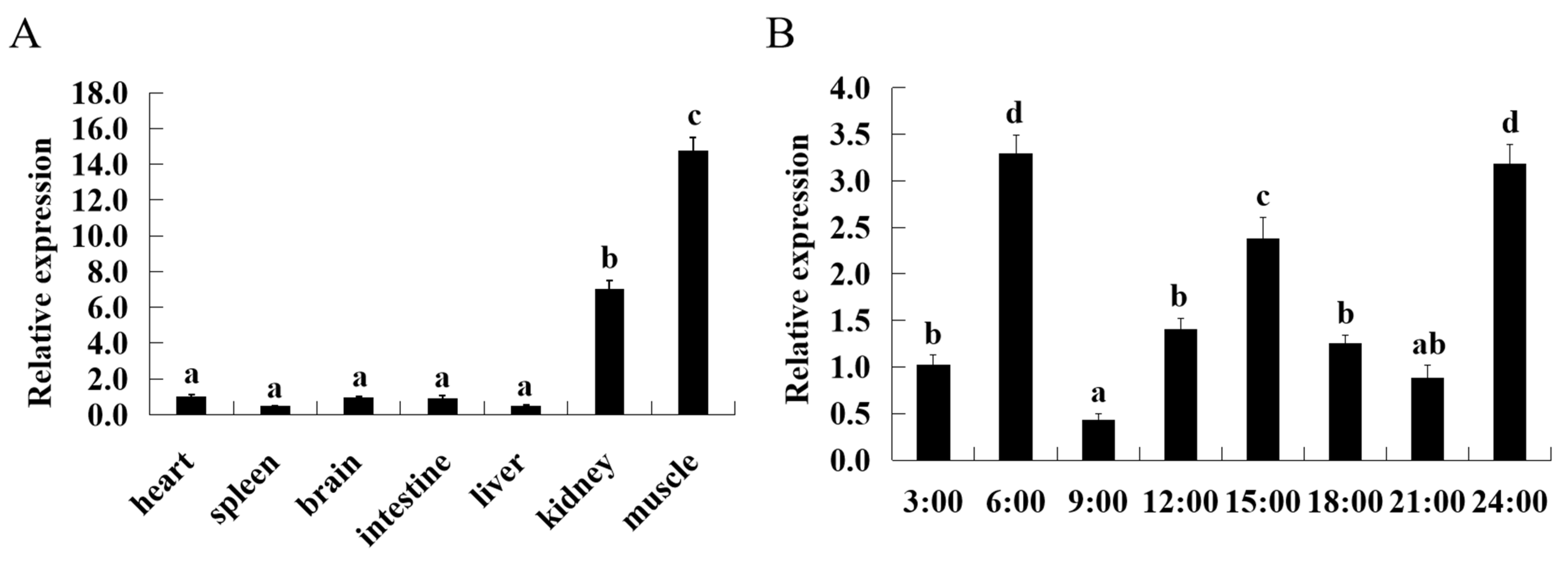
3.2. Tissue Expression Patterns and Diurnal Variation of ASCT2
3.3. Effect of Glutamate and Glutamine Levels on the ASCT2 Expression
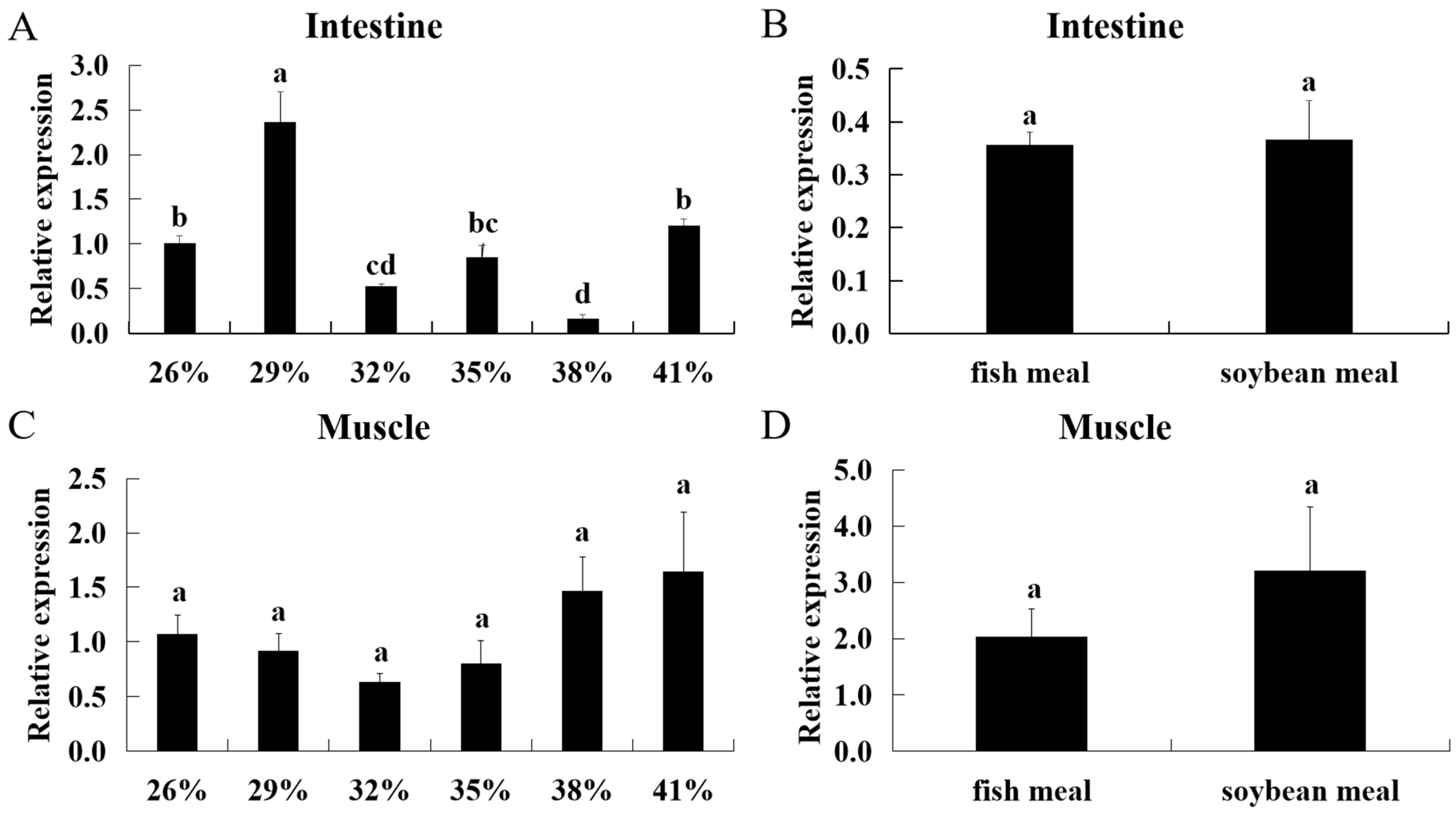
3.4. Effect of Diets with Different Protein Levels and Protein Sources on ASCT2 Expression in Triploid Crucian Carp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garaeva, A.A.; Guskov, A.; Slotboom, D.J.; Paulino, C. A one-gate elevator mechanism for the human neutral amino acid transporter ASCT2. Nat. Commun. 2019, 10, 3427. [Google Scholar] [CrossRef]
- Ndaru, E.; Garibsingh, R.; Shi, Y.Y.; Wallace, E.; Zakrepine, P.; Wang, J.; Schlessinger, A.; Grewer, C. Novel alanine serine cysteine transporter 2 (ASCT2) inhibitors based on sulfonamide and sulfonic acid ester scaffolds. J. Gen. Physiol. 2019, 151, 357–368. [Google Scholar] [CrossRef]
- Scalise, M.; Pochini, L.; Panni, S.; Pingitore, P.; Hedfalk, K.; Indiveri, C. Transport mechanism and regulatory properties of the human amino acid transporter ASCT2 (SLC1A5). Amino Acids 2014, 46, 2463–2475. [Google Scholar] [CrossRef]
- Scalise, M.; Mazza, T.; Pappacoda, G.; Pochini, L.; Cosco, J.; Rovella, F.; Indiveri, C. The human SLC1A5 neutral amino acid transporter catalyzes a pH-dependent glutamate/glutamine antiport, as well. Front. Cell Dev. Biol. 2020, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.D.; Kripke, S.A.; De, P.J.; Berman, J.M.; Settle, R.G.; Rombeau, J.L. Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. JPEN, J. Parenter. Enteral Nutr. 1988, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hardie, R.A.; Hoy, A.J.; Geldermalsen, M.V.; Gao, D.; Fazli, L.; Sadowski, M.C.; Balaban, S.; Schreuder, M.; Nagarajah, R.; et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 2015, 236, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Souba, W.W.; Klimberg, V.S.; Plumley, D.A.; Salloum, R.M.; Flynn, T.C.; Bland, K.I.; Copeland, E.M. The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J. Surg. Res. 1990, 48, 383–391. [Google Scholar] [CrossRef]
- Neu, J.; Shenoy, V.; Chakrabarti, R. Glutamine nutrition and metabolism: Where do we go from here? FASEB J. 1996, 10, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Colas, C.; Grewer, C.; Otte, N.J.; Gameiro, A.; Albers, T.; Singh, K.; Shere, H.; Bonomi, M.; Holst, J.; Schlessinger, A. Ligand discovery for the alanine-serine-cysteine transporter (ASCT2, SLC1A5) from homology modeling and virtual screening. Biophys. J. 2015, 108, 54a. [Google Scholar] [CrossRef][Green Version]
