Abstract
Bolbometopon muricatum (bumphead parrotfish, Valenciennes, 1839) is a conspicuous, iconic and ecologically important coral reef fish species. B. muricatum plays an important role in the bioerosion of the reef framework and as a result has been described as both an ecosystem engineer and keystone species. Despite the complete absence of B. muricatum from 32 years of scientific surveys across the Ningaloo Reef World Heritage Area, we recorded a total of 155 individuals of B. muricatum across 63.2 ha of reef crest surveys, equating to mean density of 2.38 ind/ha. Our observations represent the first record of this iconic species in scientific surveys at Ningaloo and in combination with qualitative observations of B. muricatum by expert witnesses, indicate B. muricatum is likely to have been present in ecologically relevant densities since 2006. The densities of B. muricatum observed at northern Ningaloo in 2021 suggest this species is removing an estimated 13.42 tonnes/ha or 1.34 kg/m2 of calcium carbonate per year, which is broadly comparable with estimates of total parrotfish bioerosion across many reefs in the central Indian and Pacific Oceans. Although not currently afforded elevated conservation status within management plans, B. muricatum possess many life-history characteristics that make them vulnerable to overfishing and may justify consideration for increased protection within the world heritage listed Ningaloo Reef Marine Park.
1. Introduction
Species that create, maintain or modify habitats perform a fundamental role in shaping the physical structure of an area and the species these areas support [1]. Such ‘ecosystem engineers’ change the environment via their own physical structures, i.e., their living and dead tissues, or by transforming living or non-living materials from one physical state to another. In some cases, these species may alter the availability of energy, food, water, or sunlight available to other species, having disproportionately large effects on overall community structure and function [2]. Examples of marine ecosystem engineers include corals, mussels, and crustose coralline algae which increase biomass and structural complexity of habitats, seagrasses and kelps which alter water flows, entrain larvae and provide refuge from predation, and urchins and fish that can cause extensive bioerosion of benthic substrata. Ecosystem engineers are generally considered disproportionately important relative to the rest of the community [3], often receiving elevated conservation status [4,5,6,7]. However, the impact of ecosystem engineer species depends largely upon the scale of their actions, which can be spatially and temporally variable. Understanding and managing the impact of ecosystem engineer species therefore requires detailed knowledge of their distribution, abundance and impact.
Bolbometopon muricatum (bumphead parrotfish, Valenciennes, 1839) is a conspicuous, iconic and ecologically important coral reef fish species. B. muricatum play an ecologically important role in the bioerosion of the reef framework and have previously been described as both ecosystem engineers [8] and keystone species [9]. Using their large beak-like jaws to feed on reef substrata, large individuals (>100 cm total length; TL) can remove an estimated 5.5 tonnes of reef carbonates annually, of which approximately 50% is live coral [9]. On the Great Barrier Reef, it is estimated that bioerosion due to B. muricatum accounts for ~85% of bioerosion by parrotfish [10], which is broadly equivalent to the estimated maximum rate of coral reef accretion. Importantly, there are no functionally equivalent species to B. muricatum as the three next largest species of parrotfish (i.e., Chlorurus microrhinos, C. strongylocephalus and C. gibbus) remove an estimated maximum of 1.015 tonnes of carbonates per individual per year, of which only ~5% is live coral [11]. Importantly, in areas where the abundance of large B. muricatum has been removed through fishing, the function of fish bioerosion is essentially lost as smaller species are unable to compensate for the loss of the larger B. muricatum [12].
Globally, there have been large population declines in B. muricatum throughout much of their range. B. muricatum live up to 40 years and attain sexual maturity between 7 and 11 years of age (40–70 cm TL) [13,14,15], approximately twice the length and age of the next largest parrotfish Chlorurus microrhinos (70 cm TL and 17 years age; [13,14]). With a habit of sleeping in large groups (>60 individuals) in shallow, sheltered reef locations at night, B. muricatum are easily harvested by spearfishers [8,16,17], making them particularly susceptible to overfishing [16,18,19]. In Pacific locations where spearfishing pressure is high, B. muricatum populations may have already declined to levels where populations are unsustainable and ecologically irrelevant [9,12]. These declines have invoked total fishing bans in some Pacific nations (i.e., Palau) to try and re-establish populations and safeguard reef resilience [20].
