Mechanism of Delayed Convulsion in Fish: The Actions of Norepinephrine in Spinal Cord
Abstract
1. Introduction
2. Results
2.1. Among-Species Differences in Delayed Convulsion Measures
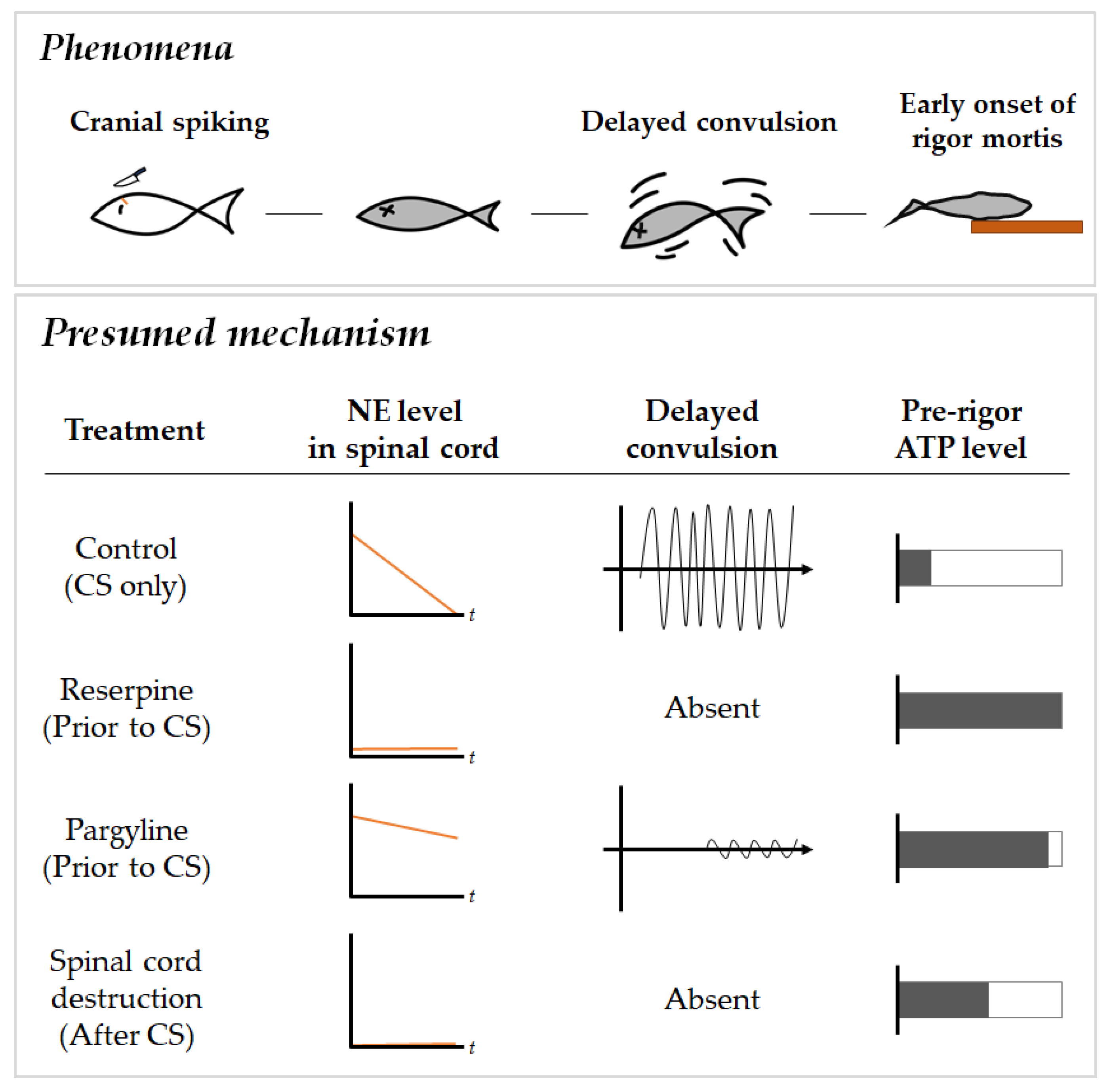
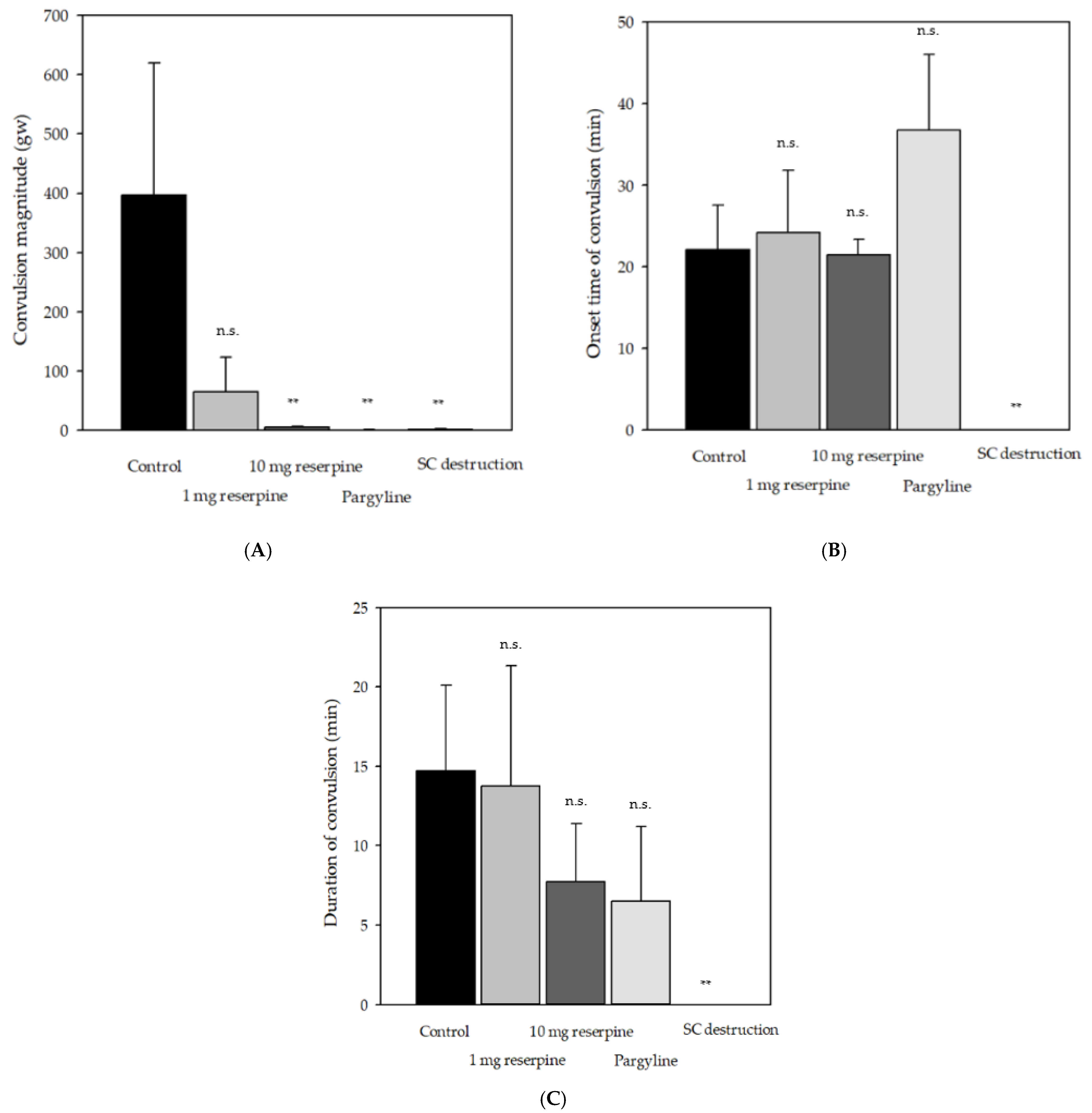
2.2. Effects of Pharmacological Compounds Involving Monoamine Metabolism on Delayed Convulsion in Red Seabreams
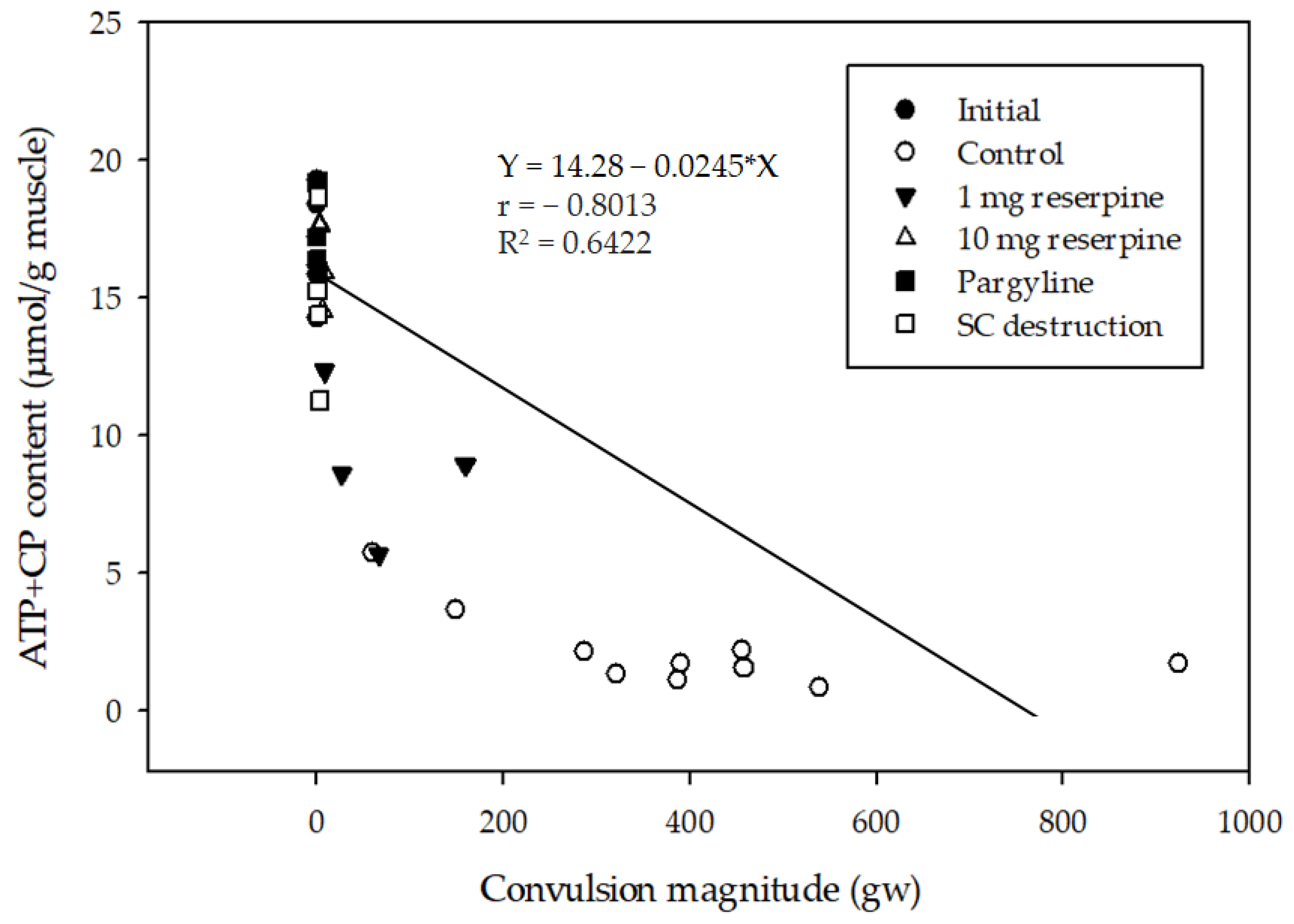
2.3. Relationship between Delayed Convulsion and Changes in ATP and Creatine Phosphate Levels
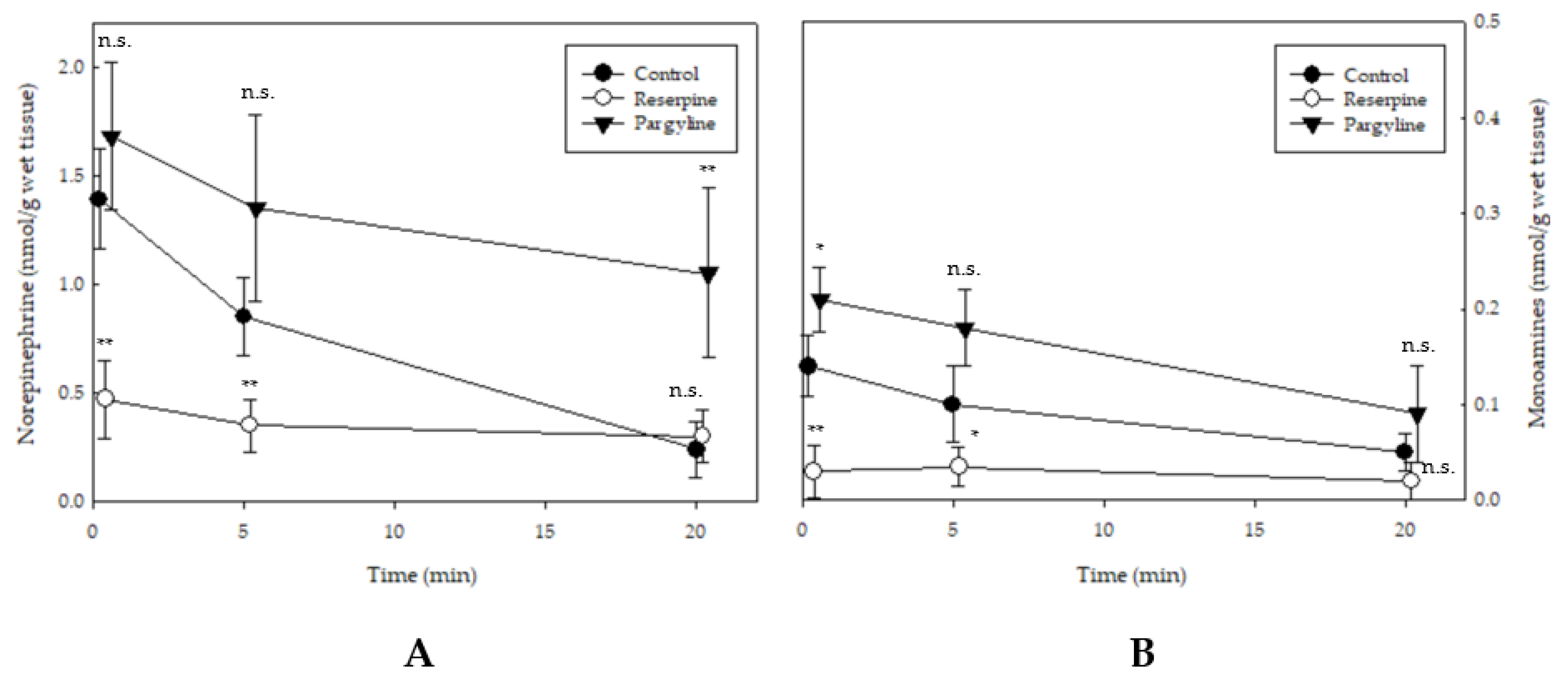
2.4. Changes in Spinal Cord Norepinephrine and Monoamine Levels
3. Discussion
4. Materials and Methods
4.1. Fish
4.2. Measurements of Delayed Convulsion, as Well as Muscles Levels of ATP and Creatine Phosphate
4.3. Determination of Norepinephrine and Monoamines in the Spinal Cord
4.4. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Fein, S.B.; Lando, A.M.; Levy, A.S.; Teisl, M.F.; Noblet, C. Trends in U.S. consumers’ safe handling and consumption of food and their risk perceptions, 1988 through 2010. J. Food Prot. 2011, 74, 1513–1523. [Google Scholar] [CrossRef]
- Lehel, J.; Yaucat-Guendi, R.; Darnay, L.; Palotás, P.; Laczay, P. Possible food safety hazards of ready-to-eat raw fish containing product (sushi, sashimi). Crit. Rev. Food Sci. Nutr. 2020, 867–888. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Ohno, K.; Ninomiya, J. Changes in texture during cold storage of cultured yellowtail meat prepared by different killing methods. Nippon Suisan Gakkaishi 1990, 56, 1673–1678. [Google Scholar] [CrossRef]
