Abstract
The integrated aquaculture-seaweed system has been identified as a bio-mitigation strategy to overcome environmental damage, improve the efficiency of nutrient use, maintain good water quality, and ensure the system’s sustainability. This study was conducted to determine the appropriate density of sea grape (Caulerpa lentillifera) in polyculture with whiteleg shrimp (Litopenaeus vannamei) in the same culture tank. Five treatments were randomly designed in triplicate tanks where shrimp was monocultured (without sea grape) as a control treatment and four polyculture treatments with different seaweed density levels (0.5, 1, 1.5, and 2 kg m−3) for 56 days. The results showed that polyculture of shrimp and sea grape significantly reduced the concentrations of total ammonia nitrogen (TAN), nitrite (NO2−), nitrate (NO3−), and phosphate (PO43−) in the rearing tanks and significantly improved (p < 0.05) the growth rate (6.67–6.76% day−1), survival (73.3–78.5%), and production of shrimp (3.44–3.87 kg m−3) compared to monoculture (6.24% day−1, 54.8%, and 2.02 kg m−3, respectively). Applying shrimp and sea grape polyculture at a density of 1 kg m−3 provided a relatively better shrimp performance and feed conversion ratio than other seaweed densities, although not significantly different among polyculture treatments. The findings suggested that sea grape could be used at densities of 0.5–2 kg m−3 in polyculture with whiteleg shrimp, of which 1 kg m−3 resulted in higher production and feed efficiency.
1. Introduction
Shrimp farming is one of the fast-growing sectors in the aquaculture industry, with a global farmed shrimp production of about 4 million tonnes in 2018, accounting for a 3–5% production increase compared to the preceding year. In 2018, the whiteleg shrimp (Litopenaeus vannamei) was ranked the most farmed shrimp species, accounting for 82% of the total world farmed shrimp production. Whiteleg shrimp farming has established itself as one of the dominant aquaculture practices in several Asian countries [1]. In Vietnam, shrimp production plays a significant role in the overall aquaculture production, with an average yield of approximately 520,000–750,000 tons per year, accounting for 11% of the global output [1,2]. The dominance of whiteleg shrimp culture in Asia has been attributed to the species superior aquaculture trait, such as fast growth, wide salinity tolerance, and the ability to grow in poor environmental conditions [3]. However, intensive shrimp farms are characterized by high stocking density (70–150 shrimp m−2) [4] and have resulted in the release of excessive nutrient loads in nitrogen and phosphorus compounds in culture systems, resulting in the rapid deterioration of water quality, hypoxic bottom water, eutrophication, and frequent outbreaks of disease, leading to reduced shrimp production [5,6,7].
In an effort to mitigate the negative impacts caused by aquaculture effluents, integrated culture or polyculture of aquatic animals with extractive species at different trophic levels is considered an environmentally friendly approach for the sustainable development of the aquaculture sector, particularly the shrimp industry [8,9,10,11]. Additionally, the integration of seaweed with shrimp has shown several benefits: reducing the risk of ecological impacts; enhancing shrimp growth, survival, and production; improving shrimp resistance to diseases; and maintaining optimum water quality in the culture system [12,13,14,15].
Sea grape (Caulerpa lentillifera) is naturally distributed in tropical and subtropical regions [16]. Sea grape is one of the most economically critical green algae due to the high nutritional value of the species known as ‘green caviar’, which is good for human health [16,17,18]. In particular, sea grape can absorb nutrients from aquaculture effluence, heavy metal wastewater, and toxic dye-contaminated wastewater [19,20,21,22]. Applying sea grapes to integrated systems makes efficient use of aquaculture effluent for their development, thus improving water quality [20,22,23,24], enhancing shrimp production, and reducing feed cost [25]. Previous studies have shown that the efficiency of integrating shrimp and seaweed systems has been affected by several factors, including the initial density of seaweed [20,26,27]. Until now, no study has been documented on the appropriate stocking density of sea grape in a polyculture system with whiteleg shrimp. Therefore, the present study aims to determine the optimal initial stocking density of sea grape co-cultured with whiteleg shrimp that ensures enhanced water quality, growth, survival, and production of shrimp in tank conditions. The study results may provide practical information for the large-scale application of a whiteleg shrimp-sea grape polyculture system.
2. Materials and Methods
2.1. Experimental Materials
Whiteleg shrimp postlarvae (PL12) were purchased from Viet-Uc Company, a commercial shrimp hatchery in Bac Lieu Province, Vietnam. PL12 were stocked in a 2 m3 tank and reared for 1 month to reach the juvenile stage with an individual weight of about 0.37 g. Sea grape stocks were bought from a private Caulerpa farm in Ninh Thuan Province, Vietnam, and acclimated to adapt to the experimental salinity (30 g L−1) in a 2 m3 tank for 3 days with continuous aeration. Healthy thalli of sea grape, which consists of creeping stolons, erect branches, and rhizoids, were selected for the experiment. Saline water of 80 g L−1 was purchased from the saltworks in Bac Lieu Province. Saline water was treated with chlorine at 30 g m−3, strongly aerated for 3 days, and then diluted with fresh water to obtain experimental salinity of 30 g L−1 before being pumped into culture tanks.
2.2. Experiment Design and Management
The experiment was performed at the College of Aquaculture and Fisheries, Can Tho University, Vietnam. Five treatments in randomly designed triplicate tanks were set up for 56 days. The five treatments included: monoculture (without sea grape in the rearing tank) as control treatment and four integrated treatments (shrimp and sea grape reared in the same tank) with four levels of sea grape density (0.5, 1, 1.5, and 2 kg m−3). Shrimp was stocked at a density of 300 individuals m−3. These five treatments are as follows:
- Treatment 1: Shrimp monoculture (Control);
- Treatment 2: Shrimp + sea grape 0.5 kg m−3(S + 0.5 kg);
- Treatment 3: Shrimp + sea grape 1 kg m−3 (S + 1 kg);
- Treatment 4: Shrimp + sea grape 1.5 kg m−3 (S + 1.5 kg);
- Treatment 5: Shrimp + sea grape 2 kg m−3 (S + 2 kg).