- Toda, K.; Nishikawa, G.; Iwamoto, M.; Itatani, Y.; Takahashi, R.; Sakai, Y.; Kawada, K. Clinical role of ASCT2 (SLC1A5) in KRAS-mutated colorectal cancer. Int. J. Mol. Sci. 2017, 18, 1632. [Google Scholar] [CrossRef] [PubMed]
- Van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.; Deng, N.; et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Shimode, S.; Nakaoka, R.; Shogen, H.; Miyazawa, T. Characterization of feline ASCT1 and ASCT2 as RD-114 virus receptor. J. Gen. Virol. 2013, 94, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, R.; Okamoto, M.; Sakaguchi, S.; Nakagawa, S.; Miura, T.; Hirai, H.; Miyazawa, T. Simian retrovirus 4 induces lethal acute thrombocytopenia in Japanese macaques. J. Virol. 2015, 89, 3965–3975. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Souba, W.W. Interorgan ammonia metabolism in health and disease: A surgeon’s view. JPEN, J. Parenter. Enteral Nutr. 1987, 11, 569–579. [Google Scholar] [CrossRef]
- Hu, R.; Wang, Y.; Qu, F.; Tang, J.; Zhou, Y.; Lu, S.; Zhang, J.; Liu, Z.; Zhou, Z.; Guo, X. Molecular characterization and dietary regulation of the AlaSerCys transporter 2 in grass carp, Ctenopharyngodon idella. J. World Aquacult. Soc. 2017, 48, 333–341. [Google Scholar] [CrossRef]
- Kanai, Y.; Hediger, M. The glutamate/neutral amino acid transporter family SLC1: Molecular, physiological and pharmacological aspects. Pfluegers Arch. 2004, 447, 469–479. [Google Scholar] [CrossRef]
- Tomé, D. The roles of dietary glutamate in the intestine. Ann. Nutr. Metab. 2018, 73, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Y.; Liu, S.; Zhao, Q.; Feng, J.; Lu, S.; Xiong, G.; Xie, D.; Zhang, J.; Liu, Y. Characterization and dietary regulation of oligopeptide transporter (PepT1) in different ploidy fishes. Peptides 2014, 52, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Y.; Wang, Y.; Lu, S.; Zhao, Q.; Wu, Q.; Xiong, G.; Hao, G.; Zhang, J. Characterization of l-type amino acid transporter 2 from crucian carp, Carassius auratus. J. World Aquacult. Soc. 2014, 45, 709–718. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Liu, S.; Zhong, H.; Zhang, C.; Kang, X.; Liu, Y. Characterization and dietary regulation of glutamate dehydrogenase in different ploidy fishes. Amino Acids 2012, 43, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, S.; Sun, Y.; Zhang, C.; Luo, K.; Tao, M.; Chen, Z.; Liu, Y. A new type of triploid crucian crap-red crucian crap (♀) × allotetraploid (♂). Prog. Nat. Sci. 2006, 16, 1348–1352. [Google Scholar]