Ningaloo Reef on the western Australian coastline (22° S, 113° E) is a fringing reef almost 300 km in length with exceptional cultural, ecological and tourism value. The area was included on UNESCO’s World Heritage List in 2011 (Ningaloo Coast World Heritage Area: NCWHA), in part due to recognition of the globally important annual aggregations of whale sharks and the high abundances of manta rays and sharks (http://whc.unesco.org/en/decisions/4278; accessed on 20 July 2021). Interactions with these iconic species are tightly regulated [21,22] and associated ecotourism activities generate over USD 20 million annually for the region [23]. However, these iconic species are not considered to be ecosystem engineers or keystone species, nor do they provide services which increase ecological resilience per se (i.e., capacity to absorb disturbances and respond to change while essentially retaining the same function) [24]. Despite recent sightings of juvenile B. muricatum at Ningaloo Reef [25], there remain no empirical data on their current distribution and/or abundance.
Here, we assess the abundance, density and size structure of B. muricatum within the world heritage listed Ningaloo Reef, western Australia. We use data obtained from three sources to assess the spatiotemporal distribution and size structure of B. muricatum populations. The three data sources include: (1) historical surveys of the abundance and density of reef fish assemblages at northern Ningaloo Reef (1987–2019); (2) qualitative observations of B. muricatum at northern Ningaloo by expert witnesses (2006–2020); and, (3) targeted timed swim surveys of B. muricatum abundance and size at northern Ningaloo (2021). We discuss the implications of our findings for the ongoing management of this iconic engineer within the world heritage listed Ningaloo Reef.
2. Materials and Methods
2.1. Study Location
Our study was focused along the western section of Ningaloo Reef (north-western Australia) along approximately 60 km of coastline (Figure 1). Ningaloo Reef is a fringing reef with well-developed reef slope, reef flat and lagoon reef zones. The reef crest is typically 0.2–1.0 km from the shore with passages (0.4–1.0 km wide; 2–5 m depth) from the reef slope to lagoon occurring at regular intervals (approximately 10–15 km) along the length of the reef. Fish movement among reef zones is largely unrestricted over the full tidal range, with the exception of shallow reef flat areas, which are inaccessible to larger fish (>50 cm TL) during spring low tides (<0.3 m deep: 20–24 h per month). Live coral cover ranges from 15–25% on the reef slope, 25–90% on the reef flat and back reef and 0–5% in the lagoon and within the reef passages [26], with Acroporidae and Poritidae corals dominating reef slope and flat zones [27]. Ningaloo Reef is relatively isolated from large human populations, with the nearest major town, Exmouth, having a population of less than 2500. As such, fishing pressure is assumed to be limited, though visitation to the area can be high [22]. Spearfishing has not been permitted on the reef slope at northern Ningaloo Reef since 2005; however, recreational line fishing is currently permitted within most zones, excluding sanctuary areas (Figure 1a). No major commercial fishing activities have occurred within the study area since the 1970s [22].
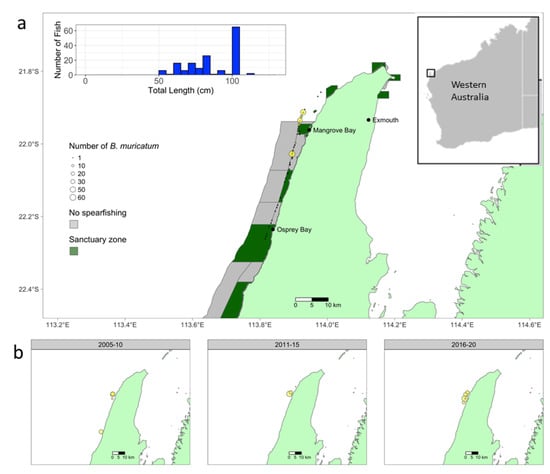
Figure 1.
(a). Number and size frequency of B. muricatum observed during timed swim surveys along the reef crest of Ningaloo Reef between February and May (yellow dots) 2021. Black dots = survey locations, grey regions = no spearfishing zones, green regions = sanctuary zones. (b) Number of B. muricatum observed by expert witnesses (yellow dots) between 2005–2010, 2011–2015 and 2016–2020 showing the majority of observations were located within 15 km of Mangrove Bay.