The experimental system was set up under a transparent roof with a natural photoperiod. Shrimp juveniles with an initial weight of 0.37 ± 0.06 g were reared in 500 L plastic tanks filled with 300 L of seawater (30 g L−1) and provided with continuous aeration. Sea grape thalli were spread over a 0.24 m2 rectangular perforated tray (0.4 m × 0.6 m × 0.1 m), covered with a large mesh-sized plastic sheet to fix them in the culture tray, and then each culture tray was hung in a shrimp tank about 20 cm from the tank bottom (Figure 1).

Figure 1.
(A) Experimental tank design, showing the relative position and orientation of the sea grape culture tray in use; (B) experimental set-up with natural photoperiod.
During the culture period, shrimps were fed commercial feed (Grobest, 40% protein, lipid 5%, and metabolizable energy 1800 kcal/kg) used for Vannamei shrimp at an initial feeding rate of 10% biomass day−1. The amount of feeds was adjusted based on the presence or absence of residual feed in the culture tank to avoid overfeeding. Feeding was conducted four times a day at 7:00, 11:00, 16:00, and 20:00 h. Changes in feed particle size (pellet size) and feeding rate were applied following the increase in shrimp size recommended by the shrimp manufacturer (Table 1). Regarding the management of the culture tanks, 10% of water was siphoned after every 2 days and refilled with new water to the initial volume. Water exchange was carried out at 14-day intervals (approximately 30% of the tank volume).

Table 1.
Feeding information based on the Vannamei shrimp manufacturer (Grobest Company).
2.3. Water Quality Parameters
Daily water temperature, dissolved oxygen (DO), and pH were recorded twice a day at 7:00 and 14:00 h using a multi-channel meter (Mettler Toledo, Columbus, OH, USA). The levels of alkalinity were determined every week using a test kit (Sera, Heinsberg, Germany). Diurnal variation in PAR (light intensity) during daytime was measured near the rearing tank’s water surface at 3-day intervals using the light meter (Extech EA31, Taiwan) at 7:00, 10:00, 13:00, and 16:00 h. The Secchi disk determined water transparency. The concentrations of total ammonia nitrogen (TAN), NO2−, NO3− and PO43− were determined at 14-day intervals using HI-83303 Aquaculture Photometer kit. Water samples for water quality monitoring were taken in the culture tanks before water exchange.
2.4. Sea Grape Biomass
The biomass of sea grape was determined at 14-day intervals during the culture period. Each sea grape tray was collected, extra water removed, and the whole sea grape biomass weighed by the electronic balance (±0.1 g precision). For the growth rate of sea grape in terms of biomass increment (BI) and relative growth rate (RGR), the following formulas were used as recommended by Shokita et al. [16]:
BI (%) = (Final biomass – Initial biomass)/Initial biomass × 100
RGR (% day−1) = [(log final weight) − (log initial weight)/cultured days] × 100
2.5. Shrimp Performance
At the beginning of the experiment, 30 whiteleg shrimps were randomly taken from the original stock to determine the initial weight and length. For the growth performance of the shrimp, sampling was conducted at a 14-day interval. Ten shrimps in each tank were randomly taken and weighed in groups using an electronic balance with a precision of 0.01 g, and then the shrimps were returned to the original tanks. At the end of the experiment, all shrimps in each culture tank were weighed in groups and counted to determine the final weight for calculating growth rate in weight. For the growth in terms of length, 30 shrimps were randomly taken from each tank to measure the total length. From this data, the survival and production of shrimp were calculated in each tank. Growth data of experimental shrimp consist of weight gain (WG), daily weight gain (DWG), the specific growth rate in weight (SGRW), the specific growth rate in length (SGRL), survival rate, production, and feed conversion ratio (FCR) were calculated using the following equations:
Weight gain (g) = Final − Initial weight
DWG (g day−1) = [(Final weight) − (Initial weight)]/Cultured days × 100
SGR-W (g day−1) = [(ln final weight) − (ln initial weight)]/Cultured days × 100
SGR-L (g day−1) = [(ln final length) − (ln initial length)]/Cultured days × 100
Survival (%) = (Final number/Initial number) × 100
Production (kg m−3) = Total shrimp weight (g/tank)/Initial shrimp weight (g)/1000
FCR = Feed provided/Weight gain
2.6. Statistical Analysis
All percentage values were normalized through an arcsine transformation before statistical analysis. For all treatments, results were statistically analyzed using a one-way analysis of variance (ANOVA) to determine the overall effect of the treatment using (SPSS for Windows version 16.0). The Duncan test was applied to identify significant differences between the mean values at a significance level of p < 0.05.
3. Results
3.1. Water Quality Parameters
Throughout the experimental period, the average daily water temperature, dissolved oxygen level, and pH in the culture tanks varied from 27.4–28.8 °C, 4.33–5.25 mg L−1, and 8.10–8.40, respectively. Additionally, alkalinity concentrations ranged between 143.2–146.2 mg CaCO3 L−1 during the culture period (Table 2). Daylight irradiances above the water surface of the culture tanks fluctuated between 55 and 212 µmol photons m−2/s−1, with the lowest value observed in the early morning (7:00 h), and then tended to increase in the afternoon (13:00 h) with peak value and sharply decline at late afternoon (16:00 h) (Table 2).