- Mao, Z.; Fu, Y.; Wang, S.; Wang, Y.; Luo, K.; Zhang, C.; Tao, M.; Liu, S. Further evidence for paternal DNA transmission in gynogenetic grass carp. Sci. China: Life Sci. 2020, 63, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Atteson, K. The performance of neighbor-joining methods of phylogenetic reconstruction. Algorithmica 1999, 25, 251–278. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Cao, S.; Mo, P.; Xiao, Y.; Chen, Y.; Shi, Y.; Hu, Y.; Tang, J.; Qu, F.; Luo, M.; Ai, X.; et al. Dietary supplementation with fermented plant meal enhances growth, antioxidant capacity and expression of TOR signaling pathway genes in gibel carp (Carassius auratus gibelio var. CAS V). Aquacult. Rep. 2021, 19, 100559. [Google Scholar] [CrossRef]
- Scalise, M.; Pochini, L.; Console, L.; Losso, M.A.; Indiveri, C. The human SLC1A5 (ASCT2) amino acid transporter: From function to structure and role in cell biology. Front. Cell Dev. Biol. 2018, 6, 96. [Google Scholar] [CrossRef]
- Yang, C.; Yang, X.; Fan, M.Z. Apical Na+-dependent neutral amino acid exchanger ASCT2/ATB0 and mTOR-signaling components are expressed along the entire jejunal crypt–villus axis in young pigs fed a liquid milk replacer. Can. J. Anim. Sci. 2016, 97, 19–29. [Google Scholar] [CrossRef]
- Kanai, Y.; Clémençon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 2013, 34, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya-Tate, N.; Endou, H.; Kanai, Y. Cloning and functional characterization of a system ASC-like Na-dependent neutral amino acid transporter. J. Biol. Chem. 1996, 271, 14883–14890. [Google Scholar] [CrossRef]
- Ochiai, H.; Onda, K.; Ogihara, K.; Naya, Y.; Sugiyama, H.; Maruo, T. cDNA sequence and tissue distribution of canine Na-dependent neutral amino acid transporter 2 (ASCT 2). J. Vet. Med. Sci. 2012, 74, 1505. [Google Scholar] [CrossRef][Green Version]
- Pelster, B.; Egg, M. Hypoxia-inducible transcription factors in fish: Expression, function and interconnection with the circadian clock. J. Exp. Biol. 2018, 221, jeb163709. [Google Scholar] [CrossRef]
- Whitmore, D.; Foulkes, N.; Strähle, U.; Sassone-Corsi, P. Zebrafish Clock rhythmic expression reveals independent peripheral circadian oscillators. Nat. Neurosci. 1998, 1, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Tamai, T.K.; Young, L.C.; Whitmore, D. Light signaling to the zebrafish circadian clock by Cryptochrome 1a. Proc. Natl. Acad. Sci. USA 2007, 104, 14712–14717. [Google Scholar] [CrossRef] [PubMed]