- Mochizuki, S.; Sato, A. Effects of various killing procedures and storage temperatures on post-mortem changes in the muscle of Horse Mackerel. Nippon Suisan Gakkaishi 1994, 60, 125–130. [Google Scholar] [CrossRef]
- Boyd, N.S.; Wilson, N.D.; Jerrett, A.R.; Hall, B.I. Effects of brain destruction on post harvest muscle metabolism in the fish Kahawai (Arripis trutta). J. Food Sci. 1984, 49, 177–179. [Google Scholar] [CrossRef]
- Mishima, T.; Nonaka, T.; Okamoto, A.; Tsuchimoto, M.; Ishiya, T.; Tachibana, K.; Tsuchimoto, M. Influence of storage temperatures and killing procedures on post-mortem changes in the muscle of horse mackerel caught near Nagasaki Prefecture, Japan. Fish. Sci. 2005, 71, 187–194. [Google Scholar] [CrossRef]
- Nakayama, T.; Toyoda, T.; Ooi, A. Delay in rigor mortis of red sea-bream by spinal cord destruction. Fish. Sci. 1996, 62, 478–482. [Google Scholar] [CrossRef]
- Eichbaum, F.W.; Slemer, O.; Yasaka, W.J. Postdecapitation convulsions and their inhibition by drugs. Exp. Neurol. 1975, 49, 802–812. [Google Scholar] [CrossRef]
- Leach, T.M.; Wilkins, L.J. Observations on the physiological effects of pithing cattle at slaughter. Meat Sci. 1985, 15, 101–106. [Google Scholar] [CrossRef]
- Oliveira, S.E.O.; Gregory, N.G.; Dalla Costa, F.A.; Gibson, T.J.; Paranhos da Costa, M.J.R. Efficiency of low versus high airline pressure in stunning cattle with a pneumatically powered penetrating captive bolt gun. Meat Sci. 2017, 130, 64–68. [Google Scholar] [CrossRef]
- Casey-Trott, T.M.; Millman, S.T.; Turner, P.V.; Nykamp, S.G.; Lawlis, P.C.; Widowski, T.M. Effectiveness of a nonpenetrating captive bolt for euthanasia of 3 kg to 9 kg pigs. J. Anim. Sci. 2014, 92, 5166–5174. [Google Scholar] [CrossRef]