Table 2.
Average temperature, DO, pH, and alkalinity in rearing tanks after 56 days of culture.
Figure 2 shows that high water transparency (43–50 cm) was observed in rearing tanks during the first 9 days of culture and then had a tendency to decline with the culture period. In particular, transparency decreased sharply from day 15 to day 24 (23–32 cm) and from day 36 to the end of the experiment. Notably, the monoculture treatment (shrimp without seaweed in culture tank) had the lowest transparency (20–27 cm) and was significantly different (p < 0.05) compared to the other polyculture tanks (shrimp and sea grape in the same tank) in the later stages of the culture period, as illustrated in Figure 2.
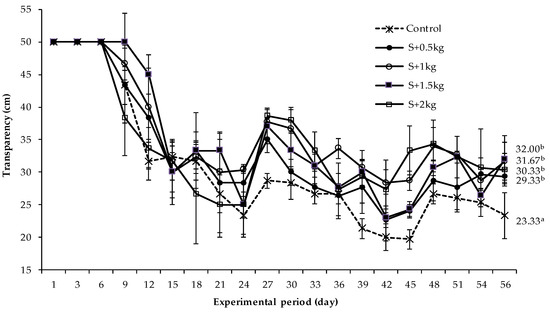
Figure 2.
The fluctuation of water transparency in rearing tanks during the culture period. The error bars stand for the mean values and standard deviation. The different superscripts a, b illustrates the statistical difference in water transparency among the treatments.
From day 14, concentrations of TAN, NO2−, NO3−, and PO43− were higher in the control group (monoculture) than in polyculture treatments, and this pattern was more distinct from day 28 onwards. At the termination of the experiment, the highest contents of TAN (2.73 mg L−1), NO2− (3.98 mg L−1), NO3− (3.59 mg L−1), and PO43− (1.53 mg L−1) were observed in the monoculture treatment that was significantly different from the polyculture groups (p < 0.05). Considering polyculture treatments, the contents of these compounds were lower at higher sea grape density levels, with the lowest and highest values detected in the S + 2 kg and S + 0.5 kg treatments, respectively, and intermediate values for the S + 1 kg and S + 1.5 kg groups. Statistical results showed that there were significant differences in the contents of nitrogen and phosphorus compounds among the seaweed densities in polyculture groups (p < 0.05), compared to the monoculture treatment (Figure 3). It was also noted that the levels of nitrogen and phosphorus compound in monoculture treatment rapidly increased (p < 0.05) than in the polyculture units over the culture duration.
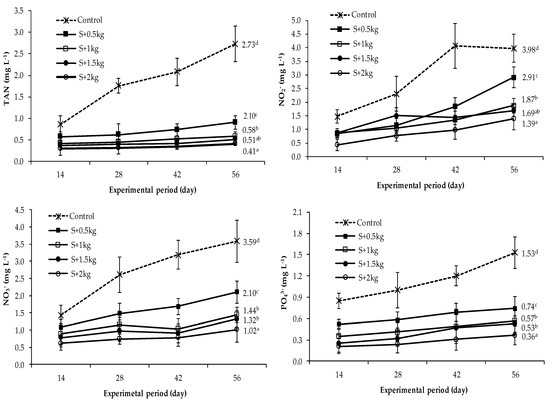
Figure 3.
Variation in concentrations of total ammonia nitrogen (TAN), nitrite (NO2−), nitrate (NO3−), and phosphate (PO43−) during the culture period. Different superscripts indicate significant differences (p < 0.05) among treatments. The error bars stand for the mean values and standard deviation. The different superscripts a, b, c, d illustrates the statistical difference in water quality parameters among the treatments.
3.2. Biomass and Growth of Sea Grape
Figure 4 displays the changes in the average biomass of sea grape in four polyculture treatments. In the first 2 weeks, the sea grape biomass rose from its initial weight in all treatments and continued to grow during the experimental period.
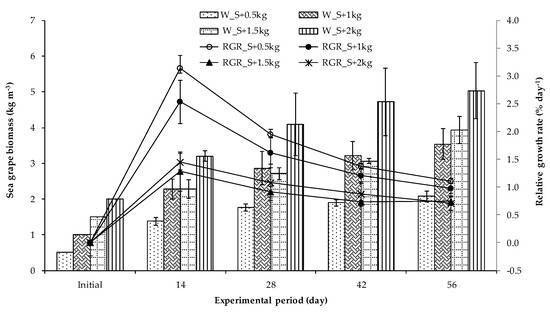
Figure 4.
Change in biomass of sea grape co-cultured with shrimp during the experimental duration. The error bars stand for the mean values and standard deviation.
After 56 days of culture, the sea grape’s mean biomass ranged from 2.08 to 5.03 kg m−3, with higher initial density resulting in higher biomass. Final biomass in S + 1 kg and S + 1.5 kg groups had similar intermediate values and were significantly different (p < 0.05) to treatment S + 0.5 kg and S + 2 kg. However, the growth rate of sea grape decreased with increasing density, the percentage increment biomass (BI) varied between 152–316%, corresponding to the relative growth rate (RGR) of 0.71–1.10% day−1. Statistical analysis showed that the growth rates of sea grape in S + 0.5 kg and S + 1.5 kg were significantly higher than others (p < 0.05) (Table 3).

Table 3.
The growth rate of sea grape integrated with whiteleg shrimp after 56 days of culture.
3.3. Growth Performance of Whiteleg Shrimp
The result indicated that the mean individual weight of shrimp between monoculture and polyculture at different sea grape densities was not distinctly affected in the first 14 days, ranging from 1.54 to 1.78 g. However, the influence from day 28 onwards was more pronounced (Figure 5). After 56 days of culture, the final weight (FW) and length (FL) of shrimp varied between 12.29–16.47 g and 12.64–13.78 cm, respectively, where the lowest and highest values were found in the control and the S + 1 kg treatments.
