Abstract
Alterations in fish developmental trajectories occur in response to genetic and environmental changes, especially during sensitive periods of development (critical windows). Embryos and larvae of Atractosteus tropicus were used as a model to study fish survival, growth, and development as a function of temperature (28 °C control, 33 °C, and 36 °C), salinity (0.0 ppt control, 4.0 ppt, and 6.0 ppt), and air saturation (control ~95% air saturation, hypoxia ~30% air saturation, and hyperoxia ~117% air saturation) during three developmental periods: (1) fertilization to hatch, (2) day 1 to day 6 post hatch (dph), and (3) 7 to 12 dph. Elevated temperature, hypoxia, and hyperoxia decreased survival during incubation, and salinity at 2 and 3 dph. Growth increased in embryos incubated at elevated temperature, at higher salinity, and in hyperoxia but decreased in hypoxia. Changes in development occurred as alterations in the timing of hatching, yolk depletion, acceptance of exogenous feeding, free swimming, and snout shape change, especially at high temperature and hypoxia. Our results suggest identifiable critical windows of development in the early ontogeny of A. tropicus and contribute to the knowledge of fish larval ecology and the interactions of individuals × stressors × time of exposure.
1. Introduction
Developmental phenotypic plasticity is the ability of an organism in its early stages to modify its phenotype as a function of intrinsic (genetic) or extrinsic (environmental) factors [1,2,3]. Modifications in the developmental trajectory as a result of phenotypic plasticity have the capacity to alter traits in adulthood, being either adaptative or detrimental [4,5,6]. Alterations in certain characteristics of developing organisms occur during specific periods of time known as critical windows, which are sensitive periods during development when phenotype is responsive to intrinsic or extrinsic factors [1,7,8,9].
Most studies analyzing critical windows have focused on the effect of one factor (stressor) at different times in development [10,11,12,13]. However, an experimental design for critical windows that is multifactorial (e.g., multiple factors varying simultaneously) offers a more realistic set of circumstances that affords the opportunity to understand and visualize the interaction of development, stressor dose, and the response on phenotypic characteristics of interest [9,14]. Most studies investigating critical windows have targeted morphological and physiological characteristics, maturation, metabolism, etc. across the different animal taxa [12,13,15,16,17].
In fishes, specifically, relatively little is known about survival and growth during critical windows, even though fish species have long been used as animal models. Some examples of studies analyzing critical windows, survival, and growth described how survival and some growth parameters are affected by shifting incubation temperature in the lake whitefish Coregonus clupeaformis [16] or decreased survival and hatch in the Japanese medaka (Ozyrias latipes) exposed to selenomethionine and hypersaline environments. These experiments identified the early neurulation as the most susceptible stage for lethality and morphological alterations [18].
In addition to determining the effect of stressors on the critical windows of a given trait, it is important to analyze how these factors can alter the developmental trajectories of organisms, especially regarding the onset and timing of developmental events. Modifications in the onset and timing of the different physiological processes of individuals have been defined as “heterokairy”, “…the plasticity in the timing of the onset of developmental events at the level of an individual during its development” [19]. However, heterokairy can also be evident in changes occurring between populations of a given species. Our understanding of heterokairy as variation at the individual or populational level and not between species remains meager [19,20,21,22]. Information on heterokairy and critical windows in air-breathing fishes—either Holosteans or Teleosteans—is especially incomplete. Most studies of these groups have focused on the morphology and physiology of the adult forms of the species [23,24,25,26,27,28]. Some studies have focused on embryonic and larval air-breathing fishes, which are the most vulnerable and where selective pressures act heavily [29]. Those studies have focused on larval and embryonic morphology and physiology of Teleosteans [30,31,32,33,34,35] and some Holosteans as Lepisosteids [36,37,38,39,40,41,42].
Lepisosteid larvae exhibit rapid embryonic and larval development, making them tractable models for studying early stages of fishes [37]. The tropical gar Atractosteus tropicus Gill 1863 is one of the seven extant Lepidosteid species and is distributed from Southern Mexico to Central America [43,44,45]. This species occurs in slow moving waters such as rivers, lakes, lagoons, and backwaters. The tropical gar can survive to low oxygen levels and moderately high temperatures [46]. In addition to the ecological role of the species in its habitat, A. tropicus represents socioeconomic and cultural significance in Tabasco (Southern Mexico) and surrounding areas with Olmec and Mayan cultures. For example, A. tropicus is a popular food item, especially because of its high nutritional quality at a low price. This species is also sold as pets, jewelry is made with their skin and scales, and whole fish are preserved as souvenirs. Moreover, A. tropicus is one of the five main fishery resources and cultured species in Mexico and it is an important species in the recreational fishing industry [43,44,45].
Our study has focused on the effect of gradients of temperature, air saturation, and salinity on survival, growth, and timing to key developmental events in embryos and larvae of the tropical gar. We employed a critical windows design comprising three early developmental periods, which we hypothesize could be developmental phases when characteristics of embryos and larvae change as a function of the dose of the stressor and the time of exposure. Furthermore, we also hypothesize that the stressors will have their greatest effects during egg incubation, which can lead to long-term affectations in the fish since embryos and early larvae are the most vulnerable stages in fish development [16,47,48]. We identified the major critical windows for survival and growth as a function of temperature, oxygen saturation, and salinity. We additionally assessed how the onset of hatching, yolk depletion, acceptance of exogenous feeding, free swimming, and snout shape change is altered by the different stressors applied during different developmental periods and treatments. The data generated by the current research contribute to the knowledge and understanding of the ecology and physiology of larval fishes and the interaction between stressors, time of exposure, and individual response.
2. Results
2.1. Survival as a Function of Stressor
2.1.1. Temperature
The highest survival rates at the end of the experimental period occurred in the control group (84%), followed by larvae from experimental groups P2-33 °C, P3-33 °C, P2-36 °C, and P3-36 °C (76–81%; p < 0.001). In contrast, fish from experimental groups P1-33 °C and P1-36 °C showed lower survival (59% and 52% of survival, respectively) and CE-36 °C the lowest (46%; p < 0.001; Figure 1a).
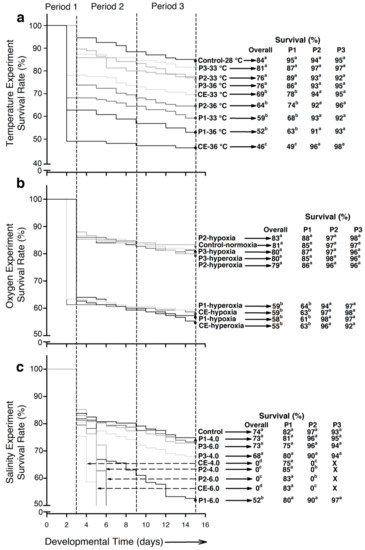
Figure 1.
Survival of embryos and larvae of Atractosteus tropicus during each experimental condition. (a) Temperature, (b) air saturation, (c) salinity. Differences in survival between groups for each experiment are represented by lowercase letters (p < 0.001). Black arrows indicate the corresponding survival percentages for each group. Dashed arrows in (c), indicate groups where all larvae died. n = 1350 fertilized eggs (150 per treatment) at the beginning of each experiment.
2.1.2. Air Saturation Level
Survival at the end of the hypoxia experiment ranged from 79% to 83% in the fish from the control and experimental groups P2-hypoxia, P3-hypoxia, P2-hyperoxia, and P3-hyperoxia. These values were significantly higher (p < 0.001) compared to larvae from experimental groups P1-hypoxia, P1-hyperoxia, CE-hypoxia, and CE-hyperoxia (55% to 59%; Figure 1b).
2.1.3. Salinity
Major differences in survival occurred (p < 0.001) due to the high mortality in experimental groups P2-4.0, P2-6.0, CE-4.0, and CE-6.0. At the end of the experiment, larvae from experimental group P1-6.0 exhibited the lowest survival (52%) and the rest of the experimental groups showed no significant differences (p > 0.05), with percentages between 68% and 74% (Figure 1c).
2.2. Survival as a Function of Development Stage
2.2.1. Period 1 (P1)—Fertilization to Hatch
- Temperature. Control larvae exhibited the highest survival (95%; p < 0.001) followed by larvae from experimental groups P2-33 °C, P3-33 °C, P2-36 °C, and P3-36 °C (74% to 89%). Lower survival (p < 0.001) was registered for fish from experimental groups P1-33 °C and P1-36 °C at 68% and 63%, respectively. The lowest survival (p < 0.001) occurred in larvae from experimental group CE-36 °C (49%; Figure 1a).
- Air saturation. Control larvae and those from experimental groups P2-hypoxia, P3-hypoxia, P2-hypeoxia, and P3-hyperoxia exhibited survival of 85–88%, which significantly differed (p < 0.001) from experimental groups P1-hypoxia, P1-hyperoxia, CE-hypoxia, and CE-hyperoxia (61–64%; Figure 1b).
- Salinity. No significant differences occurred in survival (p > 0.05), which ranged from 75% to 85% in the different groups (Figure 1c).
2.2.2. Period 2 (P2)—From 1 to 6 dph
- Temperature and air saturation. No significant differences were found (p > 0.05). Survival was >90% in both experiments (Figure 1a,b).
- Salinity. The highest salinity-induced mortality events occurred in this intermediate period (p < 0.001). By 2 days post hatch (dph), all larvae from experimental groups CE-4.0 and CE-6.0 had died. By 3 dph, the fish from experimental groups P2-4.0 and P2-6.0 had also died. The rest of the experimental groups showed no significant differences (p > 0.05; survival 90% to 97%; Figure 1c).
2.2.3. Period 3 (P3)—From 7 to 12 dph
- Developmental period 3 was the most resilient to stressors. No significant differences were found between experimental groups in any experiment (p > 0.05): temperature 91–98% survival, hypoxia-hyperoxia 92–98%, and salinity 93–97% (Figure 1a–c).
2.3. Body Morphology and Growth as a Function of Stressors and Stage of Exposure
2.3.1. Body Mass (BM)
- Temperature. At the end of developmental period 1, the control larvae displayed the lowest body mass (16.1 ± 0.6 mg), while larvae hatched in 33 °C and 36 °C exhibited the highest (BM ~19–23 mg; p < 0.001; Figure 2a). For developmental period 2, the highest BM was registered for larvae from experimental groups P1-33 °C (34.9 ± 3.9 mg) and CE-36 °C (39.2 ± 3,7 mg; p < 0.001; Figure 2a). By the end of developmental period 3, larvae from experimental groups P1-33 °C, P1-36 °C, CE-33 °C, and CE-33° showed higher BM (~38–43 mg) compared to the rest of the experimental groups (BM ~30 mg; p < 0.001; Figure 2a). Figure 2d illustrates how BM increased immediately after hatching in all the experimental groups, especially in CE-33 °C and CE-36 °C. At the beginning of developmental period 3, a slight increase in BM occurred in larvae from experimental groups P3-33 °C and P3-36 °C. However, after 2–3 days BM decreased to control values. The groups that exhibited the highest BM at the end of the experiment started increasing BM at ~9 dph.
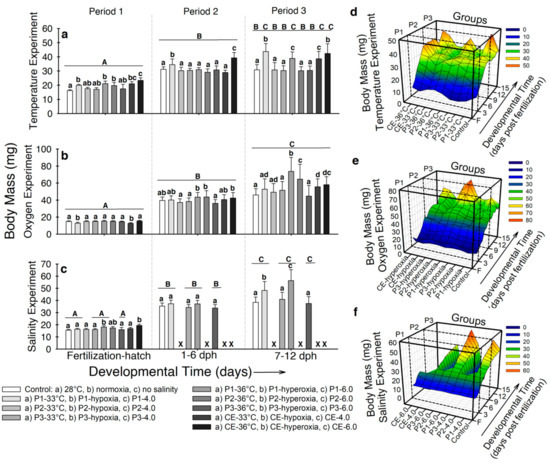 Figure 2. Body mass (BM) of Atractosteus tropicus at the end of each developmental period for (a) temperature, (b) air saturation, and (c) salinity experiments. Means ± SEM are presented. Lowercase letters represent differences between experimental groups from each developmental period (p < 0.001). Capital letters indicate differences within developmental time for each experimental group (p < 0.001). Panels (d–f) show the relation between development, treatments, and effect on the body mass of the fish in the whole experiments for temperature, air saturation, and salinity, respectively. Control conditions: 28 °C, 0.0 ppt of salinity, normoxia (95% air saturation); treatments 1: 33 °C, hypoxia (~30% air saturation), or 4.0 ppt of salinity respectively to each experiment; treatments 2 (independently for each experiment): 36 °C, hyperoxia (117% air saturation), or 6.0 ppt of salinity respectively to each experiment. Black crosses (X) in the salinity experiment represent groups where all fish died. n = 20 for each treatment.