2.2. Historic Reef Fish Surveys (1987–2019)
Data from major research and monitoring programs surveying fish at northern Ningaloo Reef over the last 32 years (1987–2019) were examined for records of B. muricatum (Table S1). All major tropical marine fishes field guides and online databases were also searched for records of B. muricatum. Historical reef fish surveys encompassed three survey methods: Underwater Visual Census (UVC: 1987–2019), Baited Remote Underwater stereo-Video (BRUV:2000–2015) and Diver Operated stereo-Video (DOV:2006–2016) (see Table S1) [28,29,30]. Historical surveys were conducted in four of the five major reef zones at Ningaloo (lagoon, back reef, reef flat, reef slope) ranging from 3 m to 20 m water depth. No historical surveys were conducted within the reef crest zone. Detailed summaries of the time and/or area surveyed within each zone and survey methods are provided in Table 1.

Table 1.
Major research and monitoring programs surveying fish at northern Ningaloo Reef (Cloates to Jurabi; 1987–2019) showing the time (hours) for BRUV surveys, area (ha) for other surveys and number of B. muricatum observed across the four major habitats. For a complete list of surveys refer to Tables S1 and S2.
2.3. Qualitative Observations of B. muricatum at Northern Ningaloo (2006–2020) Solicited from Expert Witnesses
Data on the presence and size of B. muricatum observed at Ningaloo Reef between 2006 and 2020 were solicited from expert witnesses. Using local community facebook pages (Exmouth, Coral Bay, Ningaloo Whale Shark operators), members were asked to confirm sightings of B. muricatum on Ningaloo Reef. Specifically, we asked the following four questions: “have you have seen B. muricatum (humphead parrotfish) at northern Ningaloo?”; “when have you seen B. muricatum at northern Ningaloo?”; “where have you seen B. muricatum at northern Ningaloo (coordinates or reef zone)?”; and, “how many B. muricatum did you observe and what was their approximate length?”. If group members responded positively to any of these four questions, they were contacted via phone or email and provided an opportunity to validate their responses and expertise identifying coral reef fish species by answering two further questions: “how confident are you in your response to the previous questions (0–100%)?”; and, “how many years’ experience do you have in identifying coral reef fish species (<5 years, 5–10 years, >10 years)?”. Responses were included if respondents were >80% confident in their response and had more than 5 years’ experience identifying coral reef fish species. Known experts in surveying fish communities at Ningaloo Reef were also contacted directly and asked the same questions with results anonymised and summarised for each habitat (see Table S3 for details).
2.4. Observations of B. muricatum at Northern Ningaloo (2021) from Reef Crest Timed UVC Surveys
Data on B. muricatum abundance, distribution and size on the reef crest (2–4 m depth) were obtained using a series of 20-min timed swim surveys between Osprey and Jurabi at northern Ningaloo between February and May 2021 (henceforth called timed UVC surveys). B. muricatum are largely restricted to shallow exposed habitats (reef crest and reef flat); [9,12]) so surveys were targeted within the reef crest (2–4 m depth) on calm days (<5 knots, <1.5 m swell) when conditions allowed safe access to the surf-zone. Two snorkelers separated by approximately 15 m swam parallel to the reef crest in the direction of the prevailing current for 20 min, recording the number and estimated length of each B. muricatum within a 10 m wide belt. Forty-four sites were surveyed with a minimum distance between adjacent surveys of approximately 100 m. The start and end points of each survey were recorded using handheld GPS (Garmin eTrex), and the distance swum (metres) and the total survey area (ha) were calculated for each survey (Table S2). Prior to conducting surveys, observers were trained to identify B. muricatum using region-specific field guides [25] and estimate length using calibration models.
3. Results
3.1. Historic Reef Fish Surveys (1987–2019)
No previous records of B. muricatum were recorded in monitoring programs surveying fish at northern Ningaloo Reef over the last 32 years (1987–2019). Three records of B. muricatum were recorded in tropical marine fishes field guides between 2011 and 2020. Detailed summaries of the time and/or area surveyed within each habitat and survey methods are provided in Table 1 and Table S1.