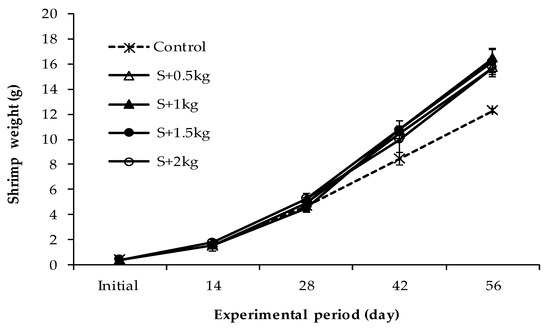
Figure 5.
Variation in shrimp weight in polyculture with sea grape during the culture duration.
Furthermore, the growth rate of shrimps in terms of weight gain (WG) and daily weight gain (DWG) and specific growth rate (SGRW), and the specific growth rate in length (SGRL) of the shrimps followed the same pattern as that observed for the final weight and length. Statistical analysis of the growth performance of the shrimp found that control treatment significantly differed from polyculture groups (Table 4). Although the shrimp growth rate was relatively higher in the S + 1 kg group than in the other integrated treatments, there were no significant differences among these treatments (Table 4).

Table 4.
Effects of polyculture of different densities of sea grape with shrimp on the growth, survival, production, and feed conversion ratio (FCR) of shrimps after 56 days of culture.
3.4. Survival, Production, and Feed Conversion Ratio
Survival, production, and feed conversion ratio (FCR) of shrimp after 56 days of culture are presented in Table 4. The mean survival and production of shrimp fluctuated in the ranges of 54.8–78.5% and 2.02–3.87 kg m−3, respectively. Polyculture groups were found to have produced similar survival and shrimp production, and two of these parameters in polyculture groups were significantly higher (p < 0.05) than in the monoculture group.
The FCR among the treatments ranged from 0.80 to 0.97, with the monoculture treatments obtaining the highest FCR, which was statistically different (p < 0.05) from the polyculture treatments. Compared to the polyculture groups, the S + 1 kg treatment (polyculture of shrimp and sea grape density of 1 kg m−3) resulted in a relatively lower FCR than other polyculture treatments. Generally, these results highlight the significance of integrating sea grape with whiteleg shrimp in the same culture tanks to improve water quality and enhance overall shrimp performance and feed efficiency.
4. Discussions
4.1. Effects of Integrating Different Densities of Sea Grape with Whiteleg Shrimp on Water Quality and Sea Grape Growth
The present study showed that temperature, dissolved oxygen, pH, and alkalinity in rearing tanks and light intensity were maintained in suitable ranges for whiteleg shrimp growth [28,29] and the development of sea grape [16].
According to previous studies, seaweeds with high nutrient absorption capacity have been used in integrated aquaculture systems as a form of bioremediation technology to absorb nitrogenous and phosphorous wastes as a nutrient source for their growth [12,13,14,30]. Because the green algae of the Caulerpa is characterized by rapid growth, high nutrient uptake, and bioaccumulation potential, it is considered an essential component in the tank and pond-based polyculture systems [16,24,31]. Mainly, sea grape has been used in shrimp ponds as a bio-filter to absorb nitrogenous and phosphorous wastes as a nutrient source for their growth, thus reducing the nutrient load, ameliorating water quality, and improving shrimp growth in the system [17,20,23]. Anh and Ngan [25] demonstrated that the co-culture of black tiger shrimp (Penaeus monodon) with sea grape improved water quality in co-culture treatments compared to monoculture treatments. The results of the present study are in line with the findings above.
The polyculture of whiteleg shrimp with sea grape significantly improved the rearing tanks’ water quality, as illustrated in Figure 3. Concentrations of nitrogen and phosphorus compounds were significantly affected by the seaweed density in culture tanks. Similarly, the study of Kha and Anh [26] evaluated the co-culture of whiteleg shrimp with different densities of Gracilaria sp. (1, 1.5, 2, and 2.5 kg m−3). The authors revealed that co-culture treatments significantly reduced contents of nitrogen (TAN, NO2, NO3-, and TN) and phosphorus compounds (PO43− and TP) compared to monoculture treatment and lower levels of these substances at higher algae density. An analogous result was reported in a recent finding, where an increase in seaweed density led to a reduction in the concentrations of total ammonia, nitrite, nitrate, and phosphate in water tanks in the integrated culture of whiteleg shrimp with red seaweed G. corticata [32].
It was noted that the highest concentrations of toxic nitrogen compounds, i.e., TAN= 2.73 mg L−1 and NO2- = 3.98 mg L−1, were detected in the monoculture treatment (without seaweed in culture tank) at the end of the experiment (Figure 3). Shrimps are more susceptible to nitrogenous compound toxicity when reared at low water salinity [33,34,35]. According to Brito et al. [36], TAN contents between 0.75 and 2.98 mg L−1 did not impact whiteleg shrimp performance integrated with Ulva lactuca in a biofloc system. High levels of ammonia in culture systems can be harmful to penaeid shrimp by causing adverse effects such as reduced growth and survival rate [33,37]. In the current study, monoculture treatment recorded the highest level of TAN content at the end of the experiment, the effects of which are reflected by the low survival rate of shrimps in the control treatment compared to the integrated treatments.