Figure 2. Body mass (BM) of Atractosteus tropicus at the end of each developmental period for (a) temperature, (b) air saturation, and (c) salinity experiments. Means ± SEM are presented. Lowercase letters represent differences between experimental groups from each developmental period (p < 0.001). Capital letters indicate differences within developmental time for each experimental group (p < 0.001). Panels (d–f) show the relation between development, treatments, and effect on the body mass of the fish in the whole experiments for temperature, air saturation, and salinity, respectively. Control conditions: 28 °C, 0.0 ppt of salinity, normoxia (95% air saturation); treatments 1: 33 °C, hypoxia (~30% air saturation), or 4.0 ppt of salinity respectively to each experiment; treatments 2 (independently for each experiment): 36 °C, hyperoxia (117% air saturation), or 6.0 ppt of salinity respectively to each experiment. Black crosses (X) in the salinity experiment represent groups where all fish died. n = 20 for each treatment. - Air saturation. Larvae from experimental groups P1-hypoxia and CE-hypoxia displayed the lowest BM (~12 mg) at hatching (p < 0.001; Figure 2b). By developmental period 2, larvae from experimental groups P1-hyperoxia, P2-hyperoxia, and CE-hyperoxia exhibited the highest BM (~42 mg; p < 0.001; Figure 2b). By developmental period 3, fish from the control and P3-hyperoxia exhibited the lowest BM (~45 mg); conversely, individuals from experimental groups P1-hyperoxia and P2-hyperoxia showed the highest (BM ~64–73 mg; p < 0.001; Figure 2b). Figure 2e shows high variation in BM for all groups since the beginning of developmental period 2. Fish from experimental groups with the highest BM at the end of the experiment started showing differences at ~9 dph (p < 0.001). Body mass of larvae from experimental group P3-hyperoxia showed little variation through developmental period 3.
- Salinity. In developmental period 1, fish from experimental groups P1-6.0 and CE-6.0 showed the highest BM (18.12 ± 1.3 mg and 19.4 ± 1.6 mg respectively; p < 0.001; Figure 2c). At the end of developmental period 2, no significant differences in BM occurred between any of the five surviving groups (BM ~32–37 mg; p > 0.05). By developmental period 3, larvae from experimental groups P1-4.0 and P1-6.0 exhibited higher BM (48.2 ± 4.1 mg and 56.3 ± 6.1 mg, respectively) than the control and the rest of the groups (~37–40 mg; p < 0.001; Figure 2c). Figure 2f shows the BM to increase in P1-4.0 and P1-6.0 at ~7 dph and continued by the end of the experiment, while fish from the control and experimental groups P3-4.0 and P3-6.0 remained constant.
In summary, body mass was significantly increased by higher temperature during the three developmental periods, decreased in hypoxic groups during incubation and increased in hyperoxic experimental groups exposed during developmental periods 1 and 2, and increased at the end of the salinity experiment in fishes incubated at either 4.0 ppt and 6.0 ppt of salinity.
2.3.2. Total Length (LT)
- Temperature. Larvae exposed to either 33 °C or 36 °C during incubation exhibited higher total length (LT) (~10–12 mm) compared to the controls (9.1 ± 0.36 mm; p < 0.001; Figure 3a). For developmental period 2, the highest LT was observed in larvae from experimental groups CE-36 °C (22.9 ± 1.3 mm; p < 0.001; Figure 3a). By developmental period 3, larvae from experimental groups P1-33 °C, P1-36 °C, CE-33 °C, and CE-36 °C showed higher LT (~20–22 mm) than the control larvae (19.1 ± 0.7 mm; p < 0.001; Figure 3a). The interaction of all variables during the complete experiment is presented in Figure 3d. Total length from fish from experimental groups P1-33 °C and CE-36 °C significantly increased from incubation through 12 dph, compared to the rest of the groups (p < 0.001). However, larvae from experimental group P1-36 °C showed increased LT by hatching, but during developmental period 2, the values were closer to the control larvae (~19 mm).
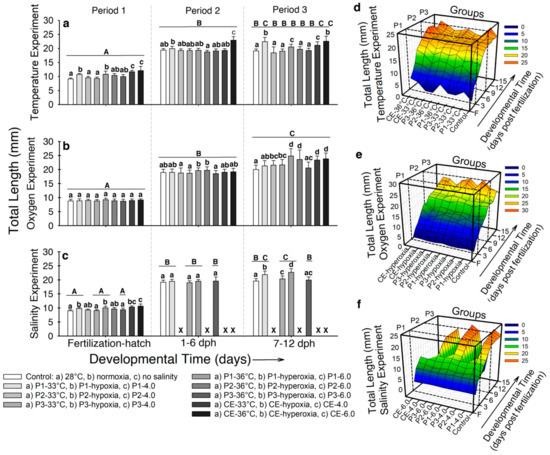 Figure 3. Total length (LT) of Atractosteus tropicus at the end of each developmental period for the experiments with (a) temperature, (b) air saturation, and (c) salinity. Means ± SEM are presented. Lowercase letters represent differences between experimental groups from each developmental period (p < 0.001). Capital letters indicate differences within developmental time for each experimental group (p < 0.001). Panels (d–f) show the relation between development, treatments, and effect on the total length of the fish in the whole experiments for temperature, air saturation, and salinity, respectively. Control conditions: 28 °C, 0.0 ppt of salinity, normoxia (95% air saturation); treatments 1: 33 °C, hypoxia (~30% air saturation), or 4.0 ppt of salinity respectively to each experiment; treatments 2 (independently for each experiment): 36 °C, hyperoxia (117% air saturation), or 6.0 ppt of salinity respectively to each experiment. Black crosses (X) in the salinity experiment represent groups where all fish died. n = 20 for each treatment.
Figure 3. Total length (LT) of Atractosteus tropicus at the end of each developmental period for the experiments with (a) temperature, (b) air saturation, and (c) salinity. Means ± SEM are presented. Lowercase letters represent differences between experimental groups from each developmental period (p < 0.001). Capital letters indicate differences within developmental time for each experimental group (p < 0.001). Panels (d–f) show the relation between development, treatments, and effect on the total length of the fish in the whole experiments for temperature, air saturation, and salinity, respectively. Control conditions: 28 °C, 0.0 ppt of salinity, normoxia (95% air saturation); treatments 1: 33 °C, hypoxia (~30% air saturation), or 4.0 ppt of salinity respectively to each experiment; treatments 2 (independently for each experiment): 36 °C, hyperoxia (117% air saturation), or 6.0 ppt of salinity respectively to each experiment. Black crosses (X) in the salinity experiment represent groups where all fish died. n = 20 for each treatment. - Air saturation. No significant differences were observed in fish LT by developmental period 1 (LT ~9 mm; p > 0.05; Figure 3b). In developmental period 2, larvae from experimental groups P1-hyperoxia and P2-hyperoxia showed higher LT (19.6 ± 0.9 mm; 19.7 ± 1.2 mm, respectively) compared to the rest (LT ~18.5 mm; p < 0.001; Figure 3b). For developmental period 3, LT was higher in larvae from experimental groups P1-hyperoxia (24.8 ± 1.6 mm), P2-hyperoxia (23.6 ± 2.3 mm), CE-hyperoxia (23.8 ± 2.2 mm), and CE-hypoxia (23.3 ± 1.3 mm; p < 0.001) compared to the control (20 ± 1.8 mm; Figure 3b). Figure 3e shows the interaction of development, LT, and time of exposure to hypoxia and hyperoxia throughout the experiment. All treatments showed a similar tendency in the LT increase from incubation to ~9 dph. However, at this point, LT of fishes from experimental group P1-hyperoxia, P2-hyperoxia, CE-hyperoxia, and CE-hypoxia showed major increase in LT than the fish in the rest of the groups.
- Salinity. By the end of developmental period 1, larvae from experimental groups P1-4.0, CE-4.0, P1-6.0, and CE-6.0 showed higher LT (~9.6-10 mm) compared to fish from the control and experimental groups P2-4.0, P3-4.0, P2-6.0, and P3-6.0 (LT ~9.2 mm; p < 0.001; Figure 3c). No significant differences in LT were observed at developmental period 2 between experimental groups (LT ~19.2 mm; p > 0.05; Figure 3c). At the end of the experiment, only larvae from experimental groups P1-4.0 and P1-6.0 showed significantly higher LT values (~20.3–21-8 mm) compared to the control and larvae from experimental groups P3-4.0 and P3-6.0 (~19.7 mm; p < 0.001; Figure 3c). The interaction of LT, development, and the groups during the whole experiment is presented in Figure 3f. From incubation to ~9 dph, all groups showed similar patterns of increase LT. Right after this point, fish from experimental groups P1-4.0 and P1-6.0 showed higher values of LT (~21 mm) than the control (19.2 ± 1.1 mm; p < 0.001), which continued through the experiment.
In summary, total length was increased by higher temperature, especially during continuous exposure. Hyperoxia during developmental periods 1 and 2, and continuous hypoxia and hyperoxia increased LT increased LT at the end of the experiment. Salinities of 4.0 ppt and 6.0 ppt during incubation increased LT at the end of the experiment.
2.3.3. Specific Growth Rate (SGR)
- Temperature. During developmental period 1, fish from the control and experimental group P2-33 °C showed the lowest specific growth rate (SGR) (~0.7% d−1), while the highest SGR was observed in larvae from experimental group CE-36 °C (1.3 ± 0.2% d−1; p < 0.001; Figure 4a).
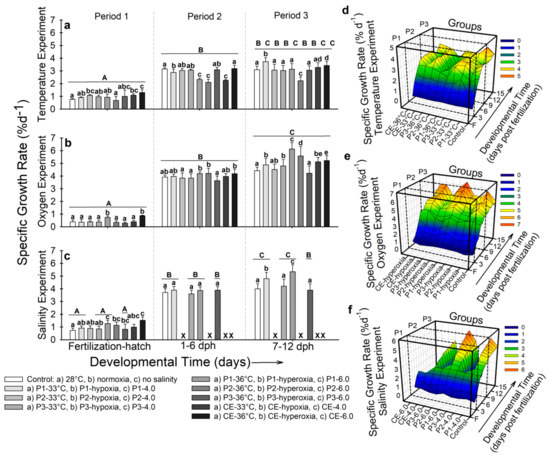 Figure 4. Specific growth rate (SGR) of Atractosteus tropicus. (a) Temperature, (b) air saturation, and (c) salinity experiments at the end of each developmental period. Means ± SEM are presented. Lowercase letters represent differences between experimental groups from each developmental period (p < 0.001). Capital letters indicate differences within developmental time for each experimental group (p < 0.001). Panels (d–f) show the relation between development, treatments, and effect on the specific growth rate of the fish in the whole experiments for temperature, air saturation, and salinity, respectively. Control conditions: 28 °C, 0.0 ppt of salinity, normoxia (95% air saturation); treatments 1: 33 °C, hypoxia (~30% air saturation), or 4.0 ppt of salinity respectively to each experiment; treatments 2 (independently for each experiment): 36 °C, hyperoxia (117% air saturation), or 6.0 ppt of salinity respectively to each experiment. Black crosses (X) in the salinity experiment represent groups where all fish died. n = 20 for each treatment.