- Pappas, B.A.; Breese, G.R.; Mailman, R.B.; Mueller, R.A. Importance of the Locus coeruleus and involvement of α-adrenergic receptors in the post-decapitation reflex in the rat. Psychopharmacology 1980, 69, 163–171. [Google Scholar] [CrossRef]
- Roberts, D.C.S.; Mason, S.T.; Fibiger, H.C. Selective depletion of spinal noradrenaline abolishes post-decapitation convulsions. Life Sci. 1978, 23, 2411–2413. [Google Scholar] [CrossRef]
- Fukuda, T.; Araki, Y.; Suenaga, N. Inhibitory effects of 6-hydroxydopamine on the clonic convulsions induced by electroshock and decapitation. Neuropharmacology 1975, 14, 579–583. [Google Scholar] [CrossRef]
- Yamada, K.; Matsuo, N.; Kumagai, M.; Nagashima, M.; Nojima, H.; Hashizume, N.; Oguro, K.; Fukuda, T.; Furukawa, T. Inhibition of post-decapitation convulsions in the rat by dibenzothiepin neuroleptics via α1-adrenoceptor blockade. Eur. J. Pharmacol. 1988, 148, 205–212. [Google Scholar] [CrossRef]
- Krahl, S.E.; Clark, K.B.; Smith, D.C.; Browning, R.A. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 1998, 39, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, D. The contribution of norepinephrine and orexigenic neuropeptides to the anticonvulsant effect of the ketogenic diet. Epilepsia 2008, 49, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Szot, P.; Weinshenker, D.; White, S.S.; Robbins, C.A.; Rust, N.C.; Schwartzkroin, P.A.; Palmiter, R.D. Norepinephrine-deficient mice have increased susceptibility to seizure- inducing stimuli. J. Neurosci. 1999, 19, 10985–10992. [Google Scholar] [CrossRef] [PubMed]
- Zemková, H.; Svoboda, P.; Teisinger, J.; Vyskočil, F. On the mechanism of catecholamine-induced hyperpolarization of skeletal muscle cells. Naunyn-Schmiedebergs Arch. Pharmacol. 1985, 329, 18–23. [Google Scholar] [CrossRef]