The toxicity effect of nitrite (NO2−) in shrimp ponds substantially depends on water salinity, with NO2− being less toxic at high salinity [35]. Compared to ammonia, nitrite is considered more toxic than ammonia in low salinity waters, while its toxicity increases with a decrease in salinity [38]. According to Furtado et al. [39], nitrite content between 3.1 and 4.3 mg L−1 did not adversely affect the performance of whiteleg shrimp cultured in a biofloc system. In this study, whiteleg shrimp was stocked at a salinity of 30 g/L, though the high nitrite level in monoculture may not be harmful to shrimp.
The growth of sea grape is not only affected by the abiotic factors (temperature, salinity, light, and nutrients) [16,40,41] but also by the culture conditions and technical factors [17,40]. Rabia [42] reported that sea grape cultured in a tray method was able to gain an accrued weight increment of 649 g that is a 58% biomass increment. Paul et al. [43] found that sea grape cultured in a tray method for 6 weeks yielded an average biomass production of 2 kg week−1. A comparative study on the growth of Caulerpa spp. (C. lentillifera, C. taxifolia, C. serrulata, C. racemosa) cultured in tank systems revealed a growth rate that ranged between 3% to 7% day−1, with C. lentillifera recording the lowest growth rate [17]. In the current study, the growth rate of sea grape was lower than the above-mentioned studies, a phenomenon that might be attributed to the grazing effect of omnivorous whiteleg shrimp [44] on sea grapes, hence the reduced growth. Likewise, Porchas-Cornejo [45] ascertained that the stomach contents of whiteleg shrimp reared in ponds with and without the enhancement of natural productivity contained formulated feed, diatoms, filamentous algae, macroalgae, protozoans, crustaceans, detritus, polychaetes, and rotifers, suggesting that shrimp can ingest seaweed as supplemental food. Moreover, a decline in water transparency in the later stages of the culture period (Figure 2) might have inhibited the photosynthetic efficiency and nutrient uptake of the sea grape, contributing to the slow growth of sea grape. This result corresponds to reported findings [16,46], which revealed that light intensity in the culture system greatly affects the growth of sea grapes.
4.2. Effects of Integrating Different Densities of Sea Grape with Whiteleg Shrimp on Shrimp Performance and Feed Efficiency
Previous studies have revealed that the integrated culture of shrimp and seaweed is associated with numerous benefits. Among them are enhanced shrimp growth, survival, and production; improved shrimp resistance to diseases; and improved water quality in the culture system [12,13,14]. Studies have also shown that the initial seaweed biomass plays a critical role in determining the efficiency of shrimp-seaweed integrated systems [20,26,27].
The growth parameters, survival rate, and total production of whiteleg shrimp co-cultured with seaweed have been reported to be considerably higher compared to those reared in tanks without seaweed [47]. Similarly, Anh and Ngan [25] reported that polyculture of P. monodon with sea grape attained a significantly higher survival rate (88.3–96.7%) compared to the monoculture treatment (78.3%). In another study, the integration of black tiger shrimp PL and G. tenuistipitata marginally improved the growth rate, survival, and production of shrimp in polyculture tanks compared to shrimp reared in the monoculture treatments (shrimp reared without seaweed) [15]. Similar results were observed in a polyculture system of whiteleg shrimp with G. verrucosa, which revealed that the presence of seaweed in the co-culture treatments helped increase the survival rate, shrimp final weight, specific growth rate, and shrimp production from 45.2% to 94.6%, 9.57 to 12.97 g, 4.75% to 5.07% day−1, and 182 to 884 g, respectively [48]. The current study results reveal a superior growth rate (6.24–6.76% day−1) of whiteleg shrimp when co-cultured with sea grape unlike when co-cultured with Gracilaria corticata (1.69–1.97% day−1) [47] or when co-cultured with G. verrucosa (4.75–5.07% day−1) [48].
The current study exhibited that the integration of whiteleg shrimp with sea grape significantly improved the survival, growth, and production of shrimp in polyculture systems compared to those reared in monoculture tanks (without sea grape). The superior results in the polyculture tanks might be due to the shrimps’ exposure to favorable environmental conditions evident in the integrated systems compared to the relatively poor culture conditions in the monoculture systems (mentioned earlier). Moreover, the use of sea grape in the integrated system might have offered substrate and hiding place for the shrimps, minimizing the adverse effects of cannibalism during the molting phase and contributing to the noticeably enhanced survival rate and total production output in the integrated units compared to the monoculture units. Our results correspond to the previously mentioned studies reporting improved growth rate, survival, and production of whiteleg shrimp in the presence of seaweed in a polyculture system.
Concerning the effects of the integrated culture of the different densities of sea grape and whiteleg shrimp on the FCR, it was determined that the integrated units showed improved FCR compared to the higher FCR value in the monoculture unit. This phenomenon ultimately emphasizes the importance of the polyculture of shrimp and seaweed. A combination of micro-algae and artificial feed [49] significantly improved the growth rate of whiteleg shrimp due to the nutritional supplement provided by seaweed, improving the utilization of nutrients from artificial feeds. The lower FCR value in S + 1 kg treatment (polyculture of shrimp and sea grape density of 1 kg m−3) suggests that the shrimp might have consumed sea grape as supplemental food. This highlights the importance of the high nutritional value of sea grapes [17] in the improved shrimp growth and production.