3.2. Qualitative Observations of B. muricatum at Northern Ningaloo (2006–2020) Solicited from Expert Witnesses
A total of 27 observations of B. muricatum between 2007 and 2020 were solicited from expert witnesses using social media (Figure 1b, Table S3). The frequency of observations increased between 2005 and 2020 with 10 reported sightings between 2005 and 2015 and seventeen reported sightings between 2016 and 2020 (Figure 1b). The mean size of fish in sightings was 80 cm (range = 6–100 cm) and the majority of sightings occurred on the reef flat or reef crest within 15 km of Mangrove Bay (Figure 1b).
3.3. Observations of B. muricatum at Northern Ningaloo (2021) from Reef Crest Timed UVC Surveys
A total of 155 individuals of B. muricatum were recorded across 63.2 ha of reef crest surveys in 2021, equating to mean density of 2.38 ind/ha (Figure 1a). This is in contrast to their complete absence from 32 years of historical scientific surveys. Bolbometopon muricatum distributions at northern Ningaloo were highly spatially restricted with individuals located exclusively in reef crest habitats adjacent (within 10 km) to Mangrove Bay (Figure 1). Maximum densities of B. muricatum (42.8 ind/ha) were observed on the reef crest within 10 km of Mangrove Bay, where encounter rates were also high (Figure 1a: B. muricatum observed on 6 of the total 20 transects adjacent Mangrove Bay). The mean TL length of individuals was 80 cm (±1.6 cm SE) and the range 50–110 cm, with only 7 of the 155 individuals less than 60 cm TL (Figure 1a).
4. Discussion
Bolbometopon muricatum were recorded in moderate densities across 63.2 ha of reef crest surveys in 2021, equating to mean density of 2.38 ind/ha (range 0.0–42.8 ind/ha). Their absence from 32 years of historical scientific surveys is likely due to the lack of surveys in the surf/reef crest zone and the sampling biases of historical survey methods (e.g., DOV, BRUV). Regular sightings of B. muricatum (up to 100 individuals and 120 cm TL) by expert witnesses between 2007 to 2020, in combination with the large size of individuals observed during 2021 surveys (mean ± SE = 80.9 ± 1.2 cm, range = 50–110 cm), indicates B. muricatum are unlikely to have recently arrived at northern Ningaloo and may have been present for many years. Furthermore, increased sightings of B. muricatum by expert witnesses between 2005 and 2020 could suggest their abundance at northern Ningaloo may be increasing. Based on estimates of rates of calcium carbonate bioerosion by individual B. muricatum from the Great Barrier Reef (5.69 ± 0.53 tonnes/yr/individual: [10]), B. muricatum at northern Ningaloo are removing an estimated 13.42 tonnes/ha or 1.34 kg/m2 of calcium carbonate per year, which is broadly comparable with estimates of total parrotfish bioerosion at reefs in the central Indian (i.e., Seychelles, Chagos, Maldives, Mozambique [31]) and Pacific Oceans (Moorea, Pohnpei, Northern Sulawesi: [9]). Although we did not quantify feeding rates during this study, we observed B. muricatum feeding scars on live coral colonies during surveys in 2021 (authors pers. obs). B. muricatum regularly feeds on living corals (48.2% of bites on outer shelf GBR reefs; [10]), and at northern Ningaloo, is likely to be influencing live coral cover, vertical reef growth, as well as coral growth rates and colony shapes. Moreover, the redistribution of an estimated 1.34 kg/m2/yr of reef sediment through B. muricatum feeding and excretion at northern Ningaloo would have a marked impact on the deposition and accumulation of reef sediments. Adult B. muricatum are therefore currently present at northern Ningaloo at densities that are likely to be ecologically relevant.
