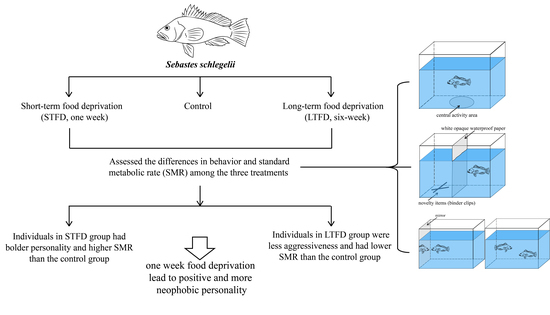
Effects of Food Deprivation Duration on the Behavior and Metabolism of Black Rockfish (Sebastes schlegelii)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Behavior and Activity Observation and Quantification
2.2.1. Indicators of Activity Ability during Rearing
2.2.2. Boldness Assessment
2.2.3. Neophobia Assessment
2.2.4. Aggressiveness Assessment
2.3. Measurement of Metabolic Rate
2.4. Data Analysis
3. Results
3.1. Effect of Food Deprivation on Locomotor Activity and Aggression during Rearing
3.2. Effect of Food Deprivation on Personalities after Rearing
3.2.1. Boldness
3.2.2. Neophobia
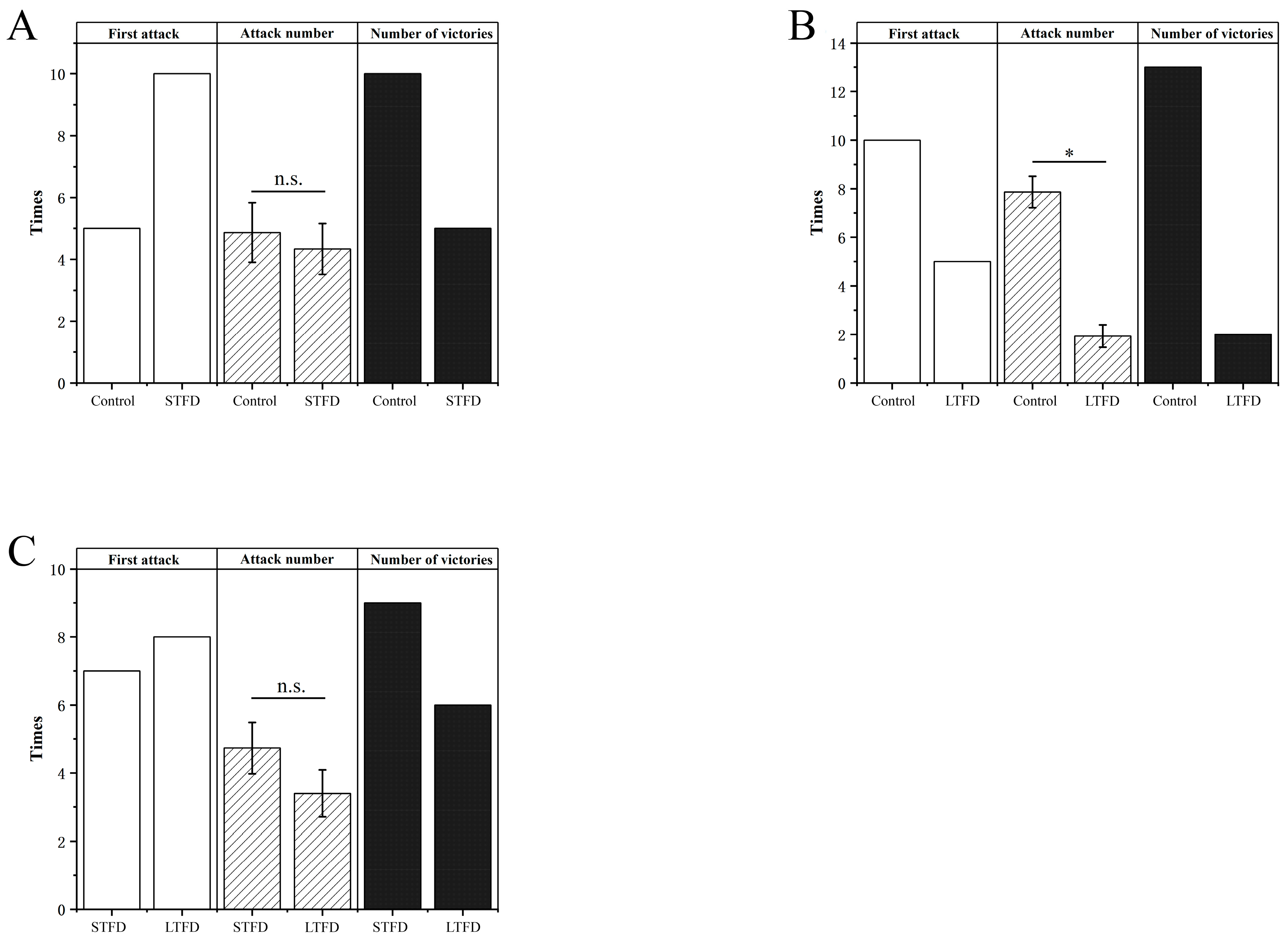
3.2.3. Aggressiveness
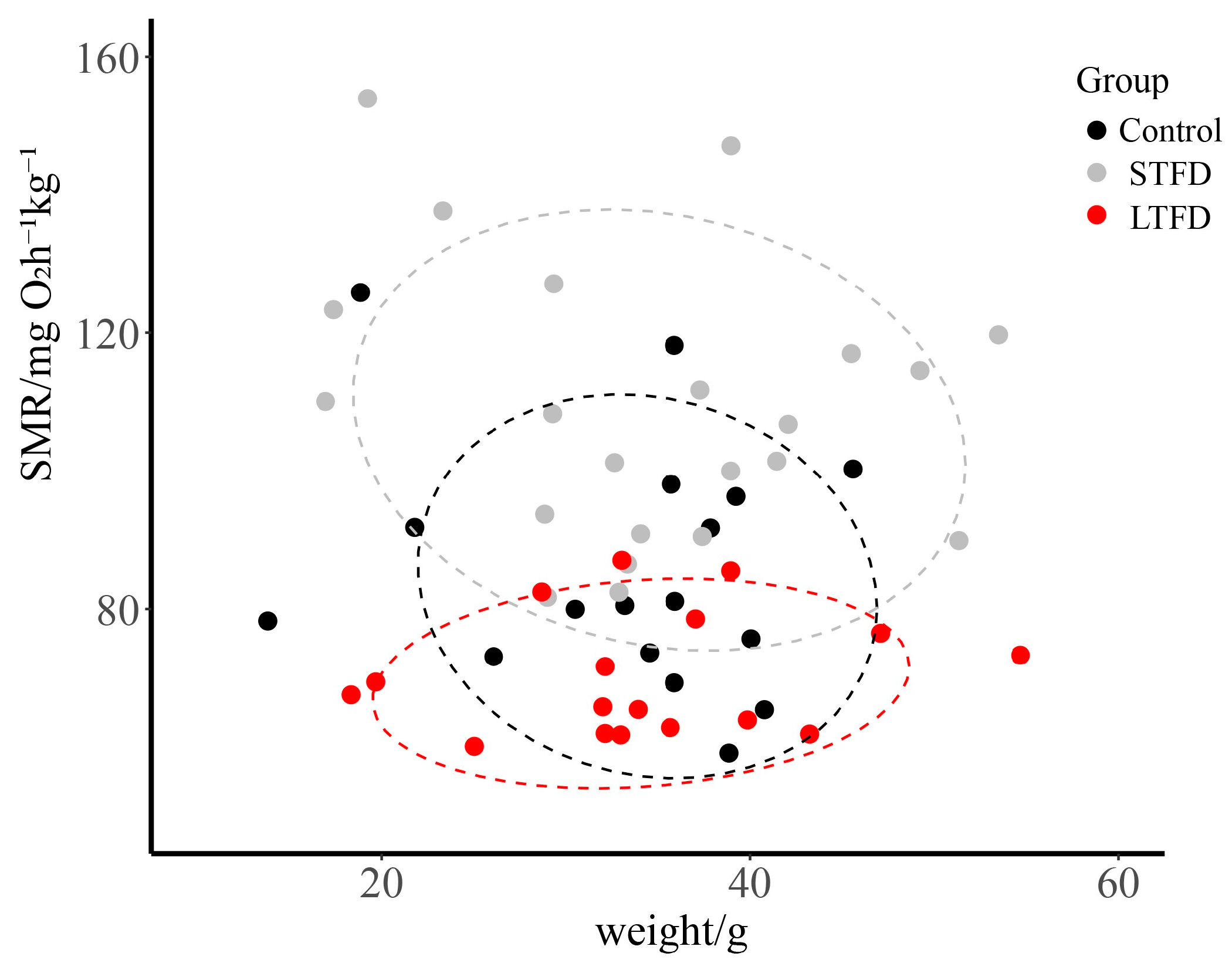
3.3. Effect of Food Deprivation on SMR
4. Discussion
4.1. Effects of Food Deprivation on Fish Personalities
4.2. Behavioral Mechanisms for the Personalities Effects of Food Deprivation
4.3. Metabolic Mechanisms for the Personality Effects of Food Deprivation
4.4. The Innovation and Deficiency of this Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Zhang, A.; Killen, S.S.; Cao, Z.; Wang, Y.; Fu, S. Standard metabolic rate predicts growth trajectory of juvenile crucian carp (Carassius auratus) under changing food availability. Biol. Open 2017, 6, 1305–1309. [Google Scholar] [CrossRef] [Green Version]
- Martin-Smith, K.M.; Armstrong, J.D. Growth Rates of Wild Stream-Dwelling Atlantic Salmon Correlate with Activity and Sex but Not Dominance. J. Anim. Ecol. 2002, 71, 413–423. [Google Scholar] [CrossRef]
- Powers, S.P.; Peterson, C.H.; Christian, R.R.; Sullivan, E.; Powers, M.J.; Bishop, M.J.; Buzzelli, C.P. Effects of eutrophication on bottom habitat and prey resources of demersal fishes. Mar. Ecol. Prog. Ser. 2005, 302, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Auer, S.K.; Salin, K.; Anderson, G.J.; Metcalfe, N.B. Flexibility