Figure 4. Specific growth rate (SGR) of Atractosteus tropicus. (a) Temperature, (b) air saturation, and (c) salinity experiments at the end of each developmental period. Means ± SEM are presented. Lowercase letters represent differences between experimental groups from each developmental period (p < 0.001). Capital letters indicate differences within developmental time for each experimental group (p < 0.001). Panels (d–f) show the relation between development, treatments, and effect on the specific growth rate of the fish in the whole experiments for temperature, air saturation, and salinity, respectively. Control conditions: 28 °C, 0.0 ppt of salinity, normoxia (95% air saturation); treatments 1: 33 °C, hypoxia (~30% air saturation), or 4.0 ppt of salinity respectively to each experiment; treatments 2 (independently for each experiment): 36 °C, hyperoxia (117% air saturation), or 6.0 ppt of salinity respectively to each experiment. Black crosses (X) in the salinity experiment represent groups where all fish died. n = 20 for each treatment.
In developmental period 2, the highest SGR was observed in the control (3.15 ± 0.1% d−1) and experimental group CE-36 °C (3.17 ± 0.3% d−1), and the lowest by experimental groups P1-36 °C, P2-36 °C, and CE-33 °C (~2.1–2.3% d−1; p < 0.001; Figure 4a). At the end of developmental period 3, the highest SGR was registered in larvae from experimental groups P1-33 °C (3.7 ± 0.3% d−1) and the lowest in larvae from experimental group P2-36 °C (SGR = 2.2 ± 0.2% d−1; p < 0.001; Figure 4a). Figure 4d shows the interaction of temperature, time of exposure, and SGR. High variation can be observed in most groups compared to the control. Larvae from P2-36 °C (lowest SGR) showed low increases in SGR since the beginning of developmental period 2 (p < 0.001). Larvae from the group with highest SGR (P1-33 °C) at the end of the experiment started increasing its values at ~10 dph.
- Air saturation. At the end of developmental period 1, fish from experimental groups P1-hyperoxia and CE-hyperoxia showed the highest SGR (0.74–0.86% d−1; p < 0.001; Figure 4b). By developmental period 2, fish from experimental groups P1-hyperoxia, P2-hyperoxia, and CE-hyperoxia exhibited the highest SGR (~4.5% d−1) and the lowest was registered in larvae from P3-hyperoxia (3.5 ± 0.4% d−1), which did not present significant differences compared to the control (3.9 ± 0.2% d−1; p > 0.05; Figure 4b). At the end of the experiment, fishes from experimental groups P1-hyperoxia and P2-hyperoxia showed the highest SGR values (6.1 ± 0.4% d−1 and 5.4 ± 0.8% d−1 respectively; Figure 4b). Figure 4e shows the overall interactions in this experiment. From ~6 dph, larvae from experimental group P3-hyperoxia showed no increase in SGR throughout 12 dph. Fish from groups with the highest SGR (P1-hyperoxia and P2-hyperoxia) increased their SGR at ~9 dph. For the rest of the groups, a continuous but lower increase than P1-hyperoxia and P2-hyperoxia occurred (p < 0.001).
- Salinity. By developmental period 1, SGR in experimental groups P1-6.0 (1.28 ± 0.1% d−1) and CE-6.0 (1.53 ± 0.2% d−1) was significantly higher than larvae from the control (0.73 ± 0.1% d−1; p < 0.001; Figure 4c). During developmental period 2, no significant differences (p > 0.05) were observed (SGR ~3.4–3.9% d−1; Figure 4c). By developmental period 3, higher SGR was observed in experimental groups P1-4.0 (4.8 ± 0.6% d−1) and P1-6.0 (5.3 ± 0.8% d−1) compared to the rest of the fish (SGR ~3.9–4.2% d−1; p < 0.001; Figure 4c). Figure 4f shows similar values of SGR in all the groups by hatching and 1 dph for the seven surviving populations. The control group and the four surviving groups show a similar pattern in SGR along the experiment. However, by 8 dph, fish from P1-4.0 and P1-6.0 started to show higher SGR values (p < 0.001).
In summary, specific growth rate was increased by higher temperature during developmental periods 1 and 2 and slightly increased by the end of the experiment in continuous exposure groups and fish incubated at 33 °C. Hypoxia during developmental periods 1 and 3 slightly increased SGR at the end of the experiment, while hyperoxia increased SGR during developmental periods 1 and 2, which was constant at the end of the experiment. Salinities of 4.0 ppt and 6.0 ppt increased SGR at the end of the experiment in fish incubated under these conditions.
2.3.4. Fulton’s Condition Factor (K)
- Temperature. In developmental period 1, larvae from the control group and experimental groups P2-33 °C and P3-33 °C showed the highest Fulton’s condition factor (K) values (2.13 ± 0.3) and the larvae from experimental group CE-33 °C presented the lowest (1.32 ± 0.2; p < 0.001; Figure 5a). No significant differences were exhibited by developmental periods 2 and 3 (p > 0.05; Figure 5a). Figure 5d shows the interaction of K values, temperature, and time of exposure. Condition factor decreases in all the groups as development progresses. Peaks in experimental groups P3-33 °C, P3-36 °C, and CE-33 °C occurred at different times of development (7 dph, 9 dph, and 4 dph respectively). However, by the end of developmental periods 2 and 3, no difference was observed (p > 0.05).
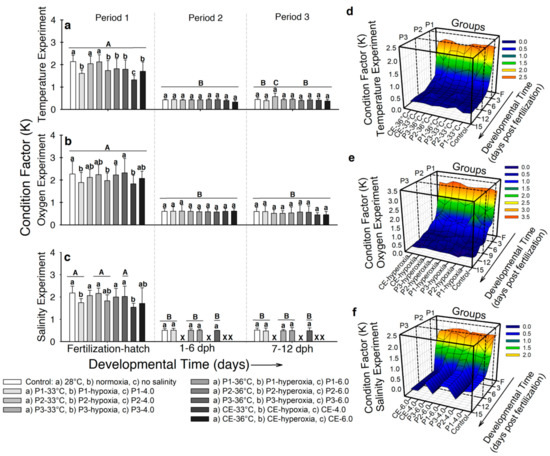 Figure 5. Fulton’s condition factor (K) of Atractosteus tropicus at the end of each developmental period. (a) Temperature, (b) air saturation, and (c) salinity experiments. Means ± SEM are presented. Lowercase letters represent differences between experimental groups from each developmental period (p < 0.001). Capital letters indicate differences within developmental time for each experimental group (p < 0.001). Panels (d–f) show the relation between development, treatments, and effect on condition factor of the fish in the whole experiments for temperature, air saturation, and salinity, respectively. Control conditions: 28 °C, 0.0 ppt of salinity, normoxia (95% air saturation); treatments 1: 33 °C, hypoxia (~30% air saturation), or 4.0 ppt of salinity respectively to each experiment; treatments 2 (independently for each experiment): 36 °C, hyperoxia (117% air saturation), or 6.0 ppt of salinity respectively to each experiment. Black crosses (X) in the salinity experiment represent groups where all fish died. n = 20 for each treatment.
Figure 5. Fulton’s condition factor (K) of Atractosteus tropicus at the end of each developmental period. (a) Temperature, (b) air saturation, and (c) salinity experiments. Means ± SEM are presented. Lowercase letters represent differences between experimental groups from each developmental period (p < 0.001). Capital letters indicate differences within developmental time for each experimental group (p < 0.001). Panels (d–f) show the relation between development, treatments, and effect on condition factor of the fish in the whole experiments for temperature, air saturation, and salinity, respectively. Control conditions: 28 °C, 0.0 ppt of salinity, normoxia (95% air saturation); treatments 1: 33 °C, hypoxia (~30% air saturation), or 4.0 ppt of salinity respectively to each experiment; treatments 2 (independently for each experiment): 36 °C, hyperoxia (117% air saturation), or 6.0 ppt of salinity respectively to each experiment. Black crosses (X) in the salinity experiment represent groups where all fish died. n = 20 for each treatment. - Air saturation. In developmental period 1, larvae from the control and experimental groups P2-hypoxia, P3-hypoxia, P2-hyperoxia, and P3-hyperoxia showed higher K values (K~2.1) than the larvae incubated in hypoxia and hyperoxia (K~1.8; p < 0.001; Figure 5b). No significant differences in K occurred in developmental periods 2 and 3 and K values occurred as ~0.57 (p > 0.05; Figure 5b). Figure 5e shows how K values decreased in most of the groups from hatch to 5 dph. Two peaks were registered at 2 dph and 3 dph for experimental groups P1-hypoxia and P2-hypoxia, respectively. However, one day later K decreased to values close to the control group (K = 1.2 ± 0.1).
- Salinity. At the end of developmental period 1, the control and larvae from experimental groups P2-4.0, P3-4.0, P2-6.0, and P3-6.0 showed higher K values (~2.1) than larvae incubated in higher salinity (K ~1.7–1.9; p < 0.001; Figure 5c). No differences in K occurred by developmental periods 2 and 3 (K ~0.5; p > 0.05; Figure 5c). Figure 5f shows how K values decreased from hatching to 5 dph and remained constant for the rest of the experiment in the five surviving groups.
In summary, Fulton’s condition factor was decreased by the three stressors during incubation. However, no differences occurred in developmental periods 2 and 3 for any experiment.
2.4. Timing of Developmental Events as a Function of Stressors and Exposure Stage
The experimental treatments resulted in significant changes in the timing of developmental events (Table 1).

Table 1.
Time of occurrence (hours post fertilization) of key developmental events in Atractosteus tropicus. Significant differences between groups from every experiment for time to hatching, exogenous feeding, yolk depletion, free swimming, and mouth opening are denoted by lowercase letters. Mean ± SD are presented. n = 30 fish per experiment.
2.4.1. Time to Hatching
Time to hatching was significantly decreased by increasing temperature (p < 0.001). Embryos incubated at 28 °C hatched at ~63 h post fertilization (hpf), while embryos incubated in 33 °C and 36 °C hatched at ~48 hpf and ~36 hpf, respectively (Table 1). Temperature sensitivity for hatching showed higher Q10 values between 33 °C and 36 °C (Q10 = 2.5) while lower values were exhibited by intervals 28–33 °C (Q10 = 1.5 ± 0.24) and 28–36 °C (Q10 = 0.89 ± 0.09; p < 0.001; Table 2).

Table 2.
Temperature sensitivity in key developmental events in the tropical gar in the temperature experiment. Mean ± SD are presented. Differences between temperature intervals for each developmental event are indicated with different lowercase letters. n = 30 fish per group.
2.4.2. Time to Exogenous Feeding
In the temperature experiment, larvae from the control and experimental groups P2-33 °C, P3-33 °C, P2-36 °C, and P-36 °C began exogenous feeding at ~109–116 hpf. The larvae from experimental groups P1-33 °C, CE-33 °C, P1-36 °C, and CE-36 °C started feeding significantly earlier (~ 82–98 hpf; p < 0.001; Table 1). The largest temperature sensitivity occurred between 33 °C and 36 °C (Q10 = 3.1; p < 0.001; Table 2), with primarily temperature insensitivity at the highest temperature range. All larvae in the air saturation experiment showed no differences in the onset of exogenous feeding (~122 hpf; p > 0.05; Table 1). Fish from the control and the surviving larvae in the salinity experiment did not exhibit significant differences in the onset of feeding (~122 hpf; p > 0.05).
2.4.3. Time to Yolk Depletion
Larvae that hatched in 33 °C and 36 °C depleted the yolk sac in ~118 hpf and ~96 hpf, respectively, which was significantly sooner than the control (~ 124 hpf; p < 0.001; Table 1). Individuals from experimental groups P2-33 °C, P3-33 °C, P2-36 °C, and CE-36 °C depleted the yolk at ~122 hpf (Table 1). Temperature sensitivity was observed only between 33 °C and 36 °C (Q10 = 3.05; p < 0.001; Table 2). In the air saturation experiment, yolk sac depletion was significantly delayed in larva from experimental groups P1-hypoxia and CE-hypoxia (~132 hpf; p < 0.001; Table 1). However, fish from experimental group P2-hyperoxia exhibited the shortest depletion time (125 ± 2.5 hpf). No significant differences were observed in the salinity experiment (p > 0.05), with a time to yolk depletion of ~126 hpf (Table 1).