The reduced FCR value in the integrated systems corresponds to the studies above, which revealed that seaweeds in polyculture systems reduced FCR compared to monoculture systems (without seaweed). The present results are congruent with the study by Anh and Ngan [25]. The authors revealed a reduction in FCR within black tiger shrimp and sea grape co-culture treatments fed 50% satiation compared to the monoculture group. Similarly, whiteleg shrimp co-cultured with red seaweed Gracilaria corticata was singled out for reduced FCR compared to the monoculture treatment [47]. Analogous results were observed by Cruz-Suárez et al. [50], who demonstrated that the intake of seaweed by shrimps helped to improve FCR, with 10% or 45% less commercial feed used, diminishing partial reliance for artificial feeds in shrimp-seaweed co-culture systems. Additionally, a study by Anh et al. [51] reported that integrated culture of L. vannamei with gut weed (Enterormorpha sp.) and blanket weed (Cladophoraceae) enhanced shrimp production at 50% and 75% feed ratio in the control treatment while reducing FCR compared to the control treatment fed ad libitum, thus helping to reduce feed supply up to 50%. Similarly, another study found that when black tiger shrimp was co-cultured with green seaweed (Chaetomorpha sp.) for 10 weeks, the final weight of the shrimps in co-culture was 50% larger and the FCR was 38.9% lower than those in monoculture [52].
5. Conclusions
Polyculture of whiteleg shrimp (300 individuals m−3) with different densities of sea grape biomass (0.5, 1, 1.5, and 2 kg m−3) significantly reduced the concentrations of nitrogen and phosphorous compounds in culture tanks, subsequently improving water quality in the culture medium together with enhancing the survival and total production of shrimps. Sea grape of densities 0.5–2 kg m−3 could be used in whiteleg shrimp and sea grape polyculture systems. However, sea grape density 1 kg m−3 resulted in a relatively higher production output and feed efficiency among the integrated systems, and is thus identified as the suitable density for polyculture of whiteleg shrimp with sea grape. This study’s results may be of great importance to the establishment of sustainable integrated shrimp-seaweed systems that provide promising solutions for maintaining optimum water quality in culture systems while enhancing the production output of culture species. Further studies should be conducted at a larger scale to evaluate the financial efficiency of whiteleg shrimp and sea grape integrated culture.
Author Contributions
K.V.L., D.K.M., and N.A.T.N. implemented experimental design and culture system. D.K.M. and D.P.N. managed the experiment and data collection. K.V.L., D.P.N., and N.A.T.N. performed sample analysis and data analysis interpretation. D.K.M., K.V.L., D.P.N., and N.A.T.N. prepared the outline of the manuscript structure, and D.K.M. and K.V.L. wrote the initial version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Can Tho University, Vietnam, for the financial support assigned to the scientific research programs. We appreciate Lieu The and Lam Thanh Thoai for their assistance while conducting the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Fishery Statistical Collections—Global Aquaculture Production. Food and Agriculture Organisation of the United Nations-Fisheries and Aquaculture Department. 2019. Available online: http://fao.org/fishery/statistics/global-aquaculture-production/ (accessed on 15 December 2020).
- Rubel, H.; Woods, W.; Pérez, D.; Unnikrishnan, S.; Meyer, A. A strategic approach to sustainable shrimp production in Vietnam: The case for improved economics and sustainability. Boston Consulting Group (BCG). 2019. Available online: http://media-publications.bcg.com/BCG-A-Strategic-Approach-to-Sustainable-Shrimp-Production-in-Vietnam-Aug-2019.pdf (accessed on 13 January 2021).
- Liao, I.C.; Chien, Y.H. The pacific white shrimp, Litopenaeus vannamei, in Asia: The world’s most widely cultured alien crustacean. In The Wrong Place-Alien Marine Crustaceans: Distribution, Biology and Impacts; Springer: Dordrecht, The Netherlands, 2011; pp. 489–519. [Google Scholar]
- Nguyen, T.A.T.; Nguyen, K.A.T.; Jolly, C. Is super-intensification the solution to shrimp production and export sustainability? Sustainability 2019, 11, 5277. [Google Scholar] [CrossRef]
- Anh, P.T.; Kroeze, C.; Bush, S.R.; Mol, A.P.J. Water pollution by intensive brackish shrimp farming in southeast Vietnam: Causes and options for control. Agric. Water Manag. 2010, 97, 872–882. [Google Scholar] [CrossRef]
- Zhang, Y.; Bleeker, A.; Liu, J. Nutrient discharge from China’s aquaculture industry and associated environmental impacts. Environ. Res. Lett. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Chaikaew, P.; Rugkarn, N.; Pongpipatwattana, V.; Kanokkantapong, V. Enhancing ecological-economic efficiency of intensive shrimp farm through in-out nutrient budget and feed conversion ratio. Sustain. Environ. Res. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Troell, M.; Halling, C.; Neori, A.; Chopin, T.; Buschmann, A.H.; Kautsky, N.; Yarish, C. Integrated mariculture: Asking the right questions. Aquaculture 2003, 226, 69–90. [Google Scholar] [CrossRef]