in metabolic rate and activity level determines individual variation in overwinter performance. Oecologia 2016, 182, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Chapman, B.B.; Morrell, L.J.; Krause, J. Unpredictability in food supply during early life influences boldness in fish. Behav. Ecol. 2010, 21, 501–506. [Google Scholar] [CrossRef]
- Frommen, J.G.; Luz, C.; Bakker, T.C.M. Nutritional state influences shoaling preference for familiars. Zoology 2007, 110, 369–376. [Google Scholar] [CrossRef]
- Krause, J.; Hartmann, N.; Pritchard, V.L. The influence of nutritional state on shoal choice in zebrafish, Danio rerio. Anim. Behav. 1999, 57, 771–775. [Google Scholar] [CrossRef] [Green Version]
- Miki, T.; Nakatsukasa, H.; Takahashi, N.; Murata, O.; Ishibashi, Y. Aggressive behaviour and cannibalism in greater amberjack, Seriola dumerili: Effects of stocking density, feeding conditions and size differences. Aquac. Res. 2011, 42, 1339–1349. [Google Scholar] [CrossRef]
- Killen, S.S.; Fu, C.; Wu, Q.; Wang, Y.X.; Fu, S.J. The relationship between metabolic rate and sociability is altered by food deprivation. Funct. Ecol. 2015, 30, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Auer, S.K.; Salin, K.; Rudolf, A.M.; Anderson, G.J.; Metcalfe, N.B. The optimal combination of standard metabolic rate and aerobic scope for somatic growth depends on food availability. Funct. Ecol. 2015, 29, 479–486. [Google Scholar] [CrossRef]
- DeAngelis, D.L. Intraspecific trait variation and its effects on food chains. Math. Biosci. 2013, 244, 91–97. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef]
- Johnston, I.A. Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 2006, 209, 2249–2264. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A. Clever fish. Nature 2015, 521, 412–414. [Google Scholar] [CrossRef] [Green Version]