- Kamat, U.G.; Sheth, U.K. The role of central monoamines in decapitation convulsions of mice. Neuropharmacology 1971, 10, 571–579. [Google Scholar] [CrossRef]
- Bessman, S.P.; Geiger, P.J. Transport of energy in muscle: The phosphorylcreatine shuttle. Science 1981, 211, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, N.; Oishi, R.; Fukuda, T. The role of locus coeruleus in decapitation convulsions of rats. Brain Res. 1979, 177, 83–93. [Google Scholar] [CrossRef]
- Eichbaum, F.W.; Yasaka, W.J. Inhibition of post-decapitation convulsions by reserpine. Experientia 1973, 29, 816–817. [Google Scholar] [CrossRef] [PubMed]
- Klein, A. The curious case of the decapitated frog: On experiment and philosophy. Br. J. Hist. Philos. 2018, 26, 890–917. [Google Scholar] [CrossRef]
- Pflüger, E.F.W. Nebst einer neuen Lehre über die Leitungsgesetze der Reflexionen. In Die Sensorischen Functionen des Rückenmarks der Wirbelthiere; August Hirschwald: Berlin, Germany, 1853. [Google Scholar]
- Pafilis, P.; Valakos, E.D.; Foufopoulos, J. Comparative postautotomy tail activity in six Mediterranean Lacertid lizard species. Physiol. Biochem. Zool. 2005, 78, 828–838. [Google Scholar] [CrossRef]
- Hedgecock, T.; Phillips, A.; Ludrick, B.; Golden, T.; Wu, N. Molecular mechanisms and applications of a reserpine-induced rodent model. SSR Inst. Int. J. Life Sci. 2019, 5, 2160–2167. [Google Scholar] [CrossRef]
- Steckler, T. Reserpine BT—Encyclopedia of Psychopharmacology; Stolerman, I.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 1154. ISBN 978-3-540-68706-1. [Google Scholar]