Bolbometopon muricatum distributions at northern Ningaloo were spatially restricted with individuals observed exclusively in reef crest zones. Moreover, B. muricatum was spatially aggregated across just 15 km/26.7 ha of reef crest zone and maximum densities (42.8 ind/ha) were observed within 10 km of Mangrove Bay. Within this area, encounter rates were also high (Figure 1: B. muricatum observed on six of the total 20 transects adjacent to Mangrove Bay) during timed swims and among expert witnesses. The reason for this spatial concentration is not known but may relate to proximity of habitats suitable for juvenile B. muricatum and/or adult resting sites. B. muricatum typically settle to branching corals in sheltered lagoons adjacent to mangroves [32], before moving to high energy forereef foraging habitat as adults [12]. Adults are also known to seek shelter in deep inter-reef passages at night [33,34]. Reef crest zones at northern Mangrove Bay are currently characterised by moderate live coral cover (mean ± SE = 19% ± 5.8%: [27]) and close proximity to the only mangroves stands at northern Ningaloo. It is therefore possible that the highly restricted spatial extent of observations of B. muricatum observed in this study are due to the co-location of ideal habitats for feeding and recruitment, although we are unable to rule other factors such as temperature (see Table S4 for additional factors considered).
The size frequency structure of B. muricatum observed at northern Ningaloo indicates all individuals were likely to be reproductively mature adults (>40 cm) [32]. Of the 155 individuals observed, the mean TL was 80.9 cm (±1.6 cm SE) and the range 50–110 cm, with only seven of the 155 individuals less than 60 cm TL (Figure 1a). Unlike most parrotfishes, B. muricatum are gonochoric (single sex), with females reaching sexual maturity at about 55–65 cm TL and 7–9 years of age [32]. The lack of juvenile sized B. muricatum (<40 cm TL) at northern Ningaloo is consistent with other studies on the GBR where Bellwood and Choat reported just four juveniles (<35 cm TL) among 664 individuals recorded during >5000 h of surveys [33]. Noteworthy records of juvenile B. muricatum at Ningaloo Reef include those by Denise Jenkins in June 2011 (a single juvenile, 6 cm, 5 km north Mangrove Bay) [25] and Glen Whisson in August 2018 and May 2020 (three juveniles ranging 6–10 cm, 120 km south Mangrove Bay) (iNaturalist.org 2020). Both of these photographic records indicate that juvenile B. muricatum do occur at Ningaloo Reef and these juveniles may be recruiting from the populations of reproductively mature local adults observed during the present study.
The lack of B. muricatum from historical scientific fish surveys may relate to the methods used being inappropriate for highly mobile, sparsely distributed reef taxa. Alternatively, it may reflect the poleward expansion of this species along the western Australian coast as has been documented for other tropical reef fish species [35]. B. muricatum are highly gregarious, feeding and sleeping in groups of up to 100 individuals [36] with spawning aggregations of up to 1200 individuals [34]. Schools can move up to 6 km per day [32] but they are often sparsely distributed, making them difficult to detect using certain survey techniques [37]. For example, traditional UVC and DOV techniques generally survey small areas (<1000 m2 per survey; [38]) and as a result, often fail to detect large, highly mobile and/or sparsely distributed fish species. Improved density estimates of sparsely distributed taxa usually require sampling over larger scales (>1000 m2). The exclusive observations of B. muricatum on the reef crest during our timed swims (>11,000 m2 per survey) suggests B. muricatum, like many populations of large-bodied, sparsely distributed reef fishes, are most reliably detected using spatially comprehensive survey techniques conducted within targeted habitats.
5. Conclusions
A total of 155 B. muricatum were recorded across only 63.2 ha of reef crest surveys, with densities highest in the surf zone of reef crest habitats adjacent to Mangrove Bay. By soliciting expert witness observations and combining these with timed swim surveys conducted in the preferred habitats of B. muricatum, we were able to provide the first estimate of the distribution, density and size of B. muricatum at northern Ningaloo. To our knowledge, these observations represent the first records of adult B. muricatum in scientific surveys at Ningaloo Reef. The densities of B. muricatum observed at northern Ningaloo in 2021 suggest this species is removing an estimated 13.42 tonnes/ha or 1.34 kg/m2 of calcium carbonate per year, which is broadly comparable with estimates of total parrotfish bioerosion across many reefs in the central Indian and Pacific Oceans. Regular sightings of adult B. muricatum by expert witnesses between 2007 to 2020, in combination with the large size of individuals observed during 2021 surveys, indicates B. muricatum may have been present at northern Ningaloo Reef for many years. Although not currently afforded elevated conservation status within current management plans, B. muricatum possess many life-history characteristics which make them vulnerable to overfishing that may justify consideration for species specific protection within the world heritage listed Ningaloo Reef Marine Park.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/fishes6040073/s1, Table S1: Summary of historical scientific surveys of fish assemblages at Ningaloo Reef (1987–2019), Table S2: Summary data from February and May 2021 timed-swim surveys, Table S3: Reported sightings of Bolbometapon muricatum by expert witnesses between 2007 and 2020 at Ningaloo obtained via social media, Table S4: Assessment of limiting factors on the distribution and abundance of Bolbometapon muricatum at northern Ningaloo.