- Szopa-Comley, A.W.; Duffield, C.; Ramnarine, I.W.; Ioannou, C.C. Predatory behaviour as a personality trait in a wild fish population. Anim. Behav. 2020, 170, 51–64. [Google Scholar] [CrossRef]
- Woodward, M.; Winder, L.; Watt, P. Enrichment Increases Aggression in Zebrafish. Fishes 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Chuard, P.J.C.; Brown, G.E.; Grant, J.W.A. The effects of adult sex ratio on mating competition in male and female guppies (Poecilia reticulata) in two wild populations. Behav. Process 2016, 129, 1–10. [Google Scholar] [CrossRef]
- Manley, C.B.; Rakocinski, C.F.; Lee, P.G.; Blaylock, R.B. Stocking density effects on aggressive and cannibalistic behaviors in larval hatchery-reared spotted seatrout, Cynoscion nebulosus. Aquaculture 2014, 420–421, 89–94. [Google Scholar] [CrossRef]
- Biro, P.A.; Post, J.R. Rapid Depletion of Genotypes with Fast Growth and Bold Personality Traits from Harvested Fish Populations. Proc. Natl. Acad. Sci. USA 2008, 105, 2919–2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.; Burgess, F.; Braithwaite, V.A. Heritable and experiential effects on boldness in a tropical poeciliid. Behav. Ecol. Sociobiol. 2007, 62, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Chapman, B.B.; Ward, A.J.W.; Krause, J. Schooling and learning: Early social environment predicts social learning ability in the guppy, Poecilia reticulata. Anim. Behav. 2008, 76, 923–929. [Google Scholar] [CrossRef]
- Balon, E.K. Origin and domestication of the wild carp, Cyprinus carpio: From Roman gourmets to the swimming flowers. Aquaculture 1995, 129, 3–48. [Google Scholar] [CrossRef]
- Jolles, J.W.; Briggs, H.D.; Araya-Ajoy, Y.G.; Boogert, N.J. Personality, plasticity and predictability in sticklebacks: Bold fish are less plastic and more predictable than shy fish. Anim. Behav. 2019, 154, 193–202. [Google Scholar] [CrossRef]