2.4.4. Time to Free Swimming
In the temperature experiment, time to free swimming in the control was ~140 hpf and differed from fish from experimental group CE-6.0 (~96 hpf; p < 0.001). Individuals from experimental groups P1-33 °C, P2-33 °C, P2-36 °C, and P3-36 °C started swimming significantly earlier than the control (~102 hpf to ~96 hpf; p < 0.001; Table 1). Large temperature sensitivity only occurred between 33 °C and 36 °C (Q10 = 3.05; p > 0.001; Table 2). In the air saturation experiment, larvae from the control started swimming at ~130 hpf and individuals from experimental groups P1-hypoxia and P1-hyperoxia started swimming ~6 h later (p < 0.001; Table 1). In the salinity experiment, larvae from the control and experimental groups P3-4.0 and P3-6.0 started swimming at ~144 hpf, while P1-4.0 and P1-6.0 started later at ~152 hpf and ~154 hpf, respectively (p < 0.001; Table 1).
2.4.5. Time to Snout Shape Change
Change in the shape of the snout in larvae from the control occurred at ~168 hpf in the temperature experiment, at ~152 hpf in those from experimental groups P2-33 °C and P2-36 °C, and at ~120 hpf for the larvae in continuous exposure to 33 °C and 36 °C (p < 0.001; Table 1). Temperature sensitivity was exhibited primarily between 33 °C and 36 °C (Q10 = 3.12; p < 0.001; Table 2). In the air saturation experiment, snout shape change occurred at ~172 hpf in the control group and almost all the experimental groups (183 ± 1.3 in larvae from P1-hypoxia; p < 0.001; Table 1). No differences were observed for time to snout shape change in the salinity experiment (~174 hpf; p > 0.05; Table 1)
3. Discussion
3.1. Critical Windows for Survival
Organismal survival is affected by numerous factors and their combined effects. For example, changes in salinity and dissolved oxygen come together with water warming because of climate change and anthropogenic activities [49,50]. In the present study on Atractosteus tropicus, there were highly differential effects of environmental stressors. High temperature, hypoxia, and hyperoxia decreased survival during incubation; salinities of 4.0 ppt and 6.0 ppt after hatching killed all larvae (during developmental period 2). These data suggest that critical windows for survival vary with stressor type, as well as stressor dose. Moreover, the incubation period in early development of A. tropicus represents a critical window for temperature, hypoxia, and hyperoxia in contrast to a critical window of the first two days post hatch for salinity. Several studies have demonstrated how temperature and dissolved oxygen affect survival (especially during incubation) in Teleosteans and Holosteans [34,51,52,53,54,55]. When fish are incubated in high temperatures, survival can be affected because the yolk conversion efficiency can be reduced, and the cost of development increases with increasing temperature. Moreover, surviving individuals may show long-term alterations [16]. Furthermore, temperature acts as a lethal factor when the tolerance of individuals is exceeded [56]. Temperatures used in the current research did not reach the maximum temperature of 38 °C tolerated by A. tropicus [37]. However, a temperature of 36 °C may be lethal for embryos of the tropical gar in the current study since 50% of the individuals died before hatching, suggesting that 36 °C can represent the incipient lethal level for embryos of this species. Moreover, air saturation may act as a limiting factor, and can become lethal if severe enough [56]. Decreased survival in hypoxic treatments of the current study could have occurred since embryos’ oxygen supply depends on oxygen diffusion pressure, and the rate of flow [56]. Moreover, decreased survival in hyperoxic treatments could be related to oxidant-related damage and/or minor gas bubble trauma [57,58].
Numerous primitive fishes can tolerate environmental salinities higher or lower than their plasma osmolality. However, there is little information regarding salinity and their early life stages and available information exists only for juveniles and adults [59,60,61,62,63,64]. Lack of maturity of their ionoregulatory and osmoregulatory organs likely explains why newly hatched larvae in the present study could not cope with environmental salinities that could be easily tolerated by adults, since gills are the principal organ for iono/osmoregulation in larval fishes [65,66,67,68]. Atractosteus tropicus has visible gills at hatch, but regular gill ventilation did not start until ~5 dph [37]. Gill maturation was likely not complete at 5 dph, leading to the high mortality in the hatchlings.
3.2. Growth
Growth in the developing tropical gar was shaped by all three experimental stressors, but the growth responses differed with stressor dose and time of exposure. Generally, a major increase in BM, TL, and SGR tended to occur from fertilization to 6 dph. However, slow growth was observed during 7–12 dph, which corresponds to the period of the complete transition to exogenous feeding [51]. A similar growth pattern to A. tropicus occurred in A. tristoechus [51], A. spatula [42], Lepisosteus osseus [69], and L. oculatus [70], suggesting that this pattern is conserved among Lepisosteid species. These findings suggest that the period of transition from lecithotrophy to exotrophy could represent a critical window not just for growth but also for survival, especially when the young larvae must cope with the changing environment and the development of feeding strategies [71].
Temperatures close to the upper thermal limit (38 °C) [37] of A. tropicus during 1–6 dph drastically reduced SGR (Figure 4a), which suggests that this period may represent a critical window for growth at high temperatures. Despite the differences in BM, LT, and SGR, Fulton’s condition factor was constant from 1–12 dph for A. tropicus in the current study. Similar results were reported for A. tristoechus [51]. In contrast with our results, some Teleosteans show no significant relationship between incubation temperature and mass and length at hatch [72,73,74,75].
Growth rate of freshwater fishes can increase in tolerable salinities due to reduced osmoregulatory costs [76]. However, when exposed to higher concentrations, osmoregulatory costs can interfere with growth rate. This conjecture is consistent with the findings of the current study, where larvae incubated in low salinity showed higher SGR (~5 dph). There is a relationship between growth, air breathing, and salinity in juveniles of spotted gar and alligator gar [64,77], but no data are available for embryonic or larval gars. In the present study, BM, LT, and SGR were larger in larvae incubated at salinity of 6.0. A similar result was observed in larvae of the common carp (Cyprinus carpio), where higher salinities (5.0 to 20.0) increased BM but not body length [78]. In contrast, some studies showed that larval fishes incubated and raised in higher salinity decreased body length, e.g., Gymnocephalus cernuus [79] and two endemic Mexican silversides, Chirostoma humboldtianum and C. riojai [80]. These findings of either increased or decreased mass or length have been attributed to differences in water content of the species [81], which depends on the permeability of gills and body surface to water and salts in the environment [82].
In the current study, several differences in growth rate occurred depending on the oxygen levels in the water and time of exposure. The incubation period in A. tropicus can represent a critical window for growth in fishes exposed to either hypoxia or hyperoxia. Our results suggest that (1) low oxygen during incubation causes a reduction in size of post hatched larvae, as described in other studies [83,84]; and (2) high oxygen during incubation and in pre-metamorphic larvae of the tropical gar favors growth. However, after metamorphosis high oxygen levels actually decreased growth. In the first case, a reduction in growth can occur due to a compensatory response of the embryos to prioritize other organismal activities as a function of oxygen availability [85]. Moreover, fishes with decreased growth have lower chances of survival, especially because of slower swimming, lower competitivity, the presence of deformities, and a major risk of predation [52,53,86,87].
3.3. Developmental Events
In the current study, differences in timing of occurrence of five developmental events occurred as a function of temperature, salinity, and air saturation level. However, no alterations of the sequence of these developmental events were observed, as described for mollusks [88].
3.3.1. Time to Hatching
We report that environmental factors altered time to hatch, either enhancing or delaying the event. This variation in time to hatch demonstrates that heterokairy is also dependent on stressor dose and type of stressor [9,14]. In our study, time to hatch was modified by higher temperature and is consistent with previous studies where individuals hatch between 36–72 hpf when incubated between 25 °C and 35 °C [89,90]. In other Lepisosteid species, variations in time to hatch occur in similar patterns to A. tropicus, suggesting that these responses to changes in temperature, air saturation and salinity are similar within Lepisosteids [38,41,42,51,91,92]. Moderate salinity promotes longer periods of incubation and can also promote a reduction in the developmental rate by redirecting the available energy to osmoregulation and/or as a response to the ionic/osmotic stress [93]. As in the current study, Gymnocephalus cernua show an increased incubation time and promoted morphological alterations, especially in body length [79]. In addition, some fish species incubated in different air saturations may hatch prematurely whereas hatching may be delayed in others [53]. Premature hatching in hypoxic conditions occurred in A. tropicus, as described in other fish species and in amphibians [34,53,94,95,96]. Moreover, decreased air saturation also produces a delay in developmental rate, resulting in larvae hatching at earlier stages of development with diminished body mass [34]. These data are consistent with the findings in the current study since larvae incubated as embryos in hypoxia showed decreased BM, which can be correlated with earlier hatching. Furthermore, these alterations are related to a respiratory response for coping with environmental hypoxia [34]. This is also relevant to the assumption that the oxygen levels shape the onset and maturation of gill ventilation and air-breathing in A. tropicus.
3.3.2. Time to Exogenous Feeding
The onset of exogenous feeding represents a crucial time point in the development of the fishes and is related to increased mortality [97]. As larvae develop, the energy budget provided by the yolk decreases, metabolism increases, and the developing larvae must look for prey to cope with the body’s energy demands [98]. Exogenous feeding in A. tropicus occurs around 5 dph [36], which is when the control groups in the current study started consuming prey. However, higher temperature modified the onset of predation but, surprisingly, salinity and air saturation had no effect. Incubation period could represent a critical window for the onset of feeding at higher temperatures, since treatments P1-33 °C and P1-36 °C showed decreased time to predation compared to the control group and without considering the continuous exposure treatments (Table 1). Temperature accelerates fish development, which increases the energy demand of the organism for survival, growth, and maturation. Moreover, prey capture and consumption in the current study occurred when yolk sac was not completely absorbed, which prepares the larvae for the exotropic life and assists digestive tract maturation [99,100]. Lecitoexotrophy has also been described for A. tristoechus [101]. These yolk reserves provide the necessary energy of the young fishes for foraging [102,103] and competition for resources [104,105]. In natural populations of A. tropicus, an earlier onset of exogenous feeding promotes cannibalism and this characteristic is the main reason for the high variation in mass-size relationship as reported in other studies [36,37,89].
3.3.3. Time to Yolk Depletion
In the present study, variations in the timing to this key developmental landmark occurred as a function of temperature and air saturation. Hypoxia and higher temperature during incubation, and higher temperature during the first days after hatching, could represent critical windows for yolk depletion, but further research is needed. Temperature is an environmental factor that promotes faster yolk absorption. However, there is a trade-off between rapid development and the efficient utilization of the energy provided by the yolk sac. Most of the species reared at higher temperature than their optimal face this issue. Even when temperature is combined with salinity, the effects on yolk absorption and utilization by temperature are much greater than those caused by salinity [106,107,108]. In terms of energy utilization and yolk depletion, indirect observation on the larvae incubated at 33 °C showed an increase in BM, LT, and SGR, suggesting that energy was efficiently allocated in these larvae. However, weight and/or volume of the yolk sac was not measured and this increase in BM could be related to an advanced developmental stage rather than the control group.
Studies on salinity concentrations in freshwater fishes and yolk absorption are scarce. However, some freshwater fishes (e.g., Salvelinus fontinalis) are reared in low salinity where freshwater is not readily available, increasing yolk absorption efficiency and growth consequently, but significantly decreasing survival [109].