- Russo, S.M.; Daniels, A.J.; Viveros, O.H.; Reinhard, J.F. Differences in the reserpine-sensitive storage in vivo of 1-methyl-4-phenylpyridinium in rats and mice may explain differences in catecholamine toxicity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurotoxicol. Teratol. 1994, 16, 277–281. [Google Scholar] [CrossRef]
- Kumagae, Y.; Matsui, Y.; Iwata, N. Deamination of norepinephrine, dopamine, and serotonin by type A monoamine oxidase in discrete regions of the rat brain and inhibition by RS-8359. Jpn. J. Pharmacol. 1991, 55, 121–128. [Google Scholar] [CrossRef]
- Mishra, P.K.; Kahle, E.H.; Bettendorf, A.F.; Dailey, J.W.; Jobe, P.C. Anticonvulsant effects of intracerebroventricularly administered norepinephrine are potentiated in the presence of monoamine oxidase inhibition in severe seizure genetically epilepsy-prone rats (GEPR-9s). Life Sci. 1993, 52, 1435–1441. [Google Scholar] [CrossRef]
- Danysz, W.; Jonsson, G.; Mohammed, A.K.; Archer, T. The hindlimb extension reflex is not a reliable marker of post-decapitation convulsions or spinal noradrenaline depletion in rats. Eur. J. Pharmacol. 1985, 116, 331–333. [Google Scholar] [CrossRef]
- Ghasemi, M.; Mehranfard, N. Mechanisms underlying anticonvulsant and proconvulsant actions of norepinephrine. Neuropharmacology 2018, 137, 297–308. [Google Scholar] [CrossRef]
- Borszcz, G.S.; Johnson, C.P.; Williams, D.H. Increases in vocalization and motor reflex thresholds generated by the intrathecal administration of serotonin or norepinephrine. Behav. Neurosci. 1996, 110, 809–822. [Google Scholar] [CrossRef]