Author Contributions
D.P.T.: conceptualisation, methodology, investigation, writing—original draft, writing—review and editing, funding acquisition. A.K.C.: methodology, investigation, writing—review and editing, C.D.: methodology, investigation, writing—review and editing, M.D.E.H.: methodology, investigation, writing—review and editing, visualisation, M.O.: methodology, investigation, writing—review, A.S.H.: methodology, investigation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ningaloo Outlook Marine Research Partnership. Ningaloo Outlook is a BHP-CSIRO Industry-Science Marine Research Partnership investing AUD $12.4 million over 10 years to gather new knowledge on the Ningaloo Reef and its important ecological values. BHP had no involvement in the preparation of data, manuscript or the decision to submit for publication.
Institutional Review Board Statement
This work was conducted under CSIRO ethics clearance 128/21 Ningaloo Outlook. Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting results is provided in the Supplementary Materials.
Acknowledgments
We thank staff from the Department of Biodiversity, Conservation and Attractions for providing access to historical fish datasets, assisting with social media posts and discussions about B. muricatum. We thank Brett Molony and Shaun Wilson for helping to improve the manuscript and John (Howard) Choat and Ben Fitzpatrick for providing insights into B. muricatum more broadly. We also thank Andrea Asunsolo, Beau DeGroot, Emma Westlake, Natalie Travaglione and Nicholas Gust for field assistance and the expert witnesses who provided information on sightings of B. muricatum. We acknowledge the Jinigudira people, the traditional custodians of the land and sea country on which this study was conducted.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as Ecosystem Engineers. In Ecosystem Management: Selected Readings; Springer: New York, NY, USA, 1996; pp. 130–147. [Google Scholar] [CrossRef]
- Coleman, F.C.; Williams, S.L. Overexploiting marine ecosystem engineers: Potential consequences for biodiversity. Trends Ecol. Evol. 2002, 17, 40–44. [Google Scholar] [CrossRef]
- Pogoda, B.; Brown, J.; Hancock, B.; Preston, J.; Pouvreau, S.; Kamermans, P.; Sanderson, W.; von Nordheim, H. The Native Oyster Restoration Alliance (NORA) and the Berlin Oyster Recommendation: Bringing back a key ecosystem engineer by developing and supporting best practice in Europe. Aquat. Living Resour. 2019, 32, 13. [Google Scholar] [CrossRef] [Green Version]
- Beger, M.; Babcock, R.; Booth, D.J.; Bucher, D.; Condie, S.A.; Creese, B.; Cvitanovic, C.; Dalton, S.J.; Harrison, P.; Hoey, A. Research challenges to improve the management and conservation of subtropical reefs to tackle climate change threats: (Findings of a workshop conducted in Coffs Harbour, Australia on 13 September 2010). Ecol. Manag. Restor. 2011, 12, e7–e10. [Google Scholar] [CrossRef]
- Darling, E.S.; McClanahan, T.R.; Maina, J.; Gurney, G.G.; Graham, N.A.J.; Januchowski-Hartley, F.; Cinner, J.E.; Mora, C.; Hicks, C.C.; Maire, E.; et al. Social–environmental drivers inform strategic management of coral reefs in the Anthropocene. Nat. Ecol. Evol. 2019, 3, 1341–1350. [Google Scholar] [CrossRef]