Hypoxia promotes a delayed development that can be observed through the delayed yolk absorption in several fish species [52,110,111,112]. In the present study, this association was partially true because larvae incubated under hypoxia and transferred to normoxia showed delayed yolk absorption. However, larvae continuously exposed to hypoxia showed values similar to the controls. These results suggest that it is a greater challenge for the larvae to cope with the increasing air saturation when incubated in hypoxia than when acclimating to longer hypoxic events as older individuals. This assertion is based on the ability of continuously exposed individuals to maintain similar timing for yolk depletion and growth parameters to the control group.
3.3.4. Time to Free Swimming
A crucial developmental event in fish is the onset of swimming [113]. Free swimming occurs at 5–6 dph in A. tropicus [36] and in a period of 4–10 days for A. tristoechus [101]. Lepisosteids and other species with indirect development must go through an eleuteroembryo stage and spend time attached to the substrate for the maturation of swimming structures [114]. Nonetheless, the onset of free swimming can be affected by several factors. In the present study, the first and second developmental periods could represent a critical window for the onset of swimming in the current study when embryos and larvae are exposed to high temperature, salinity, hypoxia, and hyperoxia. Acceleration in the onset of free swimming occurred due to increased temperature during incubation and the second period of exposure (Table 1). This is explained by the effect of increased temperature on muscle development, fin differentiation, and the maturation of other structures that facilitates fish kinematics for swimming [114,115].
Salinities of 4.0 ppt and 6.0 ppt delayed the timing of free swimming in larvae incubated under these conditions and then transferred to fresh water. Salinity reduces the activity of larval fish in response to ionic and osmotic disruption [81]. In A. tropicus, even though activity was not quantified, larvae that died in the second period appeared to show less activity than the rest of the treatments, as well as characteristics such as bradycardia and ischemic stress.
Hypoxia diminishes activity in larvae and delays the onset of swimming, while hyperoxia presents little to no effect on swimming onset [116,117]. In the current experiments in A. tropicus, (1) larvae exposed to hypoxia during the second period did not show differences in timing to free swimming in comparison to the control group or the hyperoxic treatments; and (2) larvae that were incubated in hypoxia exhibited a delay in the onset of free swimming. The first case can be related to the onset of air-breathing (which occurred earlier than the control group) that helped the larvae to offset the aquatic hypoxia demands, which can occur as early as 2.5 dph [37]. In the second case, future studies are needed to assess the possible critical windows during incubation that promote these affectations in the organisms after hatching.
3.3.5. Time to Snout Shape Change
The timing in development of the pronounced snout of A. tropicus in this study corresponded to the previous reports of this event at around 7 dph [36,118]. Exposure to higher temperature during incubation and from 1–6 dph, as well as hypoxia during incubation, could represent critical windows for the onset of the change in the snout of A. tropicus.
Larvae exposed to higher temperature during 1–6 dph displayed the most rapid snout change. In contrast, larvae incubated in hypoxia and transferred to normoxia experienced a delay in this characteristic. This finding supports the need to study more specialized critical windows during incubation in A. tropicus to understand alterations induced by environment. Moreover, there is scant information on this topic, especially for Lepisosteids. Differences can occur in the timing of some characteristics, for example, the timing on mouth opening between species of gars [36]. The alligator gar (A. spatula) exhibited the allometric growth of the snout towards the proportion of the size of the adults earlier in size and age than A. tropicus. This characteristic was related to the faster metamorphosis of A. spatula that enhances the opportunity to hunt and catch prey and the earlier appearance of cannibalism [36,119,120,121]. However, A. tropicus shows high rates of cannibalism [37,89] but larvae in the current study did not show this behavior due to the feeding protocol, which can also favor the larviculture of this species.
4. Materials and Methods
4.1. Ethical Statement
Animals were handled in compliance with the Norma Oficial Mexicana NOM-062-ZOO-1999 from Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, the Mexican standards for good welfare practices of laboratory animals.
4.2. Fish Acquisition and Maintenance
Eggs and larvae of the tropical gar were obtained from three artificially induced spawnings of broodstock in May 2017, May 2018, and May 2019 carried out in the Laboratorio de Acuicultura Tropical of the Universidad Juárez Autónoma de Tabasco, Mexico. Each female was anesthetized with 200 mg L−1 of tricaine methanesulfonate, MS-222® (Agent Chemical Laboratories) and then injected with 35 μg kg−1 of luteinizing hormone-releasing analog (LNRHa) to induce egg laying. Each female and five males were placed for spawning into 2000 L tanks with artificial substrate for egg adhesion.
Three major experiments—one with each spawning and employing different experimental conditions (described below)—were performed to determine critical windows for survival, growth, and development. Sex cannot be determined in the embryos or larvae of A. tropicus, so data are reported assuming random mixing of sexes. Immediately after fertilization, fertilized eggs (3.08 ± 1.2 mm each) were transferred to 27 white 80 L holding tanks for incubation and development. For each experiment, one control and eight experimental groups were created (described in the next section). Each experiment was performed in triplicate (50 larvae per triplicate, 150 per group) with a total of 1350 fertilized eggs at the beginning of each experiment.
All tanks housing larvae contained non-chlorinated normoxic water (~95% air saturation; except in one experiment as described below), pH of 8.0, at ambient temperature (27–28 °C; except when indicated in one experiment). Photoperiod was 12 h light–12 h darkness and salinity of 0.0 ppt (with the exception of one experiment). After yolk absorption, larvae were fed ad libitum with nauplii of Artemia sp. every four hours from 8:00 a.m. to 8:00 p.m. Fifty percent of the water in the tanks was replaced every two days and feces and dead artemia were cleaned by siphoning one hour after every meal.
4.3. Developmental Stages and Experimental Design
Three developmental periods of the tropical gar were examined, based on a previously described scheme [36]: (1) the time from fertilization to hatching (60–72 h from fertilization); (2) yolk depletion stage (~from 1 day post hatch (dph) to ~6 days post hatch); and (3) pre-juvenile stage (~7–12 days post hatch). These three periods correspond to incubation, metamorphosis of the eleuteroembryo, and pre-juvenile stage, respectively. These stages are important to investigate the effect of environmental factors on the biology of developing fishes because these early stages in fishes are often the most vulnerable [47,48]. Embryos and larvae were exposed to control conditions (28 °C, normoxia, and salinity of 0.0 ppt for the three controls), to two treatments of a given stressor described below during each developmental period (six in total and returned to control conditions after exposure), and a group continuously exposed to each treatment (two per experiment). This yielded a total of nine groups for each experiment. Details of the protocol are shown in Figure 6.
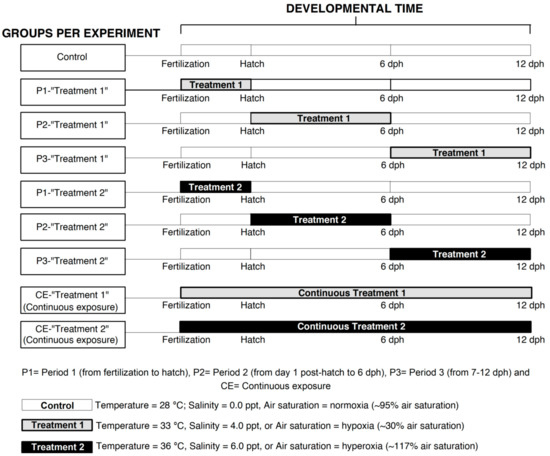
Figure 6.
Experimental design for determining specific environmental effects on critical windows for development in the early ontogeny of Atractosteus tropicus. The first developmental period is from fertilization to hatching (~72 h), the second from 1 day post hatch (dph) to 6 dph, and the third from 7 to 12 dph. White boxes indicate control conditions during the experiments; light gray boxes represent exposure to treatments 1 (33 °C, hypoxia (~30% air saturation) or salinity of 4.0 ppt); darker boxes show exposure to treatments 2 (36 °C, hyperoxia (117% air saturation) or salinity of 6.0 ppt). Boxes at the bottom explain the conditions for control populations and treatments 1/treatments 2 during each independent experiment. See text for details.
The first experiment consisted of a temperature challenge to the embryos and larvae with temperatures of 33 °C and 36 °C. These temperatures are near the upper thermal limits for larvae of this species [37] and above its optimal incubation temperature (28–30 °C) [86]. In the second experiment, fish were exposed to hypoxia (~30% air saturation) and hyperoxia (117% air saturation; Figure 6), since Lepisosteids can tolerate environments with variable dissolved oxygen levels [77,122,123]. The third experiment consisted of exposure to salinities of 4.0 ppt and 6.0 ppt, because Lepisosteids can tolerate euryhaline environments and little information is available on early life stages under these conditions, especially regarding developmental critical windows. Temperature, air saturation, and salinity switches between developmental periods (as required for each experiment) occurred gradually within a three-hour period (e.g., from 28 °C to 33 °C).
4.4. Treatment Protocols
4.4.1. Temperature
Water temperature was regulated by a 12 Johnson Controls Thermostat attached to 12 immersion heaters (Volteck 46307 CAGU-5). Temperature in the experimental tanks was maintained at a constant temperature of either 33 ± 0.5 °C or 36 ± 0.5 °C. To enhance heat dispersal, the immersion heaters were placed immediately above the aeration stones.
Embryos and larvae were exposed to control conditions (control group), to two temperature treatments (33 °C and 36 °C) during developmental period 1 (experimental groups P1-33 °C and P1-36 °C), developmental period 2 (experimental groups P2-33 °C and P2-36 °C), developmental period 3 (experimental groups P3-33 °C and P3-36 °C), and to continuous exposure to both treatments (experimental groups CE-33 °C and CE-36 °C) as shown in Figure 6.
4.4.2. Air Saturation
Two treatments with different water air saturations were employed for this experiment, hypoxia (~30.2% air saturation) and hyperoxia (~117% air saturation). Hypoxia was generated by bubbling nitrogen gas directly into each tank until air saturation decreased to 30.2% of full air saturation and remained constant for each exposure. Hyperoxia was set at 117% of full air saturation and was created using a counter current water tower with an aeration stone for O2 gas introduced to at the bottom of the tower. Water was sent from the tower to the tanks through a water pump and returned to the tower with a vent tube in the center of the tanks which was high enough to maintain a constant water level at the same air saturation. The tower promoted a high concentration of oxygen in the water simulating the function of a U-tube aeration system without the off-gas recycling mechanism [124].
Both hypoxia and hyperoxia were constantly measured with two oxygen meters YSI Pro2030. A 50% water change was performed every two days, adding water that was pre-equilibrated to the required oxygen levels.
Embryos and larvae were exposed to control conditions (control group), to two air saturation treatments (hypoxia and hyperoxia) during developmental period 1 (experimental groups P1-hypoxia and P1-hyperoxia), developmental period 2 (experimental groups P2-hypoxia and P2-hyperoxia), developmental period 3 (experimental groups P3-hypoxia and P3-hyperoxia), and to continuous exposure to both treatments (experimental groups CE-hypoxia and CE-hyperoxia) as shown in Figure 6.
4.4.3. Salinity
Water for the control population has a salinity of 0.0 ppt. Experimental salinity levels were set at 4.0 ppt for treatment 1 and 6.0 ppt for treatment 2, using industrial sea salt crystals (Grupo Industrial Roche) that were mechanically dissolved in the water of the tanks. Salinity was validated with a YSI Pro2030 multiparametric instrument. Water changes were made every 48 h at the proper validated salinity.
Embryos and larvae were exposed to control conditions (control group), to two salinity treatments (4.0 ppt and 6.0 ppt) during developmental period 1 (experimental groups P1-4.0 and P1-6.0), developmental period 2 (experimental groups P2-4.0 and P2-6.0), developmental period 3 (experimental groups P3-4.0 and P3-6.0), and to continuous exposure to both treatments (experimental groups CE-4.0 and CE-6.0) as shown in Figure 6.
4.5. Survival
Each experiment was carried out with 1350 fertilized eggs (150 fish per group, 50 fish per replicate). The unhatched embryos were counted and the number of dead larvae was recorded daily to calculate survival data. Sampled embryos for at 1 dph (described below) were not considered in this analysis.