- Garraway, S.M.; Hochman, S. Modulatory actions of serotonin, norepinephrine, dopamine, and acetylcholine in spinal cord deep dorsal horn neurons. J. Neurophysiol. 2001, 86, 2183–2194. [Google Scholar] [CrossRef]
- Myslinski, N.R.; Thut, P.D. The effect of 5,6-dihydroxytryptamine on post-decapitation convulsions. Life Sci. 1977, 21, 1475–1482. [Google Scholar] [CrossRef]
- Bosland, M.C.; Versteeg, D.H.G.; de Jong, W. Selective depletion of spinal noradrenaline inhibits post-decapitation convulsions in rats. Experientia 1980, 36, 224. [Google Scholar] [CrossRef] [PubMed]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabil. Neural Repair 2010, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rank, M.M.; Murray, K.C.; Stephens, M.J.; D’Amico, J.; Gorassini, M.A.; Bennett, D.J. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J. Neurophysiol. 2011, 105, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.R.; Yaksh, T.L. Changes in sensitivity to intrathecal norepinephrine and serotonin after 6-hydroxydopamine (6-OHDA), 5,6-dihydroxytryptamine (5,6-DHT) or repeated monoamine administration. J. Pharmacol. Exp. Ther. 1982, 220, 311–321. [Google Scholar] [PubMed]
- Ten Cate, J. Spontaneous electrical activity of the spinal cord. Electroencephalogr. Clin. Neurophysiol. 1950, 2, 445–451. [Google Scholar] [CrossRef]
- Rumping, J.M.; Jayne, B.C. Muscle activity in autotomized tails of a lizard (Gekko gecko): A naturally occurring spinal preparation. J. Comp. Physiol. A 1996, 179, 525–538. [Google Scholar] [CrossRef]
- Higham, T.E.; Lipsett, K.R.; Syme, D.A.; Russell, A.P. Controlled chaos: Three-dimensional kinematics, fiber histochemistry, and muscle contractile dynamics of autotomized lizard tails. Physiol. Biochem. Zool. 2013, 86, 611–630. [Google Scholar] [CrossRef][Green Version]