- Frøland Steindal, I.A.; Whitmore, D. Circadian clocks in fish—What have we learned so far? Biology 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Ladeuix, B.; Golik, M.; Koeners, M.; Asher, G. Rhythmic oxygen levels reset circadian clocks through HIF1α. Cell Metab. 2017, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Oehme, M.; Grammes, F.; Takle, H.; Zambonino-Infante, J.-L.; Refstie, S.; Thomassen, M.S.; Rørvik, K.-A.; Terjesen, B.F. Dietary supplementation of glutamate and arginine to Atlantic salmon (Salmo salar L.) increases growth during the first autumn in sea. Aquaculture 2010, 310, 156–163. [Google Scholar] [CrossRef]
- Qu, F.; Liu, Z.; Hu, Y.; Zhao, Q.; Zhou, Y.; Liu, Z.; Zhong, L.; Lu, S.; Li, J. Effects of dietary glutamine supplementation on growth performance, antioxidant status and intestinal function in juvenile grass carp (Ctenopharyngodon idella). Aquacult. Nutr. 2019, 25, 609–621. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 2020, 52, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Li, L.; Li, M.; Yu, Z.; Wang, H.; Wu, L. The effects of substituting fish meal with soy protein concentrate on growth performance, antioxidant capacity and intestinal histology in juvenile golden crucian carp, Cyprinus carpio × Carassius auratus—ScienceDirect. Aquacult. Rep. 2020, 18, 100435. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Seiliez, I. Protein and amino acid nutrition and metabolism in fish: Current knowledge and future needs. Aquac. Res. 2010, 41, 322–332. [Google Scholar] [CrossRef]

| Primer Names | Sequence Direction (5′ → 3′) | Use |
|---|---|---|
| ASCT2-F | CATAAGTCATTTCCACGCTGC | CDS |
| ASCT2-R | GGAATCAGCGTGAATGAGGTA | CDS |
| RT-actin-F | CTGCCCACCAACGATCTGTCCC | qRT-PCR |
| RT-actin-R | CTTATTTAGCCCCGCCCCCTCT | qRT-PCR |
| RT-ASCT2-F | GAACACTGTGCTGTCTGGAGGTA | qRT-PCR |
| RT-ASCT2-R | CTTGGGTTGGTCGTTTGAATC | qRT-PCR |
| Group | Control | Glutamate | |||||
|---|---|---|---|---|---|---|---|
| 0.5% | 1.0% | 1.5% | 2.0% | 2.5% | 3.0% | ||
| Glutamate | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| Fish meal | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Soybean meal | 34 | 34 | 34 | 34 | 34 | 34 | 34 |
| Rapeseed meal | 23.6 | 23.6 | 23.6 | 23.6 | 23.6 | 23.6 | 23.6 |