- Bell, A.M.; Hankison, S.J.; Laskowski, K.L. The repeatability of behaviour: A meta-analysis. Anim. Behav. 2009, 77, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Careau, V.; Thomas, D.; Humphries, M.M.; Réale, D. Energy Metabolism and Animal Personality. Oikos 2008, 117, 641–653. [Google Scholar] [CrossRef]
- Chabot, D.; Steffensen, J.F.; Farrell, A.P. The determination of standard metabolic rate in fishes. J. Fish Biol. 2016, 88, 81–121. [Google Scholar] [CrossRef]
- Biro, P.A.; Stamps, J.A. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 2010, 25, 653–659. [Google Scholar] [CrossRef]
- Van Leeuwen, T.E.; Rosenfeld, J.S.; Richards, J.G. Effects of food ration on SMR: Influence of food consumption on individual variation in metabolic rate in juvenile coho salmon (Onchorhynchus kisutch). J. Anim. Ecol. 2012, 81, 395–402. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Guo, H.; Zhang, X. Effect of environmental enrichment on the stress response of juvenile black rockfish Sebastes schlegelii. Aquaculture 2021, 533, 736088. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Wang, Y.; Zhang, X. Effects of environmental enrichment on growth performance, aggressive behavior and stress-induced changes in cortisol release and neurogenesis of black rockfish Sebastes schlegelii. Aquaculture 2020, 528, 735483. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Q.; Xu, X.; Guo, H.; Zhang, X. Effects of environmental enrichment on the welfare of juvenile black rockfish Sebastes schlegelii: Growth, behavior and physiology. Aquaculture 2020, 518, 734782. [Google Scholar] [CrossRef]
- Gonçalves-de-Freitas, E.; Bolognesi, M.; Gauy, A.; Brandão, M.; Giaquinto, P.; Fernandes-Castilho, M. Social Behavior and Welfare in Nile Tilapia. Fishes 2019, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Sneddon, L.U. The bold and the shy: Individual differences in rainbow trout. J. Fish Biol. 2003, 62, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Sloan, W.D.; Clark, A.B.; Coleman, K.; Dearstyne, T. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 1994, 9, 442–446. [Google Scholar] [CrossRef]