- Higham, T.E.; Russell, A.P. Flip, flop and fly: Modulated motor control and highly variable movement patterns of autotomized gecko tails. Biol. Lett. 2010, 6, 70–73. [Google Scholar] [CrossRef][Green Version]
- Ando, M.; Banno, A.; Haitani, M.; Hirai, H.; Nakagawa, T.; Makinodan, Y. Influence on post-mortem rigor of fish body and muscular ATP consumption by the destruction of spinal cord in several fishes. Fish. Sci. 1996, 62, 796–799. [Google Scholar] [CrossRef]
- Digre, H.; Erikson, U.; Skaret, J.; Lea, P.; Gallart-Jornet, L.; Misimi, E. Biochemical, physical and sensory quality of ice-stored Atlantic cod (Gadus morhua) as affected by pre-slaughter stress, percussion stunning and AQUI-S TM anaesthesia. Eur. Food Res. Technol. 2011, 233, 447–456. [Google Scholar] [CrossRef]
- Zhang, Z.; Hamada, H.; Gerk, P.M. Selectivity of dietary phenolics for inhibition of human monoamine oxidases A and B. Biomed. Res. Int. 2019, 2019, 8361858. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Schreier, T.M. Depletion of isoeugenol residues from the fillet tissue of AQUI-STM exposed rainbow trout (Oncorhynchus mykiss). Aquaculture 2009, 296, 200–206. [Google Scholar] [CrossRef]
- Fišar, Z. Inhibition of monoamine oxidase activity by cannabinoids. Naunyn-Schmiedebergs Arch. Pharmacol. 2010, 381, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Roy, A.; Jung, J.H.; Choi, J.S. Evaluation of the inhibitory effects of eckol and dieckol isolated from edible brown alga Eisenia bicyclis on human monoamine oxidases A and B. Arch. Pharm. Res. 2017, 40, 480–491. [Google Scholar] [CrossRef]