4.6. Morphological Variables
Data from morphological variables (described below) were measured daily. However, data for treatments comparison (bars plots) are presented from measurements at the end of each developmental period (hatch, 6 dph, and 12 dph) using 20 individuals per treatment.
Body mass (BM) of the fish (n = 20 from each group) at the end of each developmental period was determined to the closest milligram with an analytical balance (Denver Instruments). All individuals were taken from the tanks using an aquarium net and carefully deposited in plastic chambers (5 cm in diameter, 2 cm depth) before weighing. Then, fish were carefully taken from the chamber with a tip-truncated plastic bulb pipette and deposited on a tared plastic mesh to allow excess water drainage before weighing. Larvae were carefully deposited into a different chamber for taking high resolution photographs (14 megapixels, camera Sony Alpha 350) to measure total fish length (LT) using Image J Software version 1.50 (NIH, Bethesda, Maryland). Millimeter graph paper underlying the larva was used to generate a scale on each image. Immediately after being photographed, individuals were carefully returned to holding tanks.
The specific growth rate (SGR) of the fish of each treatment was calculated as SGR = ((Ln mt − Ln mi) × t−1)) × 100 [125]; where mt represents body mass at the end of each critical window, mi the body mass of the embryos (removed from the chorion) 24 h after fertilization, t the duration of each experiment, and 100 a constant to obtain a percentage of growth. Fulton’s condition factor was calculated for each organism as K = 100*(mass/length3) [126].
4.7. Developmental Events
The time of occurrence of five developmental events in the development of the tropical gar was recorded from the three experiments, for each control and each of the respective groups. The developmental events were hatching, exogenous feeding, yolk depletion, free swimming, and snout shape change. To measure the timing and onset of the five developmental events, 30 embryos per experiment were identified and separated from the rest using a plastic mesh. Data were collected when 100% of the fish had hatched, had accepted exogenous food, had completely absorbed the yolk sac, had started swimming, and when the shape of the snout rapidly changed from a typical larval piscine snout to that characteristic of gars. These events were previously described [36] and are important for understanding the effect of extrinsic factors in the development of A. tropicus.
Temperature sensitivity coefficient (Q10) in the temperature experiment was calculated for all the developmental events with the data from the control group and the continuous exposure treatments as Q10 = (R2/R1)(10/(T2 − T1)), where Q10 is the factor by which the time to a given developmental event increases with a rise in temperature; R1 is the time to a given developmental event at temperature 1 (when T1 < T2); R2 is the time to a given developmental event at temperature 2 (where T2 > T1); T1 in the temperature at which R1 is measured; and T2 is the temperature at which R2 was measured.
4.8. Statistical Analyses
Survival data were analyzed with the log-rank Kaplan–Meier method [127] for the overall experiments (individually) and within each developmental period per experiment. A Holm–Sidak multiple comparison test was used to assess differences between groups.
The effect of temperature, salinity, and oxygen availability on body mass, total length, SGR, and Fulton’s condition factor was analyzed with a two-way ANOVA, followed by a Holm–Sidak multiple comparison test to assess differences between fish groups at the end of each developmental period and across development (n = 20 from every group, 180 fish in total for each experiment). Data on the timing of developmental events and Q10 were analyzed with a one-way ANOVA for each event between groups from a given experiment (10 fish per replicate, 30 fish from each group, per experiment). To determine differences between groups at a given experiment, a multiple comparison Holm–Sidak test was carried out. All the analyses were made with the software SigmaPlot Version 11.0 with a significance level of 0.05.
5. Conclusions
Our results supported the hypothesis that environmental stressors early in ontogeny would affect key developmental processes. Developmental periods comprising from fertilization to hatch and 1 to 6 dph were identified as sensitive in the early ontogeny of A. tropicus. The greatest effects of high temperature, hypoxia, and hyperoxia occurred from fertilization to hatch, while for salinity occurred from 1 to 6 dph. In conclusion, developing fishes may exhibit different degrees of functional responses to stressor type, stressor dose, and time of stressor exposure. Moreover, the assumption is that if temperature increases in natural fish populations (either global warming or anthropogenic causes), then their susceptible stages of ontogeny can be affected. This increase in temperature can come with a decrease in the dissolved oxygen and a slight increase in the salinity of some brackish water environments that can drastically affect fishes, especially during their narrow critical windows for survival, development, and growth. In this regard, this study gives the first information integrating a 3D critical windows design, heterokairy, and functional developing fish responses to environmental factors. In addition, A. tropicus offers strong potential as biological model for studying fish early ontogeny. Nonetheless, identification during narrower developmental critical windows in fish early stages and the effect of intrinsic or extrinsic factors need further investigation to identify the possible morphological, physiological, and molecular responses of the fish.
Author Contributions
Conceptualization, G.M., W.B., and A.Á.; methodology, G.M.; validation, E.P., R.M., S.C., W.B., and A.Á.; formal analysis, G.M.; investigation, G.M. and E.P.; resources, A.Á.; data curation, G.M.; writing—original draft preparation, G.M.; writing—review and editing, G.M., W.B., and A.Á.; visualization, G.M.; supervision, A.Á. and W.B.; project administration, A.Á.; funding acquisition, A.Á. All authors have read and agreed to the published version of the manuscript.
Funding
National Council of Science and Technology of Mexico (CONACyT), Number CB-2016-01-282765.
Institutional Review Board Statement
Animals were handled in compliance with the Norma Oficial Mexicana NOM-062-ZOO-1999 from Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, the Mexican standards for good welfare practices of laboratory animals.
Acknowledgments
We appreciate the support of the National Council of Science and Technology in Mexico (CONACYT) for the scholarship for the doctoral studies to Martínez-Bautista G, and to the Laboratorio de Acuicultura Tropical from the Universidad Juárez Autónoma de Tabasco for providing the tropical gars for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burggren, W.W.; Reyna, K.S. Developmental trajectories, critical windows and phenotypic alteration during cardio-respiratory development. Respir. Physiol. Neurobiol. 2011, 178, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, J.A. Old wine in new bottles: Reaction norms in salmonid fishes. Heredity 2011, 106, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Burggren, W.W. Phenotypic switching resulting from developmental plasticity: Fixed or reversible? Front. Physiol. 2020, 10, 1634. [Google Scholar] [CrossRef] [PubMed]
- Vehaskari, V.M.; Aviles, D.H.; Manning, J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001, 59, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.M.; Huryn, V.M.; Downes, S.R.; Mercer, A.R. The effects of queenlessness on the maturation of the honeybee olfactory system. Behav. Brain Res. 1998, 91, 115–126. [Google Scholar] [CrossRef]
- Sallout, B.; Walker, M. The fetal origin of adult diseases. J. Obstet. Gynaecol. 2003, 23, 555–560. [Google Scholar] [CrossRef]
- Pinkerton, K.E.; Joad, J.P. The mammalian respiratory system and critical windows of exposure for children’s health. Environ. Health Perspect. 2000, 108 (Suppl. S3), 457–462. [Google Scholar] [PubMed]
- Hogan, N.S.; Duarte, P.; Wade, M.G.; Lean, D.R.; Trudeau, V.L. Estrogenic exposure affects metamorphosis and alters sex ratios in the northern leopard frog (Rana pipiens): Identifying critically vulnerable periods of development. Gen. Comp. Endocrinol. 2008, 156, 515–523. [Google Scholar] [CrossRef]
- Burggren, W.W.; Mueller, C.A. Developmental critical windows and sensitive periods as three-dimensional constructs in time and space. Physiol. Biochem. Zool. 2015, 88, 91–102. [Google Scholar] [CrossRef]
- Dzialowski, E.M.; von Plettenberg, D.; Elmonoufy, N.A.; Burggren, W.W. Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 713–724. [Google Scholar] [CrossRef]
- Chan, T.; Burggren, W.W. Hypoxic incubation creates differential morphological effects during specific developmental critical windows in the embryo of the chicken (Gallus gallus). Respir. Physiol. Neurobiol. 2005, 145, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wong-Riley, M.T. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: Implication for a sensitive period. Neuroscience 2010, 165, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Tate, K.B.; Kohl, Z.F.; Eme, J.; Rhen, T.; Crossley, D.A. Critical windows of cardiovascular susceptibility to developmental hypoxia in common snapping turtle (Chelydra serpentina) embryos. Physiol. Biochem. Zool. 2015, 88, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.A.; Willis, E.; Burggren, W.W. Salt sensitivity of the morphometry of Artemia franciscana during development: A demonstration of 3D critical windows. J. Exp. Biol. 2016, 219 Pt 4, 571–581. [Google Scholar] [CrossRef]
- Burggren, W.W.; Elmonoufy, N.A. Critical developmental windows for morphology and hematology revealed by intermittent and continuous hypoxic incubation in embryos of quail (Coturnix coturnix). PLoS ONE 2017, 12, e0183649. [Google Scholar] [CrossRef]
- Mueller, C.A.; Eme, J.; Manzon, R.G.; Somers, C.M.; Boreham, D.R.; Wilson, J.Y. Embryonic critical windows: Changes in incubation temperature alter survival, hatching phenotype, and cost of development in lake whitefish (Coregonus clupeaformis). J. Comp. Physiol. B 2015, 185, 315–331. [Google Scholar] [CrossRef]
- Lee Pow, C.S.D.; Tilahun, K.; Creech, K.; Law, J.M.; Cope, W.G.; Kwak, T.J.; Kullman, S.W. Windows of susceptibility and consequences of early life exposures to 17β–estradiol on Medaka (Oryzias latipes) reproductive success. Environ. Sci. Technol. 2017, 51, 5296–5305. [Google Scholar] [CrossRef]
- Kupsco, A.; Schlenk, D. Stage susceptibility of Japanese medaka (Oryzias latipes) to selenomethionine and hypersaline developmental toxicity. Environ. Toxicol. Chem. 2016, 35, 1247–1256. [Google Scholar] [CrossRef]
- Spicer, J.I.; Burggren, W.W. Development of physiological regulatory systems: Altering the timing of crucial events. Zoology 2003, 106, 91–99. [Google Scholar] [CrossRef]