| Wheat flour | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Fish oil | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Soybean oil | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Corn starch | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Choline chloride | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| Vitamin and mineral premix a | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Methionine | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| CMC | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Cellulose | 8.79 | 8.29 | 7.79 | 7.29 | 6.79 | 6.29 | 5.79 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Group | Control | Glutamine | |||||
|---|---|---|---|---|---|---|---|
| 0.5% | 1.0% | 1.5% | 2.0% | 2.5% | 3.0% | ||
| Glutamine | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| Fish meal | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Soybean meal | 34 | 34 | 34 | 34 | 34 | 34 | 34 |
| Rapeseed meal | 23.6 | 23.6 | 23.6 | 23.6 | 23.6 | 23.6 | 23.6 |
| Wheat flour | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Fish oil | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Soybean oil | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Corn starch | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Choline chloride | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| Vitamin and mineral premix a | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Methionine | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| CMC | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Cellulose | 8.79 | 8.29 | 7.79 | 7.29 | 6.79 | 6.29 | 5.79 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Ingredients (g/kg) | Diets of Protein Levels | |||||
|---|---|---|---|---|---|---|
| 26% | 29% | 32% | 35% | 38% | 41% | |
| Fish meal | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Soybean meal | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Rapeseed meal | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Casein | 0.00 | 3.20 | 6.50 | 9.80 | 13.10 | 16.40 |
| Fish oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Soybean oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Corn starch | 25.00 | 21.00 | 16.80 | 12.60 | 8.40 | 4.20 |
| Wheat flour | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin and mineral premix a | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| CMC | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Methyl cellulose | 5.50 | 6.30 | 7.20 | 8.10 | 9.00 | 9.00 |
| Ingredients (g/kg) | Fish Meal Group | Soybean Meal Group |
|---|---|---|
| Fish meal | 44.40 | 0.00 |
| Soybean meal | 0.00 | 37.10 |
| Rapeseed meal | 0.00 | 15.00 |
| Casein | 0.00 | 6.50 |