- Ushio, H.; Watabe, S.; Iwamoto, M.; Hashimoto, K. Ultrastructural evidence for temperature-dependent Ca2+ release from fish sarcoplasmic reticulum during rigor mortis. Food Struct. 1991, 10, 267–275. [Google Scholar]
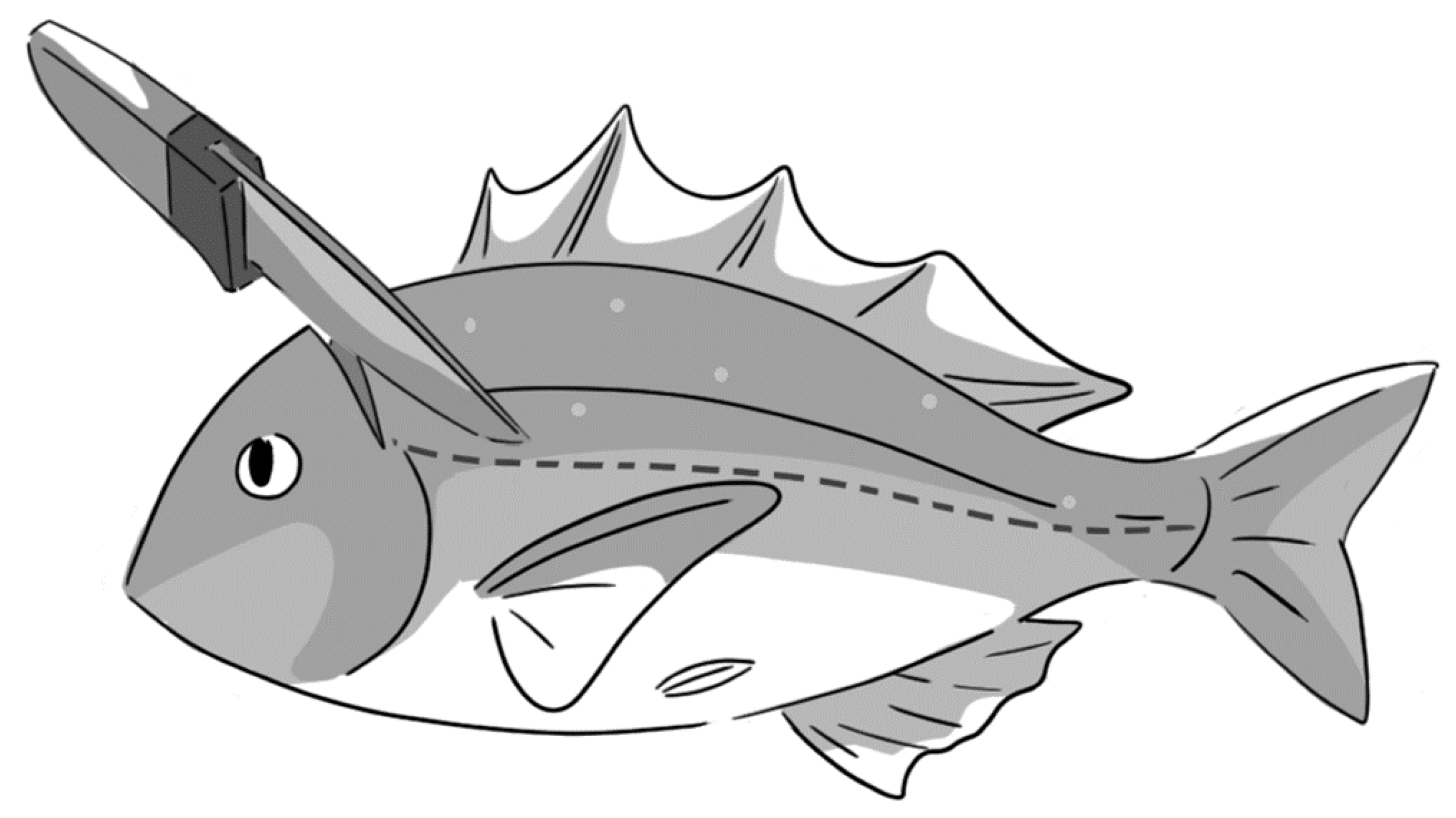
| Fish Species (Individual Numbers) | Onset Time (min) | Duration (min) | Magnitude |
|---|---|---|---|
| Freshwater | |||
| Carp Cyprinus carpio (10) | − | − | − |
| Tilapia Oreochromis niloticus (9) | − | − | − |
| Rainbow trout Oncorhynchus mykiss (10) | ~3 | ~1 | + |
| Ayu Plecoglossus altivelis (2) | ~3 | ~1 | + |
| Saltwater | |||
| Greenling (Ainame) Hexagrammos otakii (3) | ~7 | ~5 | + |
| Red seabream Pagrus major (35) | ~20 | ~15 | +++ |
| Striped jack (Shima-aji) Caranx delicatissimus (2) | ~20 | ~30 | +++ |
| Stone flounder (Ishigarei) Kareius bicoloratus (2) | ~60 | ~40 | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Kominami, Y.; Ushio, H. Mechanism of Delayed Convulsion in Fish: The Actions of Norepinephrine in Spinal Cord. Fishes 2021, 6, 12. https://doi.org/10.3390/fishes6020012
Lee C-L, Kominami Y, Ushio H. Mechanism of Delayed Convulsion in Fish: The Actions of Norepinephrine in Spinal Cord. Fishes. 2021; 6(2):12. https://doi.org/10.3390/fishes6020012
Chicago/Turabian StyleLee, Cheng-Linn, Yuri Kominami, and Hideki Ushio. 2021. "Mechanism of Delayed Convulsion in Fish: The Actions of Norepinephrine in Spinal Cord" Fishes 6, no. 2: 12. https://doi.org/10.3390/fishes6020012
APA StyleLee, C.-L., Kominami, Y., & Ushio, H. (2021). Mechanism of Delayed Convulsion in Fish: The Actions of Norepinephrine in Spinal Cord. Fishes, 6(2), 12. https://doi.org/10.3390/fishes6020012