- McCormick, M.I.; Chivers, D.P.; Allan, B.J.M.; Ferrari, M.C.O. Habitat degradation disrupts neophobia in juvenile coral reef fish. Glob. Chang. Biol. 2017, 23, 719–727. [Google Scholar] [CrossRef]
- Frost, A.J.; Winrow-Giffen, A.; Ashley, P.J.; Sneddon, L.U. Plasticity in animal personality traits: Does prior experience alter the degree of boldness? Proc. R. Soc. B Biol. Sci. 2007, 274, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Réale, D.; Dingemanse, N.J.; Kazem, A.J.N.; Wright, J. Evolutionary and ecological approaches to the study of personality. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3937–3946. [Google Scholar] [CrossRef] [Green Version]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. R. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; McCormick, M.I.; Meekan, M.G.; Chivers, D.P. Background level of risk and the survival of predator-naive prey: Can neophobia compensate for predator naivety in juvenile coral reef fishes? Proc. R. Soc. B Biol. Sci. 2015, 282, 20142197. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Guo, H.; Zhang, Z.; Wang, Y.; Qin, J.; Zhang, X. Impact of pre-aggressive experience on behavior and physiology of black rockfish (Sebastes schlegelii). Aquaculture 2021, 536, 736416. [Google Scholar] [CrossRef]
- Eisenreich, B.R.; Greene, S.; Szalda-Petree, A. Of fish and mirrors: Fluoxetine disrupts aggression and learning for social rewards. Physiol. Behav. 2017, 173, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Garland, T., Jr.; Else, P.L.; Hulbert, A.J.; Tap, P. Effects of endurance training and captivity on activity metabolism of lizards. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1987, 252, R450–R456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fu, Y.; Shen, F.; Zhang, Z.; Guo, H.; Zhang, X. Barren environment damages cognitive abilities in fish: Behavioral and transcriptome mechanisms. Sci. Total Environ. 2021, 794, 148805. [Google Scholar] [CrossRef]
- Miyazaki, T.; Masuda, R.; Furuta, S.; Tsukamoto, K. Feeding behaviour of hatchery-reared juveniles of the Japanese flounder following a period of starvation. Aquaculture 2000, 190, 129–138. [Google Scholar] [CrossRef]
- Poulsen, S.B.; Svendsen, J.C.; Jensen, L.F.; Schulz, C.; Jäger-Kleinicke, T.; Schwarten, H. Effects of food deprivation on refuge use and dispersal in juvenile North Sea houting Coregonus oxyrinchus under experimental conditions. J. Fish Biol. 2010, 77, 1702–1708. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, Q.; Fu, S. Inspection behaviour and inter-individual cooperation in juvenile qingbo: The effects of prior predator exposure and food deprivation. J. Ethol. 2018, 36, 181–190. [Google Scholar] [CrossRef]
- Burton, T.; Killen, S.S.; Armstrong, J.D.; Metcalfe, N.B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B Biol. Sci. 2011, 278, 3465–3473. [Google Scholar] [CrossRef] [Green Version]
- Wone, B.; Sears, M.W.; Labocha, M.K.; Donovan, E.R.; Hayes, J.P. Genetic variances and covariances of aerobic metabolic rates in laboratory mice. Proc. R. Soc. B Biol. Sci. 2009, 276, 3695–3704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez, D.; Nicieza, A.G. Is metabolic rate a reliable predictor of growth and survival of brown trout (Salmo trutta) in the wild? Can. J. Fish. Aquat. Sci. 2005, 62, 643–649. [Google Scholar] [CrossRef]