- Spicer, J.I.; Rundle, S.D. Plasticity in the timing of physiological development: Physiological heterokairy—What is it, how frequent is it, and does it matter? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 712–719. [Google Scholar] [CrossRef]
- Spicer, J.I.; Rundle, S.D.; Tills, O. Studying the altered timing of physiological events during development: It’s about time… or is it? Respir. Physiol. Neurobiol. 2011, 178, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rundle, S.D.; Spicer, J.I. Heterokairy: A significant form of developmental plasticity? Biol. Lett. 2016, 12, 20160509. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, S.; Bayley, M.; McKenzie, D.J. Measuring oxygen uptake in fishes with bimodal respiration. J. Fish Biol. 2016, 88, 206–231. [Google Scholar] [CrossRef] [PubMed]
- Little, C. The Colonisation of Land: Origins and Adaptations of Terrestrial Animals; Cambridge University Press: Cambridge, UK, 1983; p. 290. [Google Scholar]
- Milsom, W.K. New insights into gill chemoreception: Receptor distribution and roles in water and air breathing fish. Respir. Physiol. Neurobiol. 2012, 184, 326–339. [Google Scholar] [CrossRef]
- Perry, S.F.; Wilson, R.J.; Straus, C.; Harris, M.B.; Remmers, J.E. Which came first, the lung or the breath? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 37–47. [Google Scholar] [CrossRef]
- Randall, D.J.; Ip, Y.K. Ammonia as a respiratory gas in water and air-breathing fishes. Respir. Physiol. Neurobiol. 2006, 154, 216–225. [Google Scholar] [CrossRef]
- Shartau, R.B.; Brauner, C.J. Acid–base and ion balance in fishes with bimodal respiration. J. Fish Biol. 2014, 84, 682–704. [Google Scholar] [CrossRef]
- Johnson, D.W.; Christie, M.R.; Moye, J. Quantifying evolutionary potential of marine fish larvae: Heritability, selection, and evolutionary constraints. Evolution 2010, 64, 2614–2628. [Google Scholar] [CrossRef]
- Blank, T.; Burggren, W.W. Hypoxia-induced developmental plasticity of the gills and air-breathing organ of Trichopodus trichopterus. J. Fish Biol. 2014, 84, 808–826. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; Brauner, C.J.; Wang, Y.X.; Richards, J.G.; Patrick, M.L.; Xi, W.; Matey, V.; Val, A.L. Impact of ontogenetic changes in branchial morphology on gill function in Arapaima gigas. Physiol. Biochem. Zool. 2010, 83, 322–332. [Google Scholar] [CrossRef]
- Joss, J.M. Lungfish evolution and development. Gen. Comp. Endocrinol. 2006, 148, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sanchez, J.F.; Burggren, W.W. Environmental modulation of the onset of air breathing and survival of Betta splendens and Trichopodus trichopterus. J. Fish Biol. 2014, 84, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.A.; Joss, J.M.; Seymour, R.S. Effects of environmental oxygen on development and respiration of Australian lungfish (Neoceratodus forsteri) embryos. J. Comp. Physiol. B 2011, 181, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M. The pattern of histogenesis and growth of tooth plates in larval stages of extant lungfish. J. Anat. 1985, 140 Pt 4, 627–643. [Google Scholar]
- Aguilera, C.; Mendoza, R.; Rodríguez, G.; Márquez, G. Morphological description of alligator gar and tropical gar larvae, with an emphasis on growth indicators. Trans. Am. Fish. Soc. 2002, 131, 899–909. [Google Scholar] [CrossRef]
- Burggren, W.W.; Bautista, G.M.; Coop, S.C.; Couturier, G.M.; Delgadillo, S.P.; García, R.M.; González, C.A.A. Developmental cardiorespiratory physiology of the air-breathing tropical gar, Atractosteus tropicus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R689–R701. [Google Scholar] [CrossRef]
- Comabella, Y.; Azanza, J.; Hurtado, A.; Canabal, J.; García-Galano, T. Allometric growth in Cuban gar (Atractosteus tristoechus) larvae. Univ. Cienc. 2013, 29, 2227–2690. [Google Scholar]
- Dean, B. The early development of garpike and sturgeon. J. Morphol. 1895, 11, 1–62. [Google Scholar] [CrossRef]
- Echelle, A.A.; Riggs, C.D. Aspects of the early life history of gars (Lepisosteus) in Lake Texoma. Trans. Am. Fish. Soc. 1972, 101, 106–112. [Google Scholar] [CrossRef]
- Long, W.L.; Ballard, W.W. Normal embryonic stages of the longnose gar, Lepisosteus osseus. BMC Dev. Biol. 2001, 1, 6. [Google Scholar] [CrossRef]
- Mendoza, R.; Aguilera, C.; Rodríguez, G.; González, M.; Castro, R. Morphophysiological studies on alligator gar (Atractosteus spatula) larval development as a basis for their culture and repopulation of their natural habitats. Rev. Fish Biol. Fish. 2002, 12, 133–142. [Google Scholar] [CrossRef]
- Barrientos-Villalobos, J.; Espinosa de los Monteros, A. Genetic variation and recent population history of the tropical gar Atractosteus tropicus Gill (Pisces: Lepisosteidae). J. Fish Biol. 2008, 73, 1919–1936. [Google Scholar] [CrossRef]
- Bussing, W.A. Freshwater Fishes of Costa Rica; Editorial Universidad de Costa Rica: San José, Costa Rica, 1998; Volume 46, p. 468. [Google Scholar]
- Miller, R.R.; Minckley, W.L.; Norris, S.M. Freshwater Fishes of Mexico; University of Chicago Press: Chicago, IL, USA, 2005; p. 490. [Google Scholar]
- Mora, M.; Cabrera-Peña, J.; Galeano, G. Reproducción y alimentación del gaspar Atractosteus tropicus (Pisces: Lepisosteidae) en el refugio nacional de vida silvestre Caño Negro, Costa Rica. Rev. Biol. Trop. 1997, 45, 861–866. [Google Scholar]
- Burggren, W.W.; Bagatto, B. Cardiovascular anatomy and physiology. In Fish Larval Physiology; Finn, R.N., Kapoor, B.J., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 119–161. [Google Scholar]
- Rudneva, I. Biomarkers for Stress in Fish Embryos and Larvae; CRS Press: Boca Raton, FL, USA, 2014; p. 206. [Google Scholar]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Comabella, Y.; Hurtado, A.; Canabal, J.; García-Galano, T. Effect of temperature on hatching and growth of Cuban gar (Atractosteus tristoechus) larvae. ERA 2014, 1, 19–32. [Google Scholar]
- Del Río, A.M.; Davis, B.E.; Fangue, N.A.; Todgham, A.E. Combined effects of warming and hypoxia on early life stage Chinook salmon physiology and development. Conserv. Physiol. 2019, 7, coy078. [Google Scholar] [CrossRef]
- Hassell, K.L.; Coutin, P.C.; Nugegoda, D. Hypoxia impairs embryo development and survival in black bream (Acanthopagrus butcheri). Mar. Pollut. Bull. 2008, 57, 302–306. [Google Scholar] [CrossRef]
- Hou, Z.S.; Wen, H.S.; Li, J.F.; He, F.; Li, Y.; Qi, X. Environmental hypoxia causes growth retardation, osteoclast differentiation and calcium dyshomeostasis in juvenile rainbow trout (Oncorhynchus mykiss). Sci. Total Environ. 2020, 705, 135272. [Google Scholar] [CrossRef]
- Levesque, K.D.; Wright, P.A.; Bernier, N.J. Cross talk without cross tolerance: Effect of rearing temperature on the hypoxia response of embryonic zebrafish. Physiol. Biochem. Zool. 2019, 92, 349–364. [Google Scholar] [CrossRef]
- Fry, J. The effect of environmental factors on the physiology of fish. In Fish Physiology 5; Hoar, W.S., Randall, D.J., Eds.; Academic Press: New York, NY, USA, 1974; pp. 1–98. [Google Scholar]
- Matthews, M.D.; Prangnell, D.; Glenewinkel, H. Sensitivity of Guadalupe bass swim-up fry to hyperoxia. N. Am. J. Aquac. 2017, 79, 289–298. [Google Scholar] [CrossRef]
- Pleizier, N.; Nelson, C.; Cooke, S.; Brauner, C.J. Understanding gas bubble trauma in an era of hydropower expansion: How do fish compensate at depth? Can. J. Fish. Aquat. Sci. 2019, 77, 556–563. [Google Scholar] [CrossRef]
- Beamish, F.W.H. Osmoregulation in juvenile and adult lampreys. Can. J. Fish. Aquat. Sci. 1980, 37, 1739–1750. [Google Scholar] [CrossRef]
- Richards, J.E.; Beamish, F.W.H. Initiation of feeding and salinity tolerance in the pacific lamprey Lampetra tridentata. Mar. Biol. 1981, 63, 73–77. [Google Scholar] [CrossRef]
- McDowall, R.M. Diadromy in Fishes: Migrations between Freshwater and Marine Environments; Timber Press: Portland, OR, USA, 1988; p. 308. [Google Scholar]
- Allen, P.J.; Cech, J.J. Age/size effects on juvenile green sturgeon, Acipenser medirostris, oxygen consumption, growth, and osmoregulation in saline environments. Environ. Biol. Fishes 2007, 79, 211–229. [Google Scholar] [CrossRef]
- Allen, P.J.; McEnroe, M.; Forostyan, T.; Cole, S.; Nicholl, M.M.; Hodge, B.; Cech, J.J. Ontogeny of salinity tolerance and evidence for seawater-entry preparation in juvenile green sturgeon, Acipenser medirostris. J. Comp. Physiol. B 2011, 181, 1045–1062. [Google Scholar] [CrossRef]
- Schwarz, D.E.; Allen, P.J. Effects of salinity on growth and ion regulation of juvenile alligator gar Atractosteus spatula. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 169, 44–50. [Google Scholar] [CrossRef]
- Brauner, C.J.; Rombough, P.J. Ontogeny and paleophysiology of the gill: New insights from larval and air-breathing fish. Respir. Physiol. Neurobiol. 2012, 184, 293–300. [Google Scholar] [CrossRef]
- Kwan, G.T.; Wexler, J.B.; Wegner, N.C.; Tresguerres, M. Ontogenetic changes in cutaneous and branchial ionocytes and morphology in yellowfin tuna (Thunnus albacares) larvae. J. Comp. Physiol. B 2019, 189, 81–95. [Google Scholar] [CrossRef]
- Melo, L.H.; Martins, Y.S.; Melo, R.M.C.; Prado, P.S.; Luz, R.K.; Bazzoli, N.; Rizzo, E. Low salinity negatively affects early larval development of Nile tilapia, Oreochromis niloticus: Insights from skeletal muscle and molecular biomarkers. Zygote 2019, 27, 375–381. [Google Scholar] [CrossRef]
- Tresguerres, M.; Clifford, A.M.; Harter, T.S.; Roa, J.N.; Thies, A.B.; Yee, D.P.; Brauner, C.J. Evolutionary links between intra-and extracellular acid–base regulation in fish and other aquatic animals. J. Exp. Zool. A Ecol. Integr. Physiol. 2020, 333, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.D.; Thomas, G.A.; Clark, A.L. Early piscivory and timing of the critical period in postlarval longnose gar at mile 571 of the Ohio River. Trans. Ky. Acad. Sci. 1979, 40, 122–128. [Google Scholar]
- Simon, T.P.; Tyberghein, E.J. Contributions to the early life history of the spotted gar, Lepisosteus oculatus Winchell, from Hatchet Creek, Alabama. J. Ky. Acad. Sci. 1991, 52, 124–131. [Google Scholar]
- Hjort, J. Fluctuations in the Great Fisheries of Northern Europe Viewed in the Light of Biological Research; Andr. Fred. Høst & Søn: Copenhagen, Denmark, 1914; Volume 20, pp. 1–228. [Google Scholar]
- Jonassen, T.M.; Imsland, A.K.; Stefansson, S.O. The interaction of temperature and fish size on growth of juvenile halibut. J. Fish Biol. 1999, 54, 556–572. [Google Scholar] [CrossRef]
- Keckeis, H.; Kamler, E.; Bauer-Nemeschkal, E.; Schneeweiss, K. Survival, development and food energy partitioning of nase larvae and early juveniles at different temperatures. J. Fish Biol. 2001, 59, 45–61. [Google Scholar] [CrossRef]
- Mendiola, D.; Alvarez, P.; Cotano, U.; Martínez de Murguía, A. Early development and growth of the laboratory reared north-east Atlantic mackerel Scomber scombrus L. J. Fish Biol. 2007, 70, 911–933. [Google Scholar] [CrossRef]