- Chopin, T.; MacDonald, B.; Robinson, S.; Cross, S.; Pearce, C.; Knowler, D.; Noce, A.; Reid, G.; Cooper, A.; Speare, D.; et al. The Canadian integrated multi-trophic aquaculture network (CIMTAN)-A Network for a New Era of Ecosystem Responsible Aquaculture. Fisheries 2013, 38, 297–308. [Google Scholar] [CrossRef]
- Largo, D.B.; Diola, A.G.; Marababol, M.S. Development of an integrated multi-trophic aquaculture (IMTA) system for tropical marine species in southern Cebu, Central Philippines. Aquac. Rep. 2016, 3, 67–76. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Kitazawa, D.; Zhou, J.; Park, S.; Gao, S.; Shen, Y. Bio-mitigation based on integrated multi-trophic aquaculture in temperate coastal waters: Practice, assessment, and challenges. Lat. Am. J. Aquat. Res. 2019, 47, 212–223. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Troell, M.; Kautsky, N. Integrated algal farming: A review. Cah. Biol. Mar. 2001, 42, 83–90. [Google Scholar]
- Kang, Y.H.; Hwang, J.R.; Chung, I.K.; Park, S.R. Development of a seaweed species-selection index for successful culture in a seaweed-based integrated aquaculture system. J. Ocean Univ. China 2013, 12, 125–133. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hurd, C.L. Seaweed nutrient physiology: Application of concepts to aquaculture and bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar] [CrossRef]
- Anh, N.T.N.; An, B.N.T.; Lan, L.M.; Hai, T.N. Integrating different densities of white leg shrimp Litopenaeus vannamei and red seaweed Gracilaria tenuistipitata in the nursery phase: Effects on water quality and shrimp performance. J. Appl. Phycol. 2019, 31, 3223–3234. [Google Scholar] [CrossRef]
- Shokita, S.; Kakazu, K.; Tomori, A.; Toma, T. Mariculture of seaweeds. In Aquaculture in Tropical Area; Midori Shobo Co., Ltd.: Tokyo, Japan, 1991; pp. 31–95. [Google Scholar]
- Paul, N.A.; De Nys, R. Promise and pitfalls of locally abundant seaweeds as biofilters for integrated aquaculture. Aquaculture 2008, 281, 49–55. [Google Scholar] [CrossRef]
- Ramadas, V.; Chandralega, G. Screening of phytochemicals, fatty acid composition and in-vitro analysis of antioxidant property of green edible seaweed Caulerpa lentillifera (family: Caulerpaceae). Int. J. Pharm. Sci. Res. 2020, 11, 1495–1505. [Google Scholar]
- Marungrueng, K.; Pavasant, P. Removal of basic dye (Astrazon Blue FGRL) using macroalga Caulerpa lentillifera. J. Environ. Manag. 2006, 78, 268–274. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, Q.; Dong, S.; Tian, X. A comparative study of the nutrient uptake and growth capacities of seaweeds Caulerpa lentillifera and Gracilaria lichenoides. J. Appl. Phycol. 2016, 28, 3083–3089. [Google Scholar] [CrossRef]
- Saputra, N.R.M.; Sukoso, S.; Kartikaningsih, H. A solid waste pond tiger shrimp (Peneaus monodon) as fertilizer for Caulerpa lentillifera. J. Exp. Life Sci. 2017, 7, 17–21. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Liu, H.; Liu, S.; Qin, Y.; Li, P. Advances in cultivation, wastewater treatment application, bioactive components of Caulerpa lentillifera and their biotechnological applications. PeerJ 2019, 7, e6118. [Google Scholar] [CrossRef]
- Hamano, K.; Tsutsui, I.; Srisapoome, P. Co-Culture of Black Tiger Shrimp (Penaeus monodon) And Sea grape (Caulerpa lentillifera). Research Highlights, JICA Project. 2005. Available online: https://www.jircas.go.jp/en/publication/research_results/2005_17 (accessed on 15 January 2021).
- De Paula Silva, P.H.; McBride, S.; de Nys, R.; Paul, N.A. Integrating filamentous ‘green tide’algae into tropical pond-based aquaculture. Aquaculture 2008, 284, 74–80. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Ngan, P.T.T. Effects of different feeding rates on water quality and feed efficiency of black tiger shrimp (Penaeus monodon) co-cultured with sea grape (Caulerpa lentillifera) (in Vietnamese with abstract in English). Vietnam. J. Agric. Sci. 2017, 10, 119–124. [Google Scholar]
- Kha, N.M.; Anh, N.T.N. Efficiency of co-culture model of the white leg shrimp (Litopenaeus vannamei) with different densities of red seaweed Gracilaria sp. (in Vietnamese with abstract in English). HUAF. J. Agric. Sci. 2017, 1, 303–312. [Google Scholar]
- Bambaranda, B.V.A.S.; Sasaki, N.; Chirapart, A.; Salin, K.R.; Tsusaka, T.W. Optimization of macroalgal density and salinity for nutrient removal by Caulerpa lentillifera from aquaculture effluent. Processes 2019, 7, 303. [Google Scholar] [CrossRef]
- Chakravarty, M.S.; Ganesh, P.R.C.; Amarnath, D.; Sudha, B.S.; Babu, T.S. Spatial variation of water quality parameters of shrimp (Litopenaeus vannamei) culture ponds at Narsapurapupeta, Kajuluru and Kaikavolu villages of East Godavari district, Andhra Pradesh. Int. J. Fish. Aquat. Stud. 2016, 4, 390–395. [Google Scholar]
- Jaganmohan, P.; Kumari, C.L. Assessment of water quality in shrimp (Litopenaeus vannamei) grow out ponds in selected villages of SPSR Nellore district of Andhra Pradesh, India during winter crop season. Int. J. Fish. Aquat. Stud. 2018, 6, 260–266. [Google Scholar]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Zubia, M.; Draisma, S.G.A.; Morrissey, K.L.; Varela-Álvarez, E.; De Clerck, O. Concise review of the genus Caulerpa J.V. Lamouroux. J. Appl. Phycol. 2020, 32, 23–33. [Google Scholar] [CrossRef]