- Jenkins, A.P.; Jupiter, S.D.; Qauqau, I.; Atherton, J. The importance of ecosystem-based management for conserving aquatic migratory pathways on tropical high islands: A case study from Fiji. Aquat. Conserv. -Mar. Freshw. Ecosyst. 2010, 20, 224–238. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Graham, N.A.; MacNeil, M.A.; Muthiga, N.A.; Cinner, J.E.; Bruggemann, J.H.; Wilson, S.K. Critical thresholds and tangible targets for ecosystem-based management of coral reef fisheries. Proc. Natl. Acad. Sci. USA 2011, 108, 17230–17233. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, R.J.; Almany, G.R.; Stevens, D.; Bode, M.; Pita, J.; Peterson, N.A.; Choat, J.H. Hyperstability masks declines in bumphead parrotfish (Bolbometopon muricatum) populations. Coral Reefs 2016, 35, 751–763. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hoey, A.S.; Choat, J.H. Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol. Lett. 2003, 6, 281–285. [Google Scholar] [CrossRef]
- Hoey, A.; Bellwood, D. Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 2008, 27, 37–47. [Google Scholar] [CrossRef]
- Bonaldo, R.M.; Hoey, A.S.; Bellwood, D.R. The ecosystem roles of parrotfishes on tropical reefs. Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 81–132. [Google Scholar]
- Bellwood, D.R.; Hoey, A.S.; Hughes, T.P. Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proc. R. Soc. B Biol. Sci. 2012, 279, 1621–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choat, J.H.; Robertson, D.R. Age-based studies. Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem; Academic Press: San Diego, CA, USA, 2002; pp. 57–80. [Google Scholar]
- Choat, J.; Axe, L.; Lou, D. Growth and longevity in fishes of the family Scaridae. Mar. Ecol. Prog. Ser. 1996, 145, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Choat, J.H.; Klanten, O.S.; Van Herwerden, L.; Robertson, D.R.; Clements, K.D. Patterns and processes in the evolutionary history of parrotfishes (Family Labridae). Biol. J. Linn. Soc. 2012, 107, 529–557. [Google Scholar] [CrossRef] [Green Version]
- Dulvy, N.K.; Polunin, N.V. Using informal knowledge to infer human-induced rarity of a conspicuous reef fish. Anim. Conserv. 2004, 7, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Johannes, R.E. Working with fishermen to improve coastal tropical fisheries and resource management. Bull. Mar. Sci. 1981, 31, 673–680. [Google Scholar]
- Donaldson, T.J.; Dulvy, N.K. Threatened fishes of the world: Bolbometopon muricatum (Valenciennes 1840)(Scaridae). Environ. Biol. Fishes 2004, 70, 373. [Google Scholar] [CrossRef]
- Kobayashi, D.R.; Friedlander, A.M.; Grimes, C.B.; Nichols, R.S.; Zgliczynski, B. Bumphead parrotfish (Bolbometopon muricatum) status review. In NOAA Technical Memorandum NMFS-PIFSC; Pacific Islands Fisheries Science Center: Honolulu, HI, USA, 2011. [Google Scholar]
- Polloi, K.; Golbuu, Y.; Merep, G.; Koshiba, S.; Friedlander, A.; Koike, H. An assessment of Maml and Kemedukl in Palau and management recommendations. A Report to The Nature Conservancy Micronesia Program Technical Report No. 14-07. In The Nature Conservancy Micronesia Program Technical Report; Palau International Coral Reef Center: Koror, Palau, 2014; p. 37. [Google Scholar]
- Environment Protection and Biodiversity Conservation Act 1999. No. 91, 1999, Volume 1: Sections 1–266; The Office of the Parliamentary Counsel: Canberra, Australia, 1999.
- Management plan for the Ningaloo Marine Park and Muiron Islands Marine Management Area 2005–2015. In Perth, Western Australia: Conservation and Land Management and Marine Parks and Reserves Authority; Government of Western Australia: Perth, Australia, 2005; p. 111.