- Thünken, T.; Hesse, S.; Bakker, T.C.M.; Baldauf, S.A. Benefits of kin shoaling in a cichlid fish: Familiar and related juveniles show better growth. Behav. Ecol. 2016, 27, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Sogard, S.M.; Olla, B.L. The influence of hunger and predation risk on group cohesion in a pelagic fish, walleye pollock Theragra chalcogramma. Environ. Biol. Fish. 1997, 50, 405–413. [Google Scholar] [CrossRef]
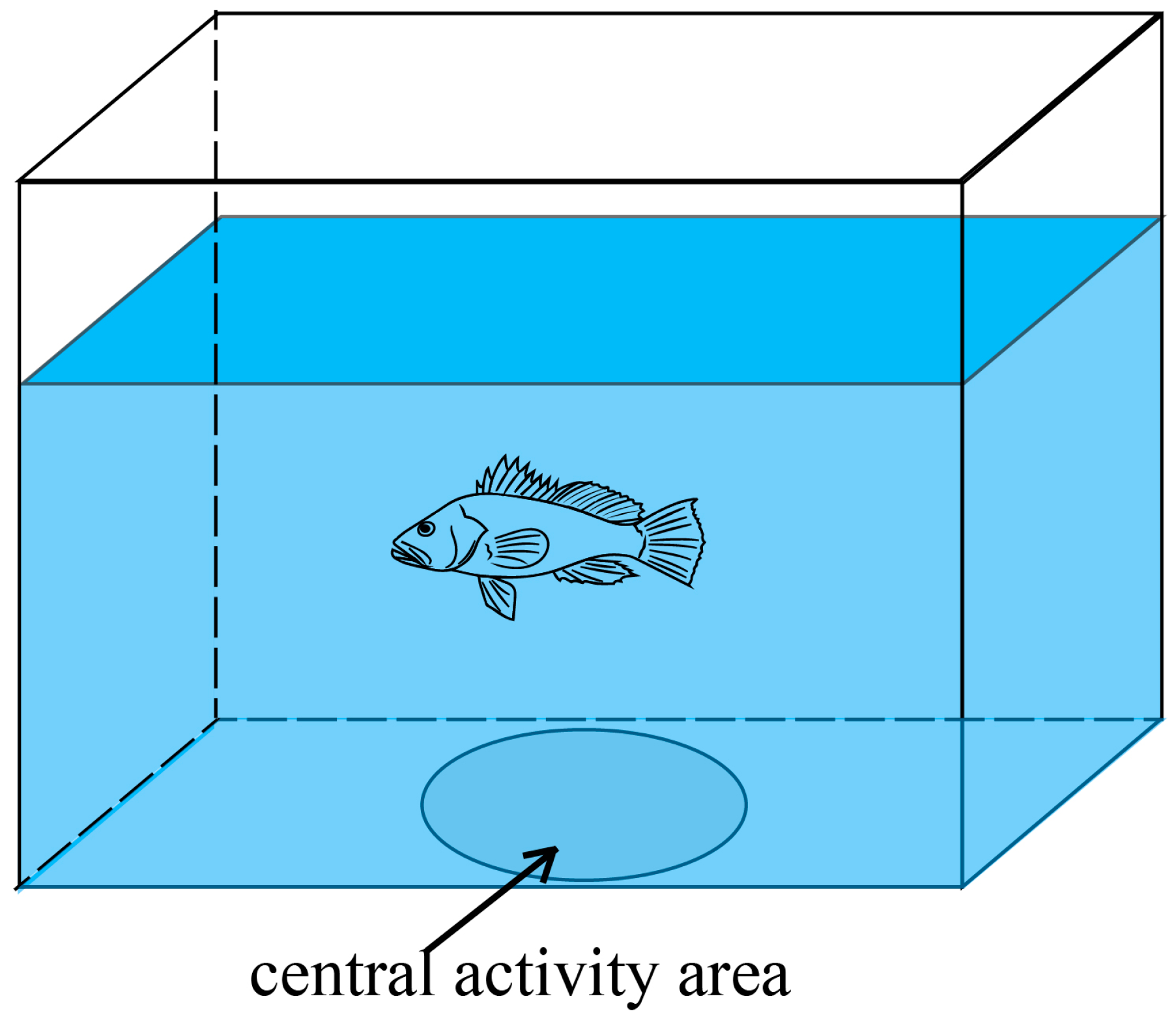
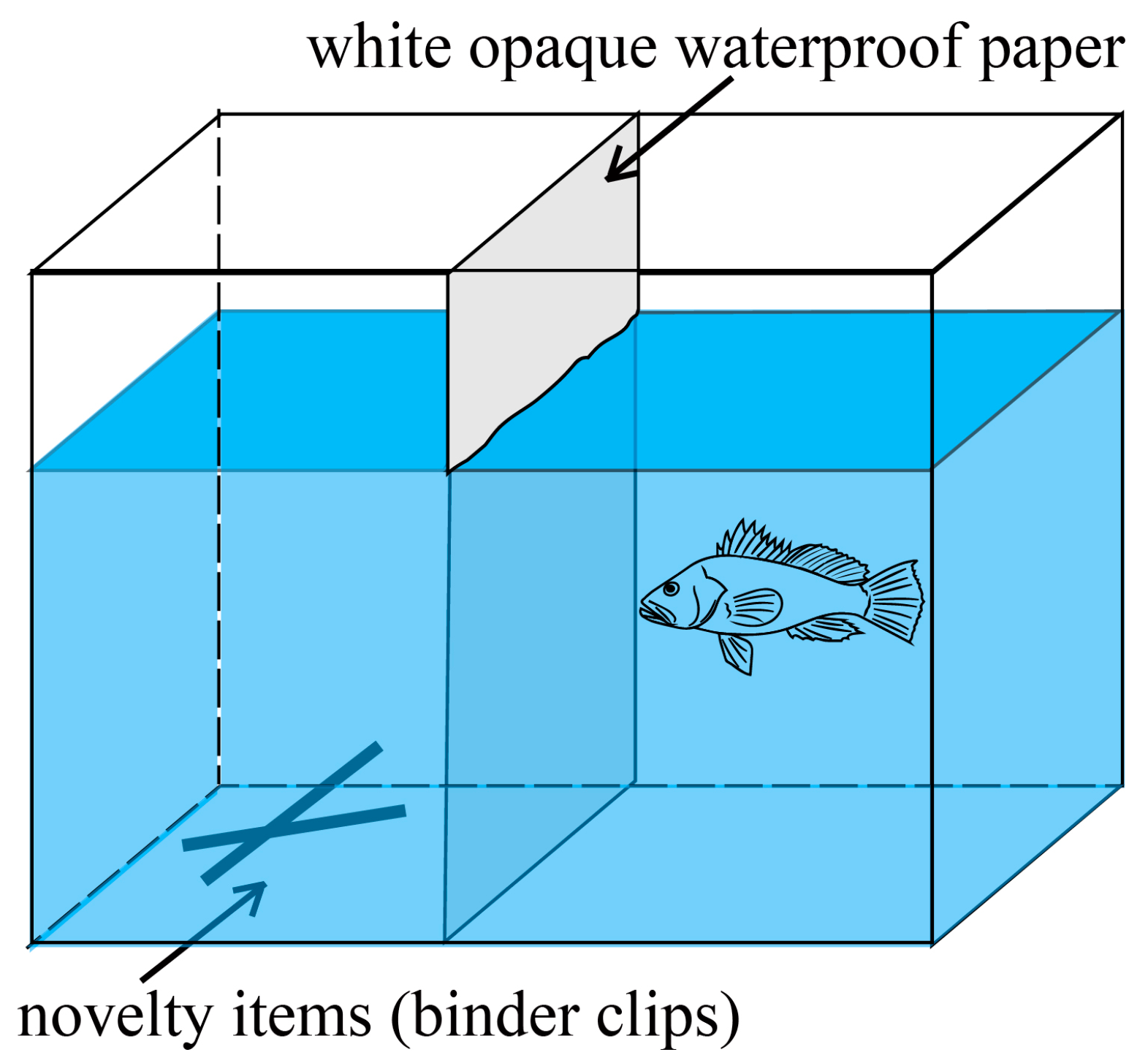
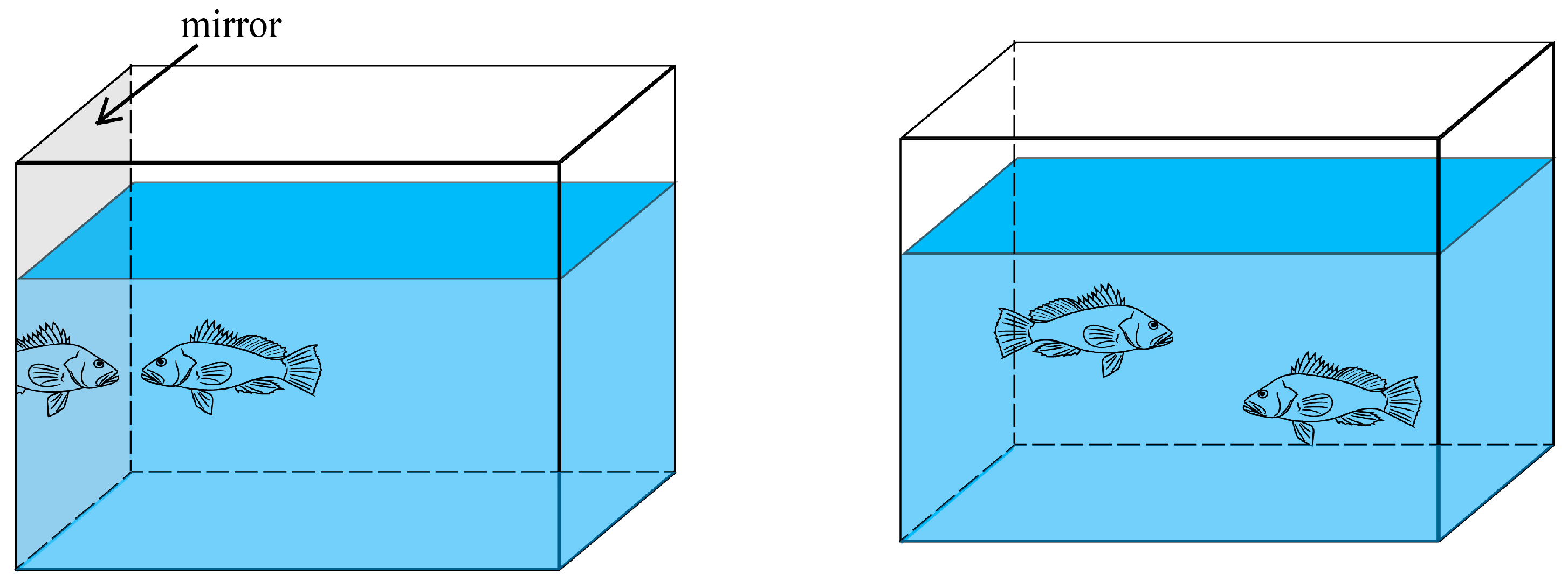
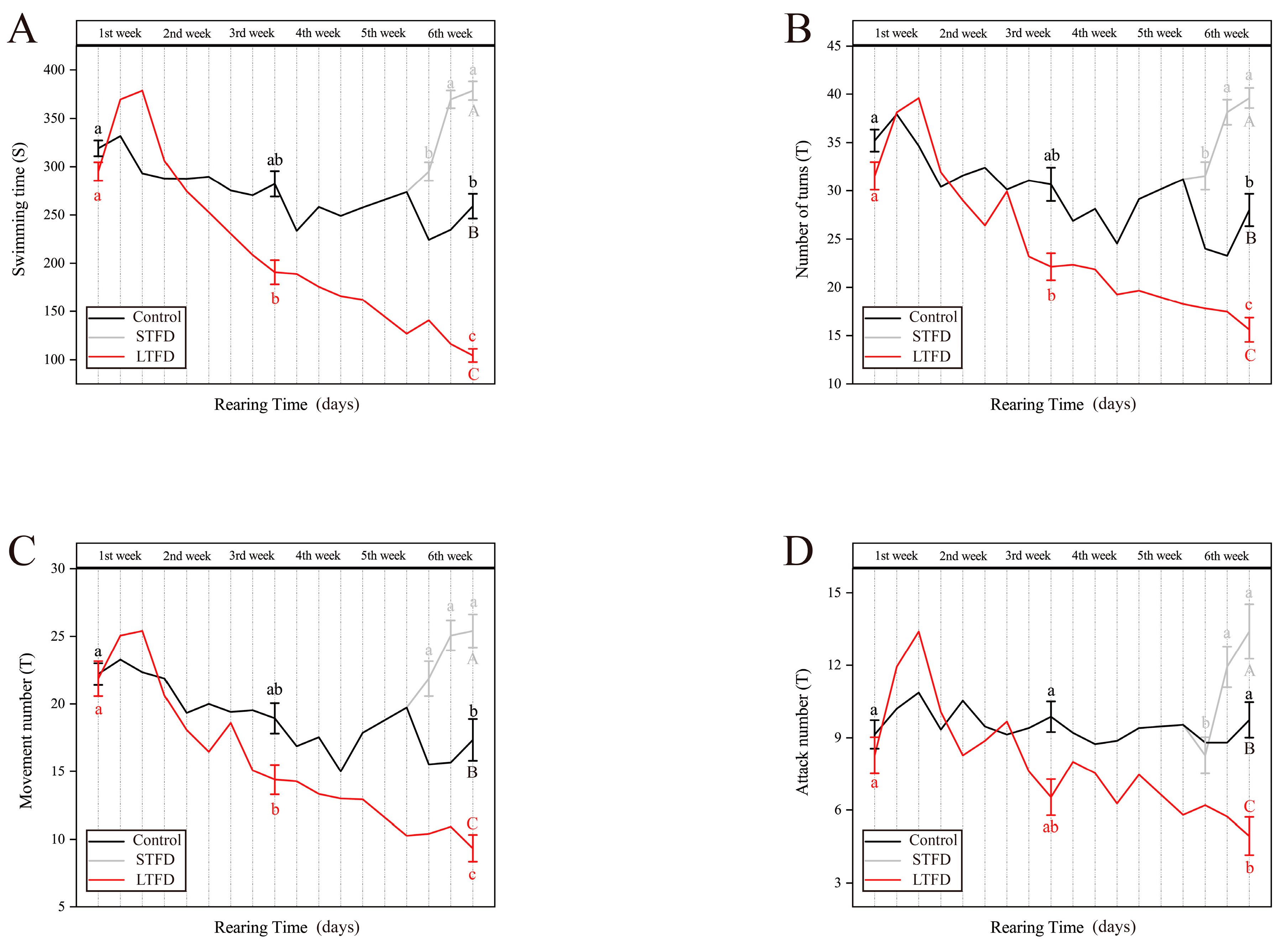
| Stage | Behavior | Indicators | Description |
|---|---|---|---|
| During rearing | - | swimming time (ST) | Non-stationary state activity duration of each fish |
| - | number of turns (NT) | Number of times fish turns more than 90° in any direction | |
| - | movement number (MN) | Number of times each fish moves back and forth (forward or backward more than one body length) | |
| - | attack number (AN) | Number of times engaged in chasing and pecking other fish in the tank | |
| After rearing | Boldness | time in the circle (CTb) | Time spent by the fish in the white circle |
| swimming time (STb) | Swimming duration of the fish | ||
| number of times through the circle (NTCb) | Number of times the fish passed through the circle | ||
| frequency in the circle (FCb) | Frequency of the fish appearing in the circle in the screenshot | ||
| Neophobia | duration in the exploratory area (Te) | Time the fish spent in the exploration area | |
| distance from novelty item (De) | Average distance of the fish from a novelty item | ||
| frequency of stay in the exploratory area (Fe) | Frequency of the experimental fish appearing in the exploration area | ||
| Aggressiveness | attack number (ANm) (mirror stimulus) | Number of times a fish committed an attack | |
| attack range frequency (ARm) | Frequency of fish appearing in the attack range area | ||
| first attack (FAp) | Number of attacks initiated first | ||
| attack number (ANp) (dyadic fights) | Number of times a fish committed an attack | ||