- Person-Le Ruyet, J.; Buchet, V.; Vincent, B.; Le Delliou, H.; Quéméner, L. Effects of temperature on the growth of pollack (Pollachius pollachius) juveniles. Aquaculture 2006, 251, 340–345. [Google Scholar] [CrossRef]
- Boeuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Smatresk, N.J.; Cameron, J.N. Respiration and acid-base physiology of the spotted gar, a bimodal breather: III. Response to a transfer from fresh water to 50% sea water, and control of ventilation. J. Exp. Biol. 1982, 96, 295–306. [Google Scholar]
- Malik, A.; Abbas, G.; Jabbar, A.; Sajjad Shah, S.; Ali Muhammad, A. Effect of different salinity level on spawning, fertilization, hatching and survival of common carp, Cyprinus carpio (Linnaeus, 1758) in semi-artificial environment. Iran. J. Fish. Sci. 2018, 17, 790–804. [Google Scholar]
- Vetemaa, M.; Saat, T. Effects of salinity on the development of fresh-water and brackish-water ruffe Gymnocephalus cernuus (L.) embryos. Ann. Zool. Fenn. 1996, 33, 687–691. [Google Scholar]
- Hernández-Rubio, M.C.; Figueroa-Lucero, G. Effects of temperature and salinity during the embryonic period of Chirostoma humboldtianum and Chirostoma riojai (Atherinopsidae) until hatching. Hidrobiológica 2013, 23, 365–373. [Google Scholar]
- Holliday, F.G.T. The effect of salinity on the eggs and larvae of teleost. Fish Physiol. 1969, 1, 293–311. [Google Scholar]
- Eddy, F.B.; Handy, R.D. Ecological and Environmental Physiology of Fishes; Oxford University Press: Oxford, UK, 2012; p. 252. [Google Scholar]
- Shumway, D.L.; Warren, C.E.; Doudoroff, P. Influence of oxygen concentration and water movement on the growth of steelhead trout and coho salmon embryos. Trans. Am. Fish. Soc. 1964, 93, 342–356. [Google Scholar] [CrossRef]
- Marks, C.; Kaut, K.P.; Moore, F.B.; Bagatto, B. Ontogenetic oxygen changes alter zebra fish size, behavior, and blood glucose. Physiol. Biochem. Zool. 2012, 85, 635–644. [Google Scholar] [CrossRef]
- Rombough, P.J. Growth, aerobic metabolism, and dissolved oxygen requirements of embryos and alevins of steelhead, Salmo gairdneri. Can. J. Zool. 1988, 66, 651–660. [Google Scholar] [CrossRef]
- Mason, J.C. Hypoxial stress prior to emergence and competition among coho salmon fry. J. Fish. Res. Board Can. 1969, 26, 63–91. [Google Scholar] [CrossRef]
- Pepin, P. Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. Can. J. Fish. Aquat. Sci. 1991, 48, 503–518. [Google Scholar] [CrossRef]
- Tills, O.; Spicer, J.I.; Rundle, S.D. Salinity-induced heterokairy in an upper-estuarine population of the snail Radix balthica (Mollusca: Pulmonata). Aquat. Biol. 2010, 9, 95–105. [Google Scholar] [CrossRef]
- Márquez-Couturier, G.; Vázquez-Navarrete, C.J.; Contreras-Sánchez, W.M.; Álvarez-González, C.A. Acuicultura Tropical Sustentable: Una Estrategia para la Producción y Conservación del Pejelagarto (Atractosteus tropicus) en Tabasco, México, 2nd ed.; Colección José Narciso Rovirosa; Universidad Juárez Autónoma de Tabasco: Tabasco, México, 2015; Volume 3, p. 223. [Google Scholar]
- Frías-Quintana, C.A.; Álvarez-González, C.A.; Márquez-Couturier, G. Diseño de microdietas para el larvicultivo de pejelagarto Atractosteus tropicus, Gill 1863. Univ. Cienc. 2010, 26, 265–282. [Google Scholar]
- Morales, G. Reproducción y Desarrollo Embriológico del Catán (Lepisosteus spatula Lacepede): Primeros Resultados; SEPES. Dirección General de Acuacultura: Ciudad de Mexico, Mexico, 1987; p. 70. [Google Scholar]
- Morales, G. Especies nativas de México. Acuavisión 1999, 12, 7–9. [Google Scholar]
- Rao, T.R. Influence of salinity on the eggs and larvae of the California killifish Fundulus parvipinnis. Mar. Biol. 1974, 24, 155–162. [Google Scholar] [CrossRef]
- Czerkies, P.; Brzuzan, P.; Kordalski, K.; Luczynski, M. Critical partial pressures of oxygen causing precocious hatching in Coregonus lavaretus and C. albula embryos. Aquaculture 2001, 196, 151–158. [Google Scholar] [CrossRef]
- Garside, E.T. Some effects of oxygen in relation to temperature on the development of lake trout embryos. Can. J. Zool. 1959, 37, 689–698. [Google Scholar] [CrossRef]
- Warkentin, K.M. Oxygen, gills, and embryo behavior: Mechanisms of adaptive plasticity in hatching. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Yúfera, M.; Darias, M.J. The onset of exogenous feeding in marine fish larvae. Aquaculture 2007, 268, 53–63. [Google Scholar] [CrossRef]
- Rønnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Sæle, Ø.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquacult. 2013, 5, S59–S98. [Google Scholar] [CrossRef]
- Camacho, S.; Carmona, R.; Llorente, J.I.; Sanz, A.; García-Gallego, M.; Domezain, A.; Dominguez, N.; Ostos-Garrido, M.V. Stomach development in the sturgeon Acipenser naccarii: Histoenzymatic and ultrastructural analysis. J. Appl. Ichthyol. 2011, 27, 693–700. [Google Scholar] [CrossRef]
- Dou, S.; Masuda, R.; Tanaka, M.; Tsukamoto, K. Feeding resumption, morphological changes and mortality during starvation in Japanese flounder larvae. J. Fish Biol. 2002, 60, 1363–1380. [Google Scholar] [CrossRef]
- Comabella Soto, Y. Desarrollo Larval del Manjuarí Atractosteus tristoechus (Bloch y Schneider, 1801) (Pisces: Lepisosteidae) en Condiciones de Cultivo: Aspectos Morfo-Fisiológicos. Ph.D. Thesis, Universidad de la Habana, La Habana, Cuba, 2011. [Google Scholar]
- Moteki, M.; Yoseda, K.; Sahin, T.; Üstündağ, C.; Kohno, H. Transition from endogenous to exogenous nutritional sources in larval black sea turbot Psetta maxima. Fish. Sci. 2001, 67, 571–578. [Google Scholar] [CrossRef][Green Version]
- Williams, K.; Papanikos, N.; Phelps, R.P.; Shardo, J.D. Development, growth, and yolk utilization of hatchery-reared red snapper Lutjanus campechanus larvae. Mar. Ecol. Prog. Ser. 2004, 275, 231–239. [Google Scholar] [CrossRef]
- Coughlin, D.J. Ontogeny of feeding behavior of first-feeding Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1991, 48, 1896–1904. [Google Scholar] [CrossRef]
- Makrakis, M.C.; Nakatani, K.; Bialetzki, A.; Sanches, P.V.; Baumgartner, G.; Gomes, L.C. Ontogenetic shifts in digestive tract morphology and diet of fish larvae of the Itaipu Reservoir, Brazil. Environ. Biol. Fishes 2005, 72, 99–107. [Google Scholar] [CrossRef]
- Collins, L.A.; Nelson, S.G. Effects of temperature on oxygen consumption, growth, and development of embryos and yolk-sac larvae of Siganus randalli (Pisces: Siganidae). Mar. Biol. 1993, 117, 195–204. [Google Scholar] [CrossRef]
- Hart, P.R.; Purser, G.J. Effects of salinity and temperature on eggs and yolk sac larvae of the greenback flounder (Rhombosolea tapirina Günther, 1862). Aquaculture 1995, 136, 221–230. [Google Scholar] [CrossRef]
- May, R.C. Effects of temperature and salinity on yolk utilization in Bairdiella icistia (Jordan & Gilbert) (Pisces: Sciaenidae). J. Exp. Mar. Biol. Ecol. 1974, 16, 213–225. [Google Scholar]
- Khan, U. Effect of salinity on yolk utilization and growth of brook trout alevins (Salvelinus fontinalis). J. Anatol. Environ. Animal Sci. 2019, 4, 93–96. [Google Scholar]
- Massa, F.; Delorme, C.; Baglinière, J.L.; Prunet, P.; Grimaldi, C. Expositions d’oeufs de truite commune (Salmo trutta) à des hypoxies temporaires ou continue: Effets sur la branchie, la résorption de la vésicule vitelline et les caractéristiques morphométriques des alevins. Bull. Français. Pêche Piscic. 1999, 355, 421–440. [Google Scholar] [CrossRef]
- Polymeropoulos, E.T.; Elliott, N.G.; Frappell, P.B. Hypoxic acclimation leads to metabolic compensation after reoxygenation in Atlantic salmon yolk-sac alevins. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 213, 28–35. [Google Scholar] [CrossRef]
- Roussel, J.M. Carry-over effects in brown trout (Salmo trutta): Hypoxia on embryos impairs predator avoidance by alevins in experimental channels. Can. J. Fish. Aquat. Sci. 2007, 64, 786–792. [Google Scholar] [CrossRef]
- Voesenek, C.J.; Muijres, F.T.; van Leeuwen, J.L. Biomechanics of swimming in developing larval fish. J. Exp. Biol. 2018, 221, jeb149583. [Google Scholar] [CrossRef] [PubMed]
- Batty, R.S. Development of swimming movements and musculature of larval herring (Clupea harengus). J. Exp. Biol. 1984, 110, 217–229. [Google Scholar]
- Vieira, V.I.A.; Johnston, I.A. Influence of temperature on muscle-fibre development in larvae of the herring Clupea harengus. Mar. Biol. 1992, 112, 333–341. [Google Scholar] [CrossRef]
- Domenici, P.; Herbert, N.A.; Lefrançois, C.; Steffensen, J.F.; McKenzie, D.J. The effect of hypoxia on fish swimming performance and behaviour. In Swimming Physiology of Fish; Springer: Berlin/Heidelberg, Germany, 2013; pp. 129–159. [Google Scholar]
- Kaufmann, R.; Wieser, W. Influence of temperature and ambient oxygen on the swimming energetics of cyprinid larvae and juveniles. In Environmental Biology of European Cyprinids; Weiser, W., Scheimer, F., Goldschmidt, A., Kotrschal, K., Eds.; Springer Science & Business Media: Luxemburg, 1992; pp. 87–96. [Google Scholar]
- Márquez-Couturier, G.; Álvarez-González, C.A.; Contreras-Sánchez, W.M.; Hernández-Vidal, U.; Hernández-Franyutti, A.A.; Mendoza-Alfaro, R.E.; Aguilera-González, C.; García-Galano, T.; Civera-Cerecedo, R.; Goytortua-Bores, E. Avances en la alimentación y nutrición del pejelagarto Atractosteus tropicus. In Avances en Nutrición Acuícola VIII, Proceedings of the VIII Simposium Internacional de Nutrición Acuícola, Mazatlán, SI, México, 15–17 November 2006; Cruz-Suárez, E., Ricque, D.M., Tapia-Salazar, M., Nieto-López, M.G., Villarreal-Cavazos, D.A., Puello-Cruz, A.C., García-Ortega, A., Eds.; Universidad Autónoma de Nuevo León: Monterrey, NL, Mexico, 2006; pp. 446–523. [Google Scholar]
- Busch, A. Transition from endogenous to exogenous nutrition: Larval size parameters determining the start of external feeding and size of prey ingested by Ruegen spring herring Clupea harengus. Mar. Ecol. Prog. Ser. 1996, 130, 39–45. [Google Scholar] [CrossRef]
- Dabrowski, K.; Bardega, R. Mouth size and predicted food size preferences of larvae of three cyprinid fish species. Aquaculture 1984, 40, 41–46. [Google Scholar] [CrossRef]
- Hecht, T.; Pienaar, A.G. A review of cannibalism and its implications in fish larviculture. J. World Aquac. Soc. 1993, 24, 246–261. [Google Scholar] [CrossRef]
- Hill, L.; Renfro, J.; Reynolds, R. Effects of dissolved oxygen tensions upon the rate of young spotted gar, Lepisosteus oculatus (Lepisosteidae). Southwest. Nat. 1972, 17, 273–278. [Google Scholar] [CrossRef]
- Rimoldi, S.; Terova, G.; Zaccone, G.; Parker, T.; Kuciel, M.; Dabrowski, K. The effect of hypoxia and hyperoxia on growth and expression of hypoxia-related genes and proteins in spotted gar Lepisosteus oculatus larvae and juveniles. J. Exp. Zool. B Mol. Dev. Evol. 2016, 326, 250–267. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture; Cayuga Aqua Ventures: Ithaca, NY, USA, 2010; p. 976. [Google Scholar]
- Brett, J.R.; Groves, D.D. Physiological energetics. In Volume VIII—Fish Physiology. Bioenergetics and Growth; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: New York, NY, USA, 1979; p. 344. [Google Scholar]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).