| Fish oil | 1.63 | 3.50 |
| Soybean oil | 1.63 | 3.50 |
| Corn starch | 31.00 | 10.00 |
| Wheat flour | 10.00 | 10.00 |
| Choline chloride | 0.50 | 0.50 |
| Vitamin and mineral premix a | 3.00 | 3.00 |
| CMC | 3.00 | 3.00 |
| Methyl cellulose | 4.80 | 7.90 |
| Nutrition level | ||
| Crude protein (%) | 32.05 | 32.03 |
| Crude lipid (%) | 8.05 | 8.06 |
| Gross energy (MJ/kg) | 18.06 | 18.00 |
| Groups | DGC(%) 1 | FCR 2 | FR (%BW/Day) 3 |
|---|---|---|---|
| Control | 0.65 ± 0.01 b | 4.50 ± 0.07 a | 1.26 ± 0.00 a |
| Glu 0.5% | 0.57 ± 0.07 b | 5.26 ± 0.62 a | 1.29 ± 0.02 ab |
| Glu 1.0% | 0.39 ± 0.03 a | 7.65 ± 0.60 b | 1.31 ± 0.01 ab |
| Glu 1.5% | 0.57 ± 0.04 b | 5.21 ± 0.34 a | 1.28 ± 0.01 ab |
| Glu 2.0% | 0.51 ± 0.05 ab | 5.86 ± 0.55 a | 1.29 ± 0.01 ab |
| Glu 2.5% | 0.57 ± 0.06 b | 5.26 ± 0.57 a | 1.28 ± 0.01 ab |
| Glu 3.0% | 0.52 ± 0.02 ab | 5.70 ± 0.19 a | 1.33 ± 0.04 b |
| Gln 0.5% | 0.46 ± 0.05 ab | 6.62 ± 0.74 ab | 1.31 ± 0.01 a |
| Gln 1.0% | 0.44 ± 0.05 a | 6.95 ± 0.75 ab | 1.30 ± 0.01 a |
| Gln 1.5% | 0.45 ± 0.08 a | 6.95 ± 1.06 ab | 1.30 ± 0.02 a |
| Gln 2.0% | 0.48 ± 0.06 ab | 6.34 ± 0.74 ab | 1.29 ± 0.01 a |
| Gln 2.5% | 0.39 ± 0.04 a | 7.92 ± 0.95 b | 1.31 ± 0.01 a |
| Gln 3.0% | 0.46 ± 0.02 ab | 6.54 ± 0.24 ab | 1.25 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Luo, S.; Zhao, D.; Zhou, X.; Zhang, Z.; Xiao, Y.; Cao, S.; Zhou, Y.; Liu, S.; Tang, J.; et al. Molecular Characterization and Nutrition Regulation of the Neutral Amino Acid Transporter ASCT2 in Triploid Crucian Carp. Fishes 2021, 6, 77. https://doi.org/10.3390/fishes6040077
Mao Z, Luo S, Zhao D, Zhou X, Zhang Z, Xiao Y, Cao S, Zhou Y, Liu S, Tang J, et al. Molecular Characterization and Nutrition Regulation of the Neutral Amino Acid Transporter ASCT2 in Triploid Crucian Carp. Fishes. 2021; 6(4):77. https://doi.org/10.3390/fishes6040077
Chicago/Turabian StyleMao, Zhuangwen, Shengwei Luo, Dafang Zhao, Xiang Zhou, Zilong Zhang, Yangbo Xiao, Shenping Cao, Yonghua Zhou, Shaojun Liu, Jianzhou Tang, and et al. 2021. "Molecular Characterization and Nutrition Regulation of the Neutral Amino Acid Transporter ASCT2 in Triploid Crucian Carp" Fishes 6, no. 4: 77. https://doi.org/10.3390/fishes6040077
APA StyleMao, Z., Luo, S., Zhao, D., Zhou, X., Zhang, Z., Xiao, Y., Cao, S., Zhou, Y., Liu, S., Tang, J., & Liu, Z. (2021). Molecular Characterization and Nutrition Regulation of the Neutral Amino Acid Transporter ASCT2 in Triploid Crucian Carp. Fishes, 6(4), 77. https://doi.org/10.3390/fishes6040077