| winner (Wp) | Number of victories |
| Behavior | Indicators | Treatment | ||
|---|---|---|---|---|
| Control | STFD | LTFD | ||
| boldness | CTb (%) | 9.80 ± 0.90 b | 15.80 ± 1.45 a | 6.13 ± 0.50 c |
| STb (s) | 263.73 ± 8.03 b | 304.07 ± 9.02 a | 173.13 ± 8.33 c | |
| NTCb (T) | 9.60 ± 0.62 b | 16.27 ± 1.62 a | 6.73 ± 0.56 b | |
| FCb | 0.09 ± 0.02 b | 0.17 ± 0.03 a | 0.02 ± 0.01 c | |
| Neophobia | Te (%) | 24.73 ± 1.87 b | 31.73 ± 2.73 b | 44.93 ± 3.17 a |
| De (cm) | 20.13 ± 0.8 b | 19.76 ± 1.43 b | 14.59 ± 1.27 a | |
| Fe | 0.35 ± 0.04 b | 0.40 ± 0.07 b | 0.63 ± 0.06 a | |
| Aggressiveness | ANm (T) | 7.50 ± 0.50 a | 7.47 ± 0.74 a | 4.07 ± 0.37 b |
| ARm | 0.58 ± 0.03 a | 0.44 ± 0.03 b | 0.34 ± 0.03 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, F.; Zhang, Z.; Fu, Y.; Zhang, Z.; Sun, X.; Dong, J.; Ding, X.; Chen, M.; Zhang, X. Effects of Food Deprivation Duration on the Behavior and Metabolism of Black Rockfish (Sebastes schlegelii). Fishes 2021, 6, 58. https://doi.org/10.3390/fishes6040058
Shen F, Zhang Z, Fu Y, Zhang Z, Sun X, Dong J, Ding X, Chen M, Zhang X. Effects of Food Deprivation Duration on the Behavior and Metabolism of Black Rockfish (Sebastes schlegelii). Fishes. 2021; 6(4):58. https://doi.org/10.3390/fishes6040058
Chicago/Turabian StyleShen, Fengyuan, Zonghang Zhang, Yiqiu Fu, Zhen Zhang, Xin Sun, Jianyu Dong, Xiayang Ding, Muyan Chen, and Xiumei Zhang. 2021. "Effects of Food Deprivation Duration on the Behavior and Metabolism of Black Rockfish (Sebastes schlegelii)" Fishes 6, no. 4: 58. https://doi.org/10.3390/fishes6040058
APA StyleShen, F., Zhang, Z., Fu, Y., Zhang, Z., Sun, X., Dong, J., Ding, X., Chen, M., & Zhang, X. (2021). Effects of Food Deprivation Duration on the Behavior and Metabolism of Black Rockfish (Sebastes schlegelii). Fishes, 6(4), 58. https://doi.org/10.3390/fishes6040058