- Deloitte Access Economics Pty Ltd. Economic contribution of Ningaloo: One of Australia’s best kept secrets. In Report Commissioned by DBCA; DBCA: Perth, Australia, 2020; p. 58. [Google Scholar]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Jenkins, D.; Morrison, S. Fishes of Ningaloo; Blue Ocean Publications: Exmouth, Australia, 2018. [Google Scholar]
- Vanderklift, M.A.; Babcock, R.C.; Barnes, P.B.; Cresswell, A.K.; Feng, M.; Haywood, M.D.; Holmes, T.H.; Lavery, P.S.; Pillans, R.D.; Smallwood, C.B. The Oceanography and Marine Ecology of Ningaloo, A World Heritage Area. In Oceanography and Marine Biology: An Annual Review; Hawkins, S.J., Allcock, A.L., Bates, A.E., Evans, A.J., Firth, L.B., McQuaid, C.D., Russel, B.D., Smith, I.P., Swearer, S.E., Todd, P.A., Eds.; Taylor & Francis: London, UK, 2020. [Google Scholar]
- Thomson, D.P.; Babcock, R.C.; Haywood, M.D.; Vanderklift, M.A.; Pillans, R.D.; Bessey, C.; Cresswell, A.; Orr, M.; Boschetti, F.; Wilson, S.K. Zone specific trends in coral cover, genera and growth-forms in the World-Heritage listed Ningaloo Reef. Mar. Environ. Res. 2020, 160, 105020. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, A.; Langlois, T.; Wilson, S.; Claudet, J.; Thomson, D.P.; Renton, M.; Fulton, C.J.; Fisher, R.; Vanderklift, M.; Babcock, R. Disentangling the response of fishes to recreational fishing over 30 years within a fringing coral reef reserve network. Biol. Conserv. 2019, 237, 514–524. [Google Scholar] [CrossRef]
- Langlois, T.J.; Harvey, E.S.; Fitzpatrick, B.; Meeuwig, J.J.; Shedrawi, G.; Watson, D.L. Cost-efficient sampling of fish assemblages: Comparison of baited video stations and diver video transects. Aquat. Biol. 2010, 9, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Murphy, H.M.; Jenkins, G.P. Observational methods used in marine spatial monitoring of fishes and associated habitats: A review. Mar. Freshw. Res. 2010, 61, 236–252. [Google Scholar] [CrossRef]
- Perry, C.T.; Alvarez-Filip, L.; Graham, N.A.; Mumby, P.J.; Wilson, S.K.; Kench, P.S.; Manzello, D.P.; Morgan, K.M.; Slangen, A.B.; Thomson, D.P. Loss of coral reef growth capacity to track future increases in sea level. Nature 2018, 558, 396–400. [Google Scholar] [CrossRef]
- Hamilton, R.; Adams, S.; Choat, J. Sexual development and reproductive demography of the green humphead parrotfish (Bolbometopon muricatum) in the Solomon Islands. Coral Reefs 2008, 27, 153–163. [Google Scholar] [CrossRef]
- Bellwood, D.; Choat, J. Dangerous demographics: The lack of juvenile humphead parrotfishes Bolbometopon muricatum on the Great Barrier Reef. Coral Reefs 2011, 30, 549–554. [Google Scholar] [CrossRef]
- Roff, G.; Doropoulos, C.; Mereb, G.; Mumby, P.J. Mass spawning aggregation of the giant bumphead parrotfish Bolbometopon muricatum. J. Fish Biol. 2017, 91, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.; Meeuwig, J.; Feng, M.; Harvey, E.; Lam, V.; Langlois, T.; Slawinski, D.; Sun, C.; Pauly, D. Climate-change induced tropicalisation of marine communities in Western Australia. Mar. Freshw. Res. 2012, 63, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Domeier, M.L.; Colin, P.L. Tropical reef fish spawning aggregations: Defined and reviewed. Bull. Mar. Sci. 1997, 60, 698–726. [Google Scholar]
- MacKenzie, D.I.; Royle, J.A.; Brown, J.A.; Nichols, J.D.; Thompson, W. Occupancy estimation and modeling for rare and elusive populations. In Sampling Rare or Elusive Species: Concepts, Designs, and Techniques for Estimating Population Parameters; Thompson, W.L., Ed.; Island Press: Washington, DC, USA, 2004; pp. 149–171. [Google Scholar]
- Colvocoresses, J.; Acosta, A. A large-scale field comparison of strip transect and stationary point count methods for conducting length-based underwater visual surveys of reef fish populations. Fish. Res. 2007, 85, 130–141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).