- Fourooghifard, H.; Matinfar, A.; Mortazavi, M.S.; Roohani Ghadikolaee, K.; Roohani Ghadikolaee, M. Nitrogen and phosphorous budgets for integrated culture of whiteleg shrimp Litopenaeus vannamei with red seaweed Gracilaria corticata in zero water exchange system. Iran. J. Fish. Sci. 2018, 17, 471–486. [Google Scholar]
- Lin, Y.C.; Chen, J.C. Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 109–119. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chen, J.C. Joint action of elevated ambient nitrite and nitrate on hemolymph nitrogenous compounds and nitrogen excretion of tiger shrimp Penaeus monodon. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 131, 303–314. [Google Scholar] [CrossRef]
- Schuler, D.J.; Boardman, G.D.; Kuhn, D.D.; Flick, G.J. Acute toxicity of ammonia and nitrite to Pacific white shrimp, Litopenaeus vannamei, at low salinities. J. World. Aquac. Soc. 2010, 41, 438–446. [Google Scholar] [CrossRef]
- Brito, L.O.; Arantes, R.; Magnotti, C.; Derner, R.; Pchara, F.; Olivera, A.; Vinatea, L. Water quality and growth of Pacific white shrimp Litopenaeus vannamei (Boone) in co-culture with green seaweed Ulva lactuca (Linaeus) in intensive system. Aquac. Int. 2014, 22, 497–508. [Google Scholar] [CrossRef]
- Cobo, M.L.; Sonnenholzner, S.; Wille, M.; Sorgeloos, P. Ammonia tolerance of Litopenaeus vannamei (Boone) larvae. Aquac. Int. 2014, 45, 470–475. [Google Scholar]
- Valencia-Castañeda, G.; Frías-Espericueta, M.G.; Vanegas-Pérez, R.C.; Pérez-Ramírez, J.A.; Chávez-Sánchez, M.C.; Páez-Osuna, F. Acute toxicity of ammonia, nitrite and nitrate to shrimp Litopenaeus vannamei postlarvae in low-salinity water. Bull. Environ. Contam. Toxicol. 2018, 101, 229–234. [Google Scholar] [CrossRef]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W. Effect of calcium hydroxide, carbonate and sodium bicarbonate on water quality and zootechnical performance of shrimp Litopenaeus vannamei reared in bio-flocs technology (BFT) systems. Aquaculture 2011, 321, 130–135. [Google Scholar] [CrossRef]
- Wang, P.Y. Effects of salinity and light intensity on the growth of Caulerpa lentillifera. J. Agric. Sci. Technol. 2011, 24, 131–132. [Google Scholar]
- Guo, H.; Yao, J.; Sun, Z.; Duan, D. Effects of salinity and nutrients on the growth and chlorophyll fluorescence of Caulerpa lentillifera. Chin. J. Oceanol. Limnol. 2015, 33, 410–418. [Google Scholar] [CrossRef]
- Rabia, M.D.S. Cultivation of Caulerpa lentillifera using tray and sowing methods in brackish water pond. Environ. Sci. 2016, 4, 23–29. [Google Scholar]
- Paul, N.A.; Dworjanyn, S.A.; De Nys, R. Green Caviar” and “Sea Grapes”: Targeted Cultivation of High-Value Seaweeds from the Genus Caulerpa. Aust. Flora Found. 2009. [Google Scholar]
- Dall, W. Food and feeding of some Australian penaeid shrimp. FAO Fish. Rep. 1968, 2, 251–258. [Google Scholar]
- Porchas-Cornejo, M.A.; Martínez-Porchas, M.; Martínez-Córdova, L.R.; Ramos-Trujillo, L.; Barraza-Guardado, R. Consumption of natural and artificial foods by shrimp (Litopenaeus vannamei) reared in ponds with and without enhancement of natural productivity. Isr. J. Aquac. 2012, 709, 1–7. [Google Scholar]
- FAO. A Guide to the Seaweed Industry; Fisheries Technical Paper; McHugh, D.J., Ed.; FAO: Rome, Italy, 2003; p. 441. [Google Scholar]
- Fourooghifard, H.; Matinfar, A.; Mortazavi, M.S.; Roohani Ghadikolaee, K.; Mirbakhsh, M. Growth parameters of whiteleg shrimp Litopenaeus vannamei and red seaweed Gracilaria corticata in integrated culturing method under zero water exchange system. Aquac. Res. 2017, 48, 5235–5242. [Google Scholar] [CrossRef]
- Susilowati, T.; Hutabarat, J.; Anggoro, S.; Zainuri, M. The improvement of the survival, growth and production of Vannamei shrimp (Litopenaeus vannamei) and seaweed (Gracilaria verucosa) based on polyculture cultivation. Int. J. Mar. Aquat. Res. Conserv. Co-Exist. 2014, 1, 6–11. [Google Scholar]
- Cruz-Suarez, L.E.; Leon, A.; Pena-Rodrıguez, A.; Rodrgıuez-Pena, G.; Mol, B.; Ricque-Marie, D. Shrimp/Ulva co-culture: A sustainable alternative to diminish the need for artificial feed and improve shrimp quality. Aquaculture 2010, 301, 64–68. [Google Scholar] [CrossRef]
- Cruz-Suárez, L.E.; Tapia-Salazar, M.; Nieto-López, M.G.; Ricque-Marie, D. A review of the effects of macroalgae in shrimp feeds and in co-culture. Avanc. En. Nutr. Acuic. 2008. [Google Scholar]
- Anh, N.T.N.; Nhung, D.T.; Hai, T.N. Feed efficiency of white leg shrimp (Litopeneaus vannamei) in co-culture with gut weed (Enterormorpha sp.) and blanket weed (Cladophoraceae). (In Vietnamese with abstract in English). Sci. J. CTU Vietnam. 2014, 31b, 98–105. [Google Scholar]
- Tsutsui, I.; Songphatkaew, J.; Meeanan, C.; Aue-umneoy, D.; Sukchai, H.; Pinphoo, P.; Klomkling, S.; Ganmanee, M.; Sudo, H.; Hamano, K. Co-culture with Chaetomorpha sp. enhanced growth performance and reduced feed conversion ratio of the giant tiger prawn, Penaeus monodon. Int. Aquat. Res. 2015, 7, 193